Obesity Status Affects the Relationship Between Protein Intake and Insulin Sensitivity in Late Pregnancy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Anthropometrics
2.2. Energy Expenditure
2.3. Insulin Sensitivity Analysis
2.4. Dietary Intake
2.5. Statistical Analysis
3. Results
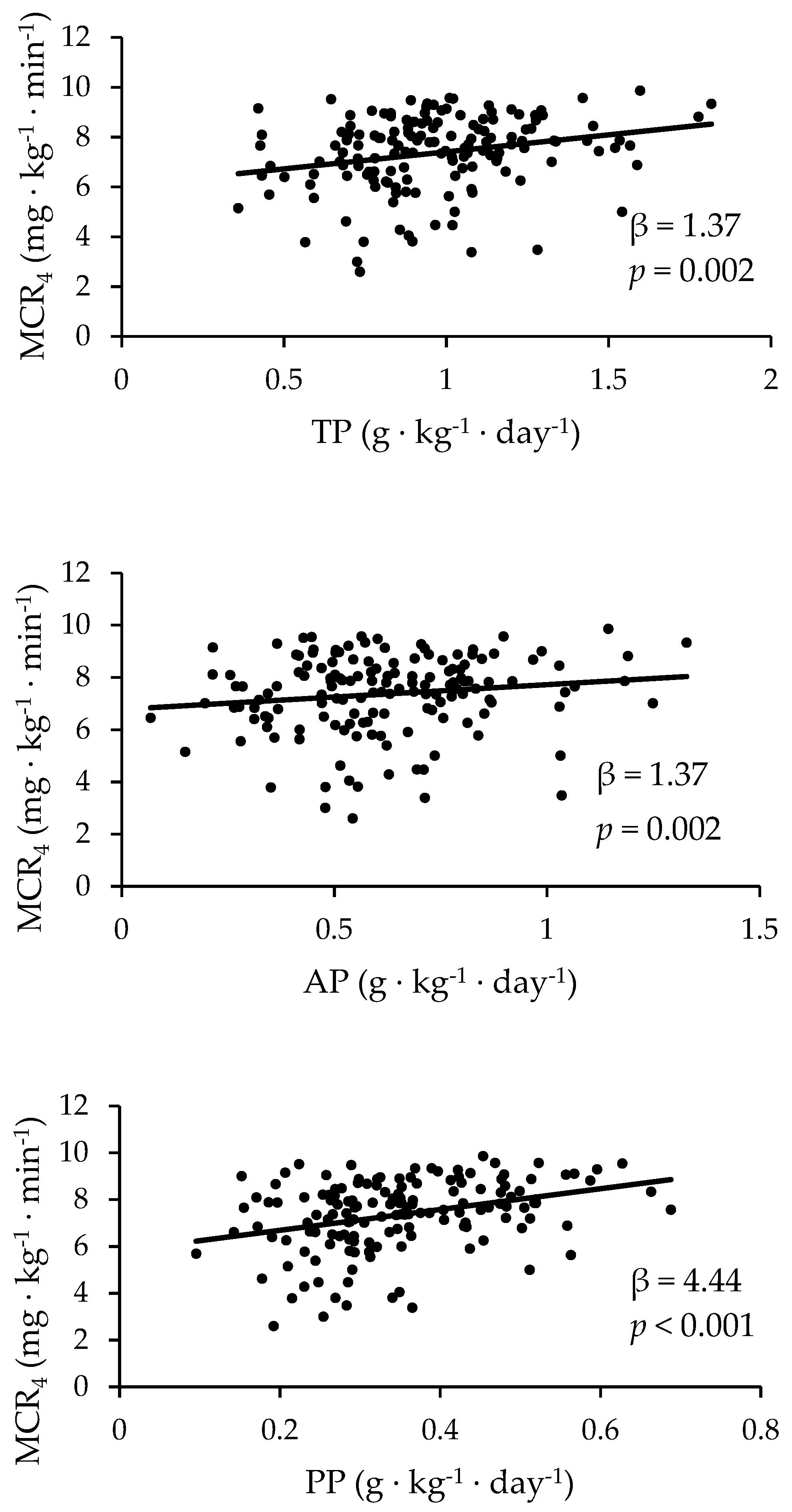
3.1. The Prediction of MCR Based on Dietary Protein Using Regressions
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Flegal, K.M.; Carroll, M.D.; Ogden, C.L.; Curtin, L.R. Prevalence and Trends in Obesity Among US Adults, 1999–2008. JAMA 2010, 303, 235. [Google Scholar] [CrossRef] [PubMed]
- DeSisto, C.L.; Kim, S.Y.; Sharma, A.J. Prevalence Estimates of Gestational Diabetes Mellitus in the United States, Pregnancy Risk Assessment Monitoring System (PRAMS), 2007–2010. Prev. Chronic Dis. 2014, 11, 130415. [Google Scholar] [CrossRef]
- Kim, S.Y.; England, L.; Wilson, H.G.; Bish, C.; Satten, G.A.; Dietz, P. Percentage of gestational diabetes mellitus attributable to overweight and obesity. Am. J. Public Health 2010, 100, 1047–1052. [Google Scholar] [CrossRef]
- Hytten, F.; Chamberlain, G. Clinical Physiology in Obstetrics; Blackwell Scientific Publications: Oxford, UK, 1991. [Google Scholar]
- Stephens, T.V.; Payne, M.; Ball, R.O.; Pencharz, P.B.; Elango, R. Protein Requirements of Healthy Pregnant Women during Early and Late Gestation Are Higher than Current Recommendations. J. Nutr. 2015, 145, 73–78. [Google Scholar] [CrossRef]
- Herring, C.M.; Bazer, F.W.; Johnson, G.A.; Wu, G. Impacts of maternal dietary protein intake on fetal survival, growth, and development. Exp. Biol. Med. 2018, 243, 525–533. [Google Scholar] [CrossRef]
- Morisaki, N.; Nagata, C.; Yasuo, S.; Morokuma, S.; Kato, K.; Sanefuji, M.; Shibata, E.; Tsuji, M.; Senju, A.; Kawamoto, T.; et al. Japan Environment and Children’s Study Group Optimal protein intake during pregnancy for reducing the risk of fetal growth restriction: The Japan Environment and Children’s Study. Br. J. Nutr. 2018, 1–9. [Google Scholar] [CrossRef]
- Te Morenga, L.; Docherty, P.; Williams, S.; Mann, J. The Effect of a Diet Moderately High in Protein and Fiber on Insulin Sensitivity Measured Using the Dynamic Insulin Sensitivity and Secretion Test (DISST). Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.-G.; Zhang, Q.-L.; Liu, X.-L.; Wu, H.; Zheng, J.-L.; Xiang, Y.-B. Dietary protein intake and risk of type 2 diabetes: A dose–response meta-analysis of prospective studies. Eur. J. Nutr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.I.; Yoshino, J.; Kelly, S.C.; Reeds, D.N.; Okunade, A.; Patterson, B.W.; Klein, S.; Mittendorfer, B. High-Protein Intake during Weight Loss Therapy Eliminates the Weight-Loss-Induced Improvement in Insulin Action in Obese Postmenopausal Women. Cell Rep. 2016, 17, 849–861. [Google Scholar] [CrossRef] [Green Version]
