In Vivo Fat Quantification: Monitoring Effects of a 6-Week Non-Energy-Restricted Ketogenic Diet in Healthy Adults Using MRI, ADP and BIA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design and Intervention
2.3. Data Acquisition
2.4. Data Post Processing
2.5. Statistics
3. Results
3.1. MRI
3.2. Modality Comparison
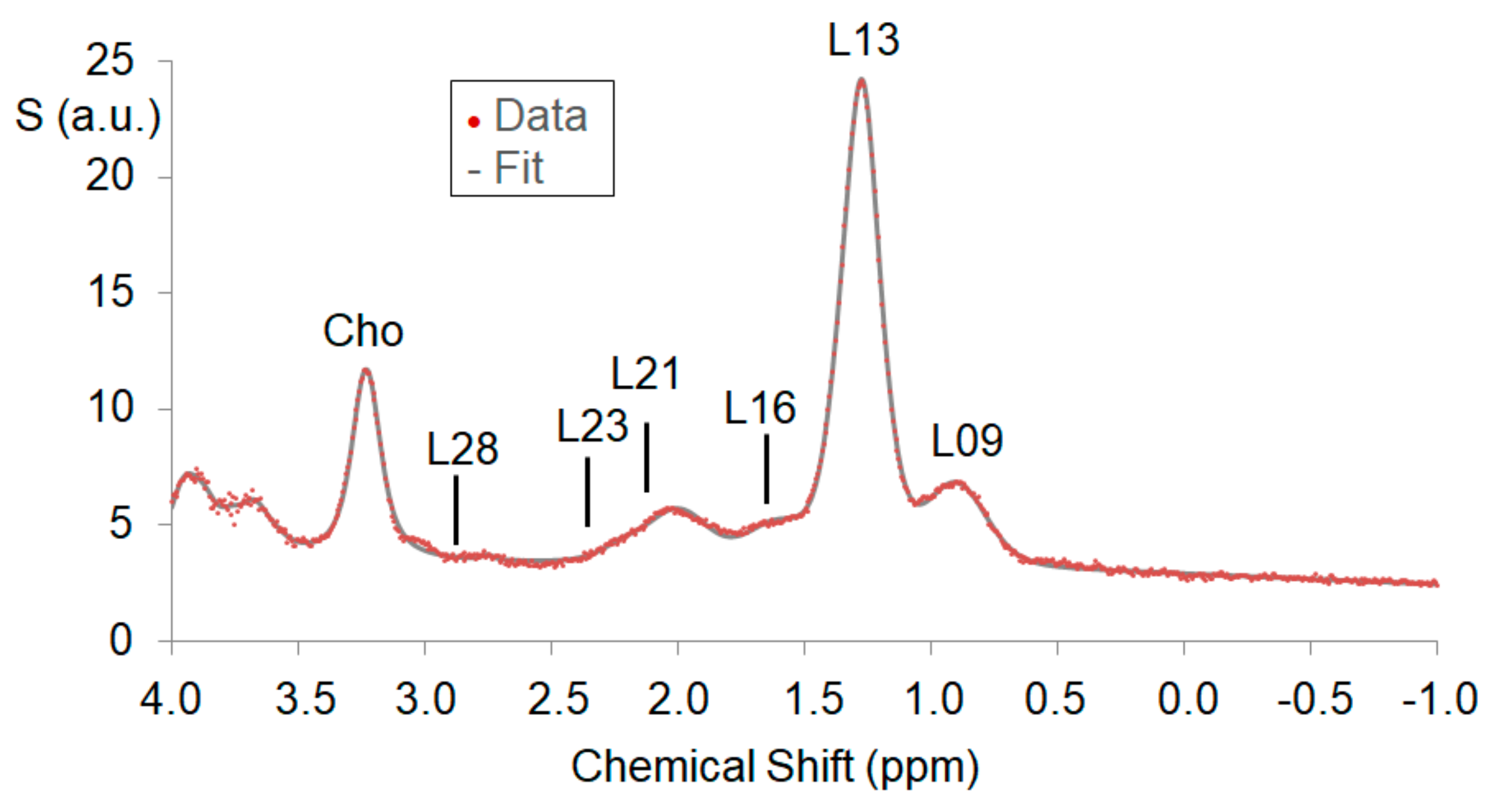
3.3. MRS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Veech, R.L. The therapeutic implications of ketone bodies: The effects of ketone bodies in pathological conditions: Ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot. Essent. Fatty Acids 2004, 70, 309–319. [Google Scholar] [CrossRef]
- Freeman, J.M.; Kossoff, E.H.; Hartman, A.L. The ketogenic diet: One decade later. Pediatrics 2007, 119, 535–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paoli, A.; Rubini, A.; Volek, J.S.; Grimaldi, K.A. Beyond weight loss: A review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur. J. Clin. Nutr. 2013, 67, 789–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krieger, J.W.; Sitren, H.S.; Daniels, M.J.; Langkamp-Henken, B. Effects of variation in protein and carbohydrate intake on body mass and composition during energy restriction: A meta-regression. Am. J. Clin. Nutr. 2006, 83, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.D.; Chen, K.Y.; Guo, J.; Lam, Y.Y.; Leibel, R.L.; Mayer, L.E.; Reitman, M.L.; Rosenbaum, M.; Smith, S.R.; Walsh, B.T.; et al. Energy expenditure and body composition changes after an isocaloric ketogenic diet in overweight and obese men. Am. J. Clin. Nutr. 2016, 104, 324–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urbain, P.; Strom, L.; Morawski, L.; Wehrle, A.; Deibert, P.; Bertz, H. Impact of a 6-week non-energy-restricted ketogenic diet on physical fitness, body composition and biochemical parameters in healthy adults. Nutr. Metab. 2017, 14, 17. [Google Scholar] [CrossRef] [Green Version]
- Phinney, S.D.; Bistrian, B.R.; Evans, W.J.; Gervino, E.; Blackburn, G.L. The human metabolic response to chronic ketosis without caloric restriction: Preservation of submaximal exercise capability with reduced carbohydrate oxidation. Metabolism 1983, 32, 769–776. [Google Scholar] [CrossRef]
- Paoli, A.; Grimaldi, K.; D’Agostino, D.; Cenci, L.; Moro, T.; Bianco, A.; Palma, A. Ketogenic diet does not affect strength performance in elite artistic gymnasts. J. Int. Soc. Sports Nutr. 2012, 9, 34. [Google Scholar] [CrossRef] [Green Version]
- Zajac, A.; Poprzecki, S.; Maszczyk, A.; Czuba, M.; Michalczyk, M.; Zydek, G. The Effects of a Ketogenic Diet on Exercise Metabolism and Physical Performance in Off-Road Cyclists. Nutrients 2014, 6, 2493–2508. [Google Scholar] [CrossRef]
- Klement, R.J.; Frobel, T.; Albers, T.; Fikenzer, S.; Prinzhausen, J. A pilot case study on the impact of a self-prescribed ketogenic diet on biochemical parameters and running performance in healthy and physically active individuals. Nutr. Med. 2013, 1, 27. [Google Scholar]