- Gannon, M.C.; Nuttall, F.Q.; Saeed, A.; Jordan, K.; Hoover, H. An increase in dietary protein improves the blood glucose response in persons with type 2 diabetes. Am. J. Clin. Nutr. 2003, 78, 734–741. [Google Scholar] [CrossRef]
- Ke, Q.; Chen, C.; He, F.; Ye, Y.; Bai, X.; Cai, L.; Xia, M. Association between dietary protein intake and type 2 diabetes varies by dietary pattern. Diabetol. Metab. Syndr. 2018, 10, 48. [Google Scholar] [CrossRef]
- Spiller, G.A.; Jensen, C.D.; Pattison, T.S.; Chuck, C.S.; Whittam, J.H.; Scala, J. Effect of protein dose on serum glucose and insulin response to sugars. Am. J. Clin. Nutr. 1987, 46, 474–480. [Google Scholar] [CrossRef]
- Lieberman, M.; Marks, A.D. Marks Basic Medical Biochemistry, 4th ed.; Lippincott Williams & Wilkens: Baltimore, MA, USA, 2013. [Google Scholar]
- Pal, S.; Ellis, V.; Dhaliwal, S. Effects of whey protein isolate on body composition, lipids, insulin and glucose in overweight and obese individuals. Br. J. Nutr. 2010, 104, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Linn, T.; Santosa, B.; Grönemeyer, D.; Aygen, S.; Scholz, N.; Busch, M.; Bretzel, R.G. Effect of long-term dietary protein intake on glucose metabolism in humans. Diabetologia 2000, 43, 1257–1265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricci, G.; Canducci, E.; Pasini, V.; Rossi, A.; Bersani, G.; Ricci, E.; Alvisi, V. Nutrient intake in Italian obese patients: Relationships with insulin resistance and markers of non-alcoholic fatty liver disease. Nutrition 2011, 27, 672–676. [Google Scholar] [CrossRef]
- Pounis, G.D.; Tyrovolas, S.; Antonopoulou, M.; Zeimbekis, A.; Anastasiou, F.; Bountztiouka, V.; Metallinos, G.; Gotsis, E.; Lioliou, E.; Polychronopoulos, E.; et al. Long-term animal-protein consumption is associated with an increased prevalence of diabetes among the elderly: The Mediterranean islands (MEDIS) study. Diabetes Metab. 2010, 36, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Morenga, L.T.; Williams, S.; Brown, R.; Mann, J. Effect of a relatively high-protein, high-fiber diet on body composition and metabolic risk factors in overweight women. Eur. J. Clin. Nutr. 2010, 64, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Rietman, A.; Schwarz, J.; Tomé, D.; Kok, F.J.; Mensink, M. High dietary protein intake, reducing or eliciting insulin resistance? Eur. J. Clin. Nutr. 2014, 68, 973–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walrand, S.; Short, K.R.; Bigelow, M.L.; Sweatt, A.J.; Hutson, S.M.; Nair, K.S. Functional impact of high protein intake on healthy elderly people. Am. J. Physiol. Metab. 2008, 295, E921–E928. [Google Scholar] [CrossRef] [Green Version]
- Tura, A.; Conte, B.; Caparrotto, C.; Spinella, P.; Maestrelli, P.; Valerio, A.; Pacini, G.; Avogaro, A. Insulin sensitivity and secretion in young, healthy subjects are not changed by Zone and Mediterranean diets. Med. J. Nutr. Metab. 2010, 3, 233–237. [Google Scholar] [CrossRef]