- Kullberg, J.; Brandberg, J.; Angelhed, J.-E.; Frimmel, H.; Bergelin, E.; Strid, L.; Ahlström, H.; Johansson, L.; Lönn, L. Whole-body adipose tissue analysis: Comparison of MRI, CT and dual energy X-ray absorptiometry. Br. J. Radiol. 2009, 82, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Becker, C. Formulierung populationsspezifischer Algorithmen zur Bestimmung der Körperfettmasse von Kindern, Jugendlichen und Erwachsenen, basierend auf Feldmethoden mit Air Displacement Plethysmography als Referenz. Ph.D. Thesis, Christian-Albrechts Universität Kiel, Kiel, Germany, 2002. [Google Scholar]
- Bray, G.A.; Jablonski, K.A.; Fujimoto, W.Y.; Barrett-Connor, E.; Haffner, S.; Hanson, R.L.; Hill, J.O.; Hubbard, V.; Kriska, A.; Stamm, E.; et al. Relation of central adiposity and body mass index to the development of diabetes in the Diabetes Prevention Program. Am. J. Clin. Nutr. 2008, 87, 1212–1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fields, D.A.; Goran, M.I.; McCrory, M.A. Body-composition assessment via air-displacement plethysmography in adults and children: A review. Am. J. Clin. Nutr. 2002, 75, 453–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchholz, A.C.; Bartok, C.; Schoeller, D.A. The Validity of Bioelectrical Impedance Models in Clinical Populations. Nutr. Clin. Pract. 2004, 19, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Manuel Gómez, J.; Lilienthal Heitmann, B.; Kent-Smith, L.; Melchior, J.-C.; Pirlich, M.; et al. Bioelectrical impedance analysis—Part II: Utilization in clinical practice. Clin. Nutr. 2004, 23, 1430–1453. [Google Scholar] [CrossRef]
- Ludwig, U.-A.; Klausmann, F.; Baumann, S.; Honal, M.; Hövener, J.-B.; König, D.; Deibert, P.; Büchert, M. Whole-body MRI-based fat quantification: A comparison to air displacement plethysmography. J. Magn. Reson. Imaging 2014, 40, 1437–1444. [Google Scholar] [CrossRef]
- Deibert, P.; Lazaro, A.; Schaffner, D.; Berg, A.; Koenig, D.; Kreisel, W.; Baumstark, M.W.; Steinmann, D.; Buechert, M.; Lange, T.; et al. Comprehensive lifestyle intervention vs soy protein-based meal regimen in non-alcoholic steatohepatitis. World J. Gastroenterol. 2019, 25, 1116–1131. [Google Scholar] [CrossRef]
- Dixon, W.T. Simple proton spectroscopic imaging. Radiology 1984, 153, 189–194. [Google Scholar] [CrossRef]
- Hernando, D.; Kellman, P.; Haldar, J.P.; Liang, Z.-P. Robust Water/Fat Separation in the Presence of Large Field Inhomogeneities Using a Graph Cut Algorithm. Magn. Reson. Med. Off. J. Soc. Magn. Reson. Med. Soc. Magn. Reson. Med. 2010, 63, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Martin, A.D.; Daniel, M.Z.; Drinkwater, D.T.; Clarys, J.P. Adipose tissue density, estimated adipose lipid fraction and whole body adiposity in male cadavers. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 1994, 18, 79–83. [Google Scholar] [CrossRef]