- Newsom, S.A.; Schenk, S.; Thomas, K.M.; Harber, M.P.; Knuth, N.D.; Goldenberg, N.; Horowitz, J.F. Energy deficit after exercise augments lipid mobilization but does not contribute to the exercise-induced increase in insulin sensitivity. J. Appl. Physiol. 2010, 108, 554–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azemati, B.; Rajaram, S.; Jaceldo-Siegl, K.; Sabate, J.; Shavlik, D.; Fraser, G.E.; Haddad, E.H. Animal-Protein Intake Is Associated with Insulin Resistance in Adventist Health Study 2 (AHS-2) Calibration Substudy Participants: A Cross-Sectional Analysis. Curr. Dev. Nutr. 2017, 1, e000299. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Xu, Q.; Jiang, R.; Han, T.; Sun, C.; Na, L. Dietary Protein Consumption and the Risk of Type 2 Diabetes: A Systematic Review and Meta-Analysis of Cohort Studies. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Viguiliouk, E.; Stewart, S.E.; Jayalath, V.H.; Ng, A.P.; Mirrahimi, A.; de Souza, R.J.; Hanley, A.J.; Bazinet, R.P.; Blanco Mejia, S.; Leiter, L.A.; et al. Effect of Replacing Animal Protein with Plant Protein on Glycemic Control in Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2015, 7, 9804–9824. [Google Scholar] [CrossRef] [PubMed]
- Allman, B.R.; Andres, A.; Børsheim, E. The Association of Maternal Protein Intake during Pregnancy in Humans with Maternal and Offspring Insulin Sensitivity Measures. Curr. Dev. Nutr. 2019. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Bowers, K.; Tobias, D.K.; Hu, F.B.; Zhang, C. Prepregnancy dietary protein intake, major dietary protein sources, and the risk of gestational diabetes mellitus: A prospective cohort study. Diabetes Care 2013, 36, 2001–2008. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Schulze, M.B.; Solomon, C.G.; Hu, F.B. A prospective study of dietary patterns, meat intake and the risk of gestational diabetes mellitus. Diabetologia 2006, 49, 2604–2613. [Google Scholar] [CrossRef] [Green Version]
- Pang, W.W.; Colega, M.; Cai, S.; Chan, Y.H.; Padmapriya, N.; Chen, L.-W.; Soh, S.-E.; Han, W.M.; Tan, K.H.; Lee, Y.S.; et al. Higher Maternal Dietary Protein Intake Is Associated with a Higher Risk of Gestational Diabetes Mellitus in a Multiethnic Asian Cohort. J. Nutr. 2017, 147, 653–660. [Google Scholar] [CrossRef]
- Liang, Y.; Gong, Y.; Zhang, X.; Yang, D.; Zhao, D.; Quan, L.; Zhou, R.; Bao, W.; Cheng, G. Dietary Protein Intake, Meat Consumption, and Dairy Consumption in the Year Preceding Pregnancy and During Pregnancy and Their Associations With the Risk of Gestational Diabetes Mellitus: A Prospective Cohort Study in Southwest China. Front. Endocrinol. 2018, 9, 596. [Google Scholar] [CrossRef]
- Catalano, P.M.; Kirwan, J.P.; Haugel-de Mouzon, S.; King, J. Gestational Diabetes and Insulin Resistance: Role in Short- and Long-Term Implications for Mother and Fetus. J. Nutr. 2003, 133, 1674S–1683S. [Google Scholar] [CrossRef]
- Stumvoll, M.; Mitrakou, A.; Pimenta, W.; Jenssen, T.; Yki-Jarvinen, H.; Van Haeften, T.; Renn, W.; Gerich, J. Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care 2000, 23, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Stumvoll, M.; Van Haeften, T.; Fritsche, A.; Gerich, J. Oral Glucose Tolerance Test Indexes for Insulin Sensitivity and Secretion Based on Various Availabilities of Sampling Times. Diabetes Care 2001, 24, 796–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krukowski, R.A.; West, D.; DiCarlo, M.; Shankar, K.; Cleves, M.A.; Tedford, E.; Andres, A. A Behavioral Intervention to Reduce Excessive Gestational Weight Gain. Matern. Child Health J. 2017, 21, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Olson, C.M. Achieving a Healthy Weight Gain During Pregnancy. Annu. Rev. Nutr. 2008, 28, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Weir, J.B.de.V. New methods for calculating metabolic rate with special reference to protein metabolism. J. Physiol. 1949, 109, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Black, A.E.; Goldberg, G.R.; Jebb, S.A.; Livingstone, M.B.; Cole, T.J.; Prentice, A.M. Critical evaluation of energy intake data using fundamental principles of energy physiology: 2. Evaluating the results of published surveys. Eur. J. Clin. Nutr. 1991, 45, 583–599. [Google Scholar] [PubMed]
- Livingstone, M.B.; Prentice, A.M.; Strain, J.J.; Coward, W.A.; Black, A.E.; Barker, M.E.; McKenna, P.G.; Whitehead, R.G. Accuracy of weighed dietary records in studies of diet and health. BMJ 1990, 300, 708–712. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, G.R.; Black, A.E.; Jebb, S.A.; Cole, T.J.; Murgatroyd, P.R.; Coward, W.A.; Prentice, A.M. Critical evaluation of energy intake data using fundamental principles of energy physiology: 1. Derivation of cut-off limits to identify under-recording. Eur. J. Clin. Nutr. 1991, 45, 569–581. [Google Scholar] [PubMed]
- Sonagra, A.D.; Biradar, S.M.; Dattatreya, K. DSJM Normal pregnancy- a state of insulin resistance. J. Clin. Diagn. Res. 2014, 8. [Google Scholar] [CrossRef]
- Sluijs, I.; Beulens, J.W.; Spijkerman, A.M.; Grobbee, D.E.; Van der Schouw, Y.T. Dietary intake of total, animal, and vegetable protein and risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-NL study. Diabetes Care 2010, 33, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Scott, D.; Hodge, A.M.; English, D.R.; Giles, G.G.; Ebeling, P.R.; Sanders, K.M. Dietary protein intake and risk of type 2 diabetes: Results from the Melbourne Collaborative Cohort Study and a meta-analysis of prospective studies. Am. J. Clin. Nutr. 2016, 104, 1352–1365. [Google Scholar] [CrossRef]