- Prior, B.M.; Modlesky, C.M.; Evans, E.M.; Sloniger, M.A.; Saunders, M.J.; Lewis, R.D.; Cureton, K.J. Muscularity and the density of the fat-free mass in athletes. J. Appl. Physiol. 2001, 90, 1523–1531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Provencher, S.W. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn. Reson. Med. 1993, 30, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, G.; Yokoo, T.; Bydder, M.; Cruite, I.; Schroeder, M.E.; Sirlin, C.B.; Middleton, M.S. In vivo characterization of the liver fat 1H MR spectrum. NMR Biomed. 2011, 24, 784–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, Q.; Danzer, C.F.; Fuchs, A.; Wolfrum, C.; Rudin, M. Hepatic lipid composition differs between ob/ob and ob/+ control mice as determined by using in vivo localized proton magnetic resonance spectroscopy. Magn. Reson. Mater. Phys. Biol. Med. 2012, 25, 381–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huxley, R.; Mendis, S.; Zheleznyakov, E.; Reddy, S.; Chan, J. Body mass index, waist circumference and waist:hip ratio as predictors of cardiovascular risk—A review of the literature. Eur. J. Clin. Nutr. 2010, 64, 16–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Artz, N.S.; Haufe, W.M.; Hooker, C.A.; Hamilton, G.; Wolfson, T.; Campos, G.M.; Gamst, A.C.; Schwimmer, J.B.; Sirlin, C.B.; Reeder, S.B.; et al. Reproducibility of MR-based liver fat quantification across field strength: Same-day comparison between 1.5T and 3T in obese subjects. J. Magn. Reson. Imaging 2015, 42, 811–817. [Google Scholar] [CrossRef] [Green Version]
- Lukaski, H.C. Biological indexes considered in the derivation of the bioelectrical impedance analysis. Am. J. Clin. Nutr. 1996, 64, 397S–404S. [Google Scholar] [CrossRef]
- Mccrory, M.; Gomez, T.; Bernauer, E.; Molé, P. Evaluation of a new air displacement plethysmograph for measuring human body composition. Med. Sci. Sports Exerc. 1995, 27, 1686–1691. [Google Scholar] [CrossRef] [Green Version]
- Xiang, Q.-S.; An, L. Water-fat imaging with direct phase encoding. J. Magn. Reson. Imaging 1997, 7, 1002–1015. [Google Scholar] [CrossRef]
- De Leva, P. Adjustments to Zatsiorsky-Seluyanov’s segment inertia parameters. J. Biomech. 1996, 29, 1223–1230. [Google Scholar] [CrossRef]
- Garrow, J.S. Is Body Fat Distribution Changed by Dieting? Acta Med. Scand. 2009, 222, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Börnert, P.; Keupp, J.; Eggers, H.; Aldefeld, B. Whole-body 3D water/fat resolved continuously moving table imaging. J. Magn. Reson. Imaging 2007, 25, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, E.J.; Nanz, D.; Leinhard, O.D.; Marcon, M.; Fischer, M.A. Whole-body adipose tissue and lean muscle volumes and their distribution across gender and age: MR-derived normative values in a normal-weight Swiss population. Magn. Reson. Med. 2018, 79, 449–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volek, J.S.; Sharman, M.J.; Love, D.M.; Avery, N.G.; Gómez, A.L.; Scheett, T.P.; Kraemer, W.J. Body composition and hormonal responses to a carbohydrate-restricted diet. Metabolism 2002, 51, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Urbain, P.; Bertz, H. Monitoring for compliance with a ketogenic diet: What is the best time of day to test for urinary ketosis? Nutr. Metab. 