- Pan, A.; Sun, Q.; Bernstein, A.M.; Schulze, M.B.; Manson, J.E.; Willett, W.C.; Hu, F.B. Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. Am. J. Clin. Nutr. 2011, 94, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Jamilian, M.; Asemi, Z. The Effect of Soy Intake on Metabolic Profiles of Women With Gestational Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2015, 100, 4654–4661. [Google Scholar] [CrossRef] [PubMed]
- Sucher, S.; Markova, M.; Hornemann, S.; Pivovarova, O.; Rudovich, N.; Thomann, R.; Schneeweiss, R.; Rohn, S.; Pfeiffer, A.F.H. Comparison of the effects of diets high in animal or plant protein on metabolic and cardiovascular markers in type 2 diabetes: A randomized clinical trial. Diabetes Obes. Metab. 2017, 19, 944–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoy, M.K.; Sebastian, R.S.; Goldman, J.D.; Wilkinson Enns, C.; Moshfegh, A.J. Consuming Vegetable-Based Salad Is Associated with Higher Nutrient Intakes and Diet Quality among US Adults, What We Eat in America, National Health and Nutrition Examination Survey 2011-2014. J. Acad. Nutr. Diet. 2019. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.; Cummings, J.H.; Englyst, H.N.; Key, T.; Liu, S.; Riccardi, G.; Summerbell, C.; Uauy, R.; van Dam, R.M.; Venn, B.; et al. FAO/WHO Scientific Update on carbohydrates in human nutrition: Conclusions. Eur. J. Clin. Nutr. 2007, 61, S132–S137. [Google Scholar] [CrossRef]
- Weickert, M.O.; Pfeiffer, A.F. Impact of Dietary Fiber Consumption on Insulin Resistance and the Prevention of Type 2 Diabetes. J. Nutr. 2018, 148, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Riccardi, G.; Giacco, R.; Rivellese, A. Dietary fat, insulin sensitivity and the metabolic syndrome. Clin. Nutr. 2004, 23, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Jeyakumar, S.M.; Vijaya Kumar, P.; Giridharan, N.V.; Vajreswari, A. Vitamin A improves insulin sensitivity by increasing insulin receptor phosphorylation through protein tyrosine phosphatase 1B regulation at early age in obese rats of WNIN/Ob strain. Diabetes Obes. Metab. 2011, 13, 955–958. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, P.; Zhao, Z.-H.; Zhang, Y.; Ma, Z.-M.; Wang, S.-X. Vitamin B6 Prevents Endothelial Dysfunction, Insulin Resistance, and Hepatic Lipid Accumulation in Apoe (-/-) Mice Fed with High-Fat Diet. J. Diabetes Res. 2016, 2016, 1748065. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Morán, M.; Guerrero-Romero, F. Oral magnesium supplementation improves insulin sensitivity and metabolic control in type 2 diabetic subjects: A randomized double-blind controlled trial. Diabetes Care 2003, 26, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef] [PubMed]
| Combined n = 173 | NW n = 72 | OW n = 71 | OB n = 30 | |
|---|---|---|---|---|
| Age (years) | 29.7 ± 0.3 | 30.0 ± 0.4 | 29.8 ± 0.4 | 29.2 ± 0.7 |
| Early Pregnancy BMI (kg/m2) | 25.8 ± 0.3 | 21.9 ± 0.2 | 26.8 ± 0.2 * | 32.2 ± 0.3 * ‡ |
| GWG (kg) | 8.6 ± 0.2 | 9.2 ± 0.2 | 8.7 ± 0.3 | 7.1 ± 0.5 * ‡ |
| Resting Energy Expenditure (kcal/day) | 1629 ± 16 | 1525 ± 21 | 1671 ± 24 * | 1794 ± 48 * ‡ |
| Fasting Insulin (µIU/mL) | 8.87 ± 0.40 | 6.23 ± 0.42 | 10.24 ± 0.65 * | 11.82 ± 0.99 * ‡ |
| Fasting Glucose (mmol/L) | 4.48 ± 0.3 | 4.35 ± 0.4 | 4.57 ± 0.04 * | 4.57 ± 0.07 * |
| MCR1 (mg·kg−1·min−1) | 5.53 ± 0.21 | 6.96 ± 0.26 | 4.46 ± 0.27 * | 3.36 ± 0.37 * ‡ |
| MCR2 (mg·kg−1·min−1) | 5.31 ± 0.19 | 6.15 ± 0.27 | 4.55 ± 0.29 * | 4.64 ± 0.47 * |
| MCR3 (mg·kg−1·min−1) | 8.07 ± 0.12 | 8.86 ± 0.13 | 7.57 ± 0.20 * | 7.33 ± 0.30 * |
| MCR4 (mg·kg−1·min−1) | 7.36 ± 0.12 | 8.10 ± 0.14 | 6.92 ± 0.18 * | 6.57 ± 0.32 * |
| HOMA2-IR | 1.12 ± 0.05 | 0.78 ± 0.05 | 1.29 ± 0.08 * | 1.49 ± 0.12 * |
| TP (g/day) | 74.28 ± 1.44 | 74.48 ± 2.37 | 74.35 ± 2.19 | 73.72 ± 3.21 |
| TP (g·kg−1·day−1) | 0.96 ± 0.02 | 1.07 ± 0.03 | 0.93 ± 0.03 * | 0.79 ± 0.04 *‡ |
| AP (g/day) | 47.99 ± 1.29 | 47.14 ± 1.99 | 48.66 ± 2.03 | 48.48 ± 3.07 |
| AP (g·kg−1·day−1) | 0.62 ± 0.02 | 0.68 ± 0.03 | 0.61 ± 0.03 * | 0.52 ± 0.03 * ‡ |
| PP (g/day) | 26.68 ± 0.58 | 27.85 ± 0.94 | 26.19 ± 0.91 | 25.17 ± 1.14 * |
| PP (g·kg−1·day−1) | 0.35 ± 0.01 | 0.40 ± 0.01 | 0.33 ± 0.01 * | 0.27 ± 0.01 * |
| n | TP | AP | PP | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No Adj. | Adj. | No Adj. | Adj. | No Adj. | Adj. | ||||||||
| β | p | β | p | β | p | β | p | β | p | β | p | ||
| MCR1 | 145 | 3.59 | 0.006 | 2.03 | 0.19 | 13.09 | <0.001 | ||||||
| MCR2 | 134 | 1.91 | 0.09 | −0.07 | 0.95 | 0.70 | 0.61 | −0.74 | 0.57 | 8.50 | 0.002 | 2.88 | 0.32 |
| MCR3 | 151 | 1.52 | 0.001 | 0.82 | 0.06 | 1.11 | 0.04 | 0.60 | 0.23 | 4.61 | <0.001 | 2.51 | 0.03 |
| MCR4 | 152 | 1.37 | 0.002 | 0.64 | 0.01 | 0.95 | 0.08 | 0.39 | 0.43 | 4.44 | <0.001 | 2.39 | 0.03 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allman, B.R.; Diaz Fuentes, E.; Williams, D.K.; Turner, D.E.; Andres, A.; Børsheim, E. Obesity Status Affects the Relationship Between Protein Intake and Insulin Sensitivity in Late Pregnancy. Nutrients 2019, 11, 2190. https://doi.org/10.3390/nu11092190
Allman BR, Diaz Fuentes E, Williams DK, Turner DE, Andres A, Børsheim E. Obesity Status Affects the Relationship Between Protein Intake and Insulin Sensitivity in Late Pregnancy. Nutrients. 2019; 11(9):2190. https://doi.org/10.3390/nu11092190
Chicago/Turabian StyleAllman, Brittany R., Eva Diaz Fuentes, D. Keith Williams, Donald E. Turner, Aline Andres, and Elisabet Børsheim. 2019. "Obesity Status Affects the Relationship Between Protein Intake and Insulin Sensitivity in Late Pregnancy" Nutrients 11, no. 9: 2190. https://doi.org/10.3390/nu11092190
APA StyleAllman, B. R., Diaz Fuentes, E., Williams, D. K., Turner, D. E., Andres, A., & Børsheim, E. (2019). Obesity Status Affects the Relationship Between Protein Intake and Insulin Sensitivity in Late Pregnancy. Nutrients, 11(9), 2190. https://doi.org/10.3390/nu11092190