2016, 13, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thamer, C.; Machann, J.; Haap, M.; Stefan, N.; Heller, E.; Schnödt, B.; Stumvoll, M.; Claussen, C.; Fritsche, A.; Schick, F.; et al. Intrahepatic Lipids Are Predicted by Visceral Adipose Tissue Mass in Healthy Subjects. Diabetes Care 2004, 27, 2726–2729. [Google Scholar] [CrossRef] [Green Version]
- Van Herpen, N.A.; Schrauwen-Hinderling, V.B.; Schaart, G.; Mensink, R.P.; Schrauwen, P. Three Weeks on a High-Fat Diet Increases Intrahepatic Lipid Accumulation and Decreases Metabolic Flexibility in Healthy Overweight Men. J. Clin. Endocrinol. Metab. 2011, 96, E691–E695. [Google Scholar] [CrossRef] [Green Version]
- Moller, L.; Stodkilde-Jorgensen, H.; Jensen, F.T.; Jorgensen, J.O.L. Fasting in healthy subjects is associated with intrahepatic accumulation of lipids as assessed by 1H-magnetic resonance spectroscopy. Clin. Sci. 2008, 114, 547–552. [Google Scholar] [CrossRef] [Green Version]
- Yki-Järvinen, H. Nutritional Modulation of Non-Alcoholic Fatty Liver Disease and Insulin Resistance. Nutrients 2015, 7, 9127–9138. [Google Scholar] [CrossRef] [Green Version]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.-C.; Pirlich, M.; et al. Bioelectrical impedance analysis—Part I: Review of principles and methods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef]
- Kershaw, E.E.; Flier, J.S. Adipose Tissue as an Endocrine Organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef] [PubMed]
- Montague, C.T.; O’Rahilly, S. The perils of portliness: Causes and consequences of visceral adiposity. Diabetes 2000, 49, 883–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kern, P.A.; Ranganathan, S.; Li, C.; Wood, L.; Ranganathan, G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E745–E751. [Google Scholar] [CrossRef] [PubMed]
- Marette, A. Molecular mechanisms of inflammation in obesity-linked insulin resistance. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 2003, 27, S46–S48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mokdad, A.H.; Ford, E.S.; Bowman, B.A.; Dietz, W.H.; Vinicor, F.; Bales, V.S.; Marks, J.S. Prevalence of Obesity, Diabetes, and Obesity-Related Health Risk Factors, 2001. JAMA 2003, 289, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Lee, K. Greater beneficial effects of visceral fat reduction compared with subcutaneous fat reduction on parameters of the metabolic syndrome: A study of weight reduction programmes in subjects with visceral and subcutaneous obesity. Diabet. Med. 2004, 22, 266–272. [Google Scholar] [CrossRef]
- Porter, S.A.; Massaro, J.M.; Hoffmann, U.; Vasan, R.S.; O’Donnel, C.J.; Fox, C.S. Abdominal Subcutaneous Adipose Tissue: A Protective Fat Depot? Diabetes Care 2009, 32, 1068–1075. [Google Scholar] [CrossRef] [Green Version]
- Volynets, V.; Machann, J.; Küper, M.A.; Maier, I.B.; Spruss, A.; Königsrainer, A.; Bischoff, S.C.; Bergheim, I. A moderate weight reduction through dietary intervention decreases hepatic fat content in patients with non-alcoholic fatty liver disease (NAFLD): A pilot study. Eur. J. Nutr. 2013, 52, 527–535. [Google Scholar] [CrossRef]
- Lange, T.; Buechert, M.; Baumstark, M.W.; Deibert, P.; Gerner, S.; Rydén, H.; Seufert, J.; Korsten-Reck, U. Value of MRI and MRS fat measurements to complement conventional screening methods for childhood obesity. J. Magn. Reson. Imaging 2015, 42, 1214–1222. [Google Scholar] [CrossRef]




| Unit | PRE | POST | Asymp. Significance (2-Tailed) | |
|---|---|---|---|---|
| Body weight | kg | 75.6 (58.5–89.7) | 73.1 (56.0–86.5) | 0.003 |
| BMI | kg/m2 | 26.1 (23.2–28.6) | 25.2 (22.2–27.3) | 0.003 |
| Bioelectrical Impedance Analysis (BIA) | ||||
| Fat mass | kg | 26.1 (17.7–35.2) | 23.9 (16.3–31.4) | 0.003 |
| Fat-free mass | kg | 47.2 (39.9–71.9) | 48.9 (38.3–70.2) | 0.003 |
| Air Displacement Plethysmography (ADP) | ||||
| Fat mass | kg | 28.7 (21.5–38.2) | 27.3 (19.2–36.5) | 0.075 |
| Fat-free mass | kg | 46.9 (36.7–68.2) | 45.8 (36.1–67.3) | 0.075 |
| Magnetic Resonance Imaging (MRI) | ||||
| Abdominal mass total | kg | 22.5 (16.1–28.2) | 21.7 (15.2–26.9) | 0.929 |
| Abdominal fat mass total | kg | 11.2 (6.5–14.6) | 10.5 (6.3–13.2) | 0.016 |
| Abdominal fat-free mass total | kg | 11.3 (9.0–15.8) | 11.3 (8.9–15.7) | 0.013 |
| Mass subcutaneous | kg | 7.1 (4.2–9.7) | 6.6 (3.9–8.9) | 0.004 |
| Fat mass subcutaneous | kg | 6.8 (3.7–9.5) | 6.2 (3.3–8.7) | 0.004 |
| Fat-free mass subcutaneous | kg | 0.3 (0.2–0.6) | 0.4 (0.2–0.7) | 0.004 |
| Mass internal | kg | 15.3 (11.8–21.3) | 15.1 (11.3–20.4) | 0.026 |
| Fat mass internal | kg | 4.4 (2.8–6.2) | 4.2 (2.7–5.5) | 0.799 |
| Fat-free mass internal | kg | 10.9 (8.8–15.2) | 10.9 (8.3–15.0) | 0.021 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buechert, M.; Lange, T.; Deibert, P.; Urbain, P. In Vivo Fat Quantification: Monitoring Effects of a 6-Week Non-Energy-Restricted Ketogenic Diet in Healthy Adults Using MRI, ADP and BIA. Nutrients 2020, 12, 244. https://doi.org/10.3390/nu12010244
Buechert M, Lange T, Deibert P, Urbain P. In Vivo Fat Quantification: Monitoring Effects of a 6-Week Non-Energy-Restricted Ketogenic Diet in Healthy Adults Using MRI, ADP and BIA. Nutrients. 2020; 12(1):244. https://doi.org/10.3390/nu12010244
Chicago/Turabian StyleBuechert, Martin, Thomas Lange, Peter Deibert, and Paul Urbain. 2020. "In Vivo Fat Quantification: Monitoring Effects of a 6-Week Non-Energy-Restricted Ketogenic Diet in Healthy Adults Using MRI, ADP and BIA" Nutrients 12, no. 1: 244. https://doi.org/10.3390/nu12010244





