C-Reactive Protein as a Possible Predictor of Trail-Making Performance in Individuals with Psychiatric Disorders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cognitive Tests
2.2. Clinical Inventories and Parameters
2.3. Biological Assays
2.4. Statistics
3. Results
3.1. Description of the Study Population
3.2. Results from Multiple Regression Analyses
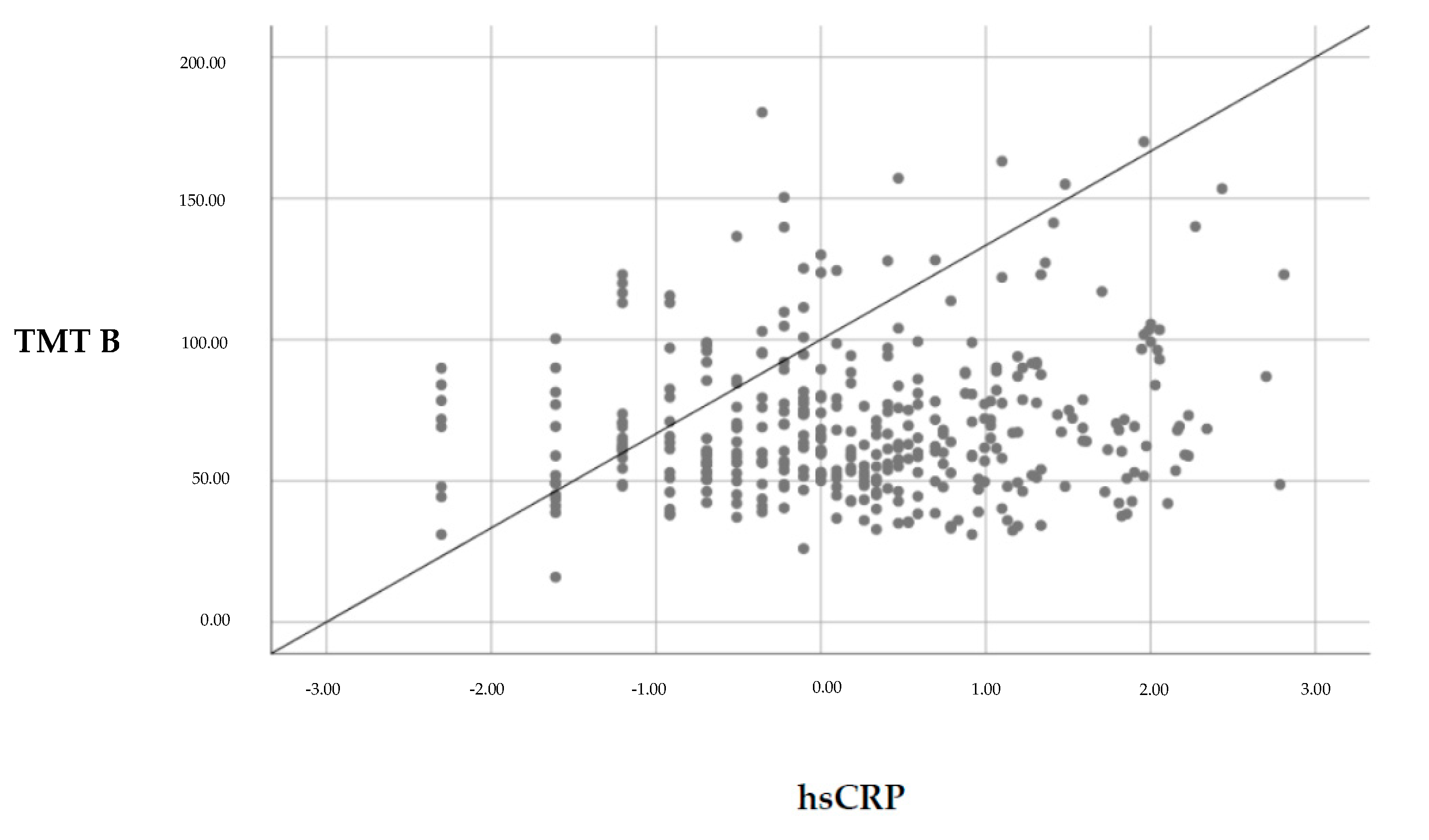
3.2.1. Associations between hsCRP and Cognitive Function
3.2.2. Associations between KYN/TRP Ratio and Cognitive Function
4. Discussion
4.1. Clinical Impact
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arts, B.; Jabben, N.; Krabbendam, L.; Van Os, J. Meta-analyses of cognitive functioning in euthymic bipolar patients and their first-degree relatives. Psychol Med. 2008, 38, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.S.; Hermens, D.F.; Porter, M.A.; Redoblado-Hodge, M.A. A meta-analysis of cognitive deficits in first-episode major depressive disorder. J. Affect. Disord. 2012, 140, 113–124. [Google Scholar] [CrossRef] [PubMed]
- McDermott, L.M.; Ebmeier, K.P. A meta-analysis of depression severity and cognitive function. J. Affect. Disord. 2009, 119, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L.J.; Thompson, J.M.; Gallagher, P.; Goswami, U.; Young, A.H.; Ferrier, I.N.; Moore, P.B. A meta-analysis of cognitive deficits in euthymic patients with bipolar disorder. J. Affect. Disord. 2006, 93, 105–115. [Google Scholar] [CrossRef]
- Snyder, H.R. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: A meta-analysis and review. Psychol Bull. 2013, 139, 81–132. [Google Scholar] [CrossRef] [Green Version]
- Millan, M.J.; Agid, Y.; Brüne, M.; Bullmore, E.T.; Carter, C.S.; Clayton, N.S.; Connor, R.; Davis, S.; DeRubeis, R.J.; Dubois, B.; et al. Cognitive dysfunction in psychiatric disorders: Characteristics, causes and the quest for improved therapy. Nat. Rev. Drug Discov. 2012, 11, 141–168. [Google Scholar] [CrossRef]
- Alvarez, J.A.; Emory, E. Executive function and the frontal lobes: A meta-analytic review. Neuropsychol. Rev. 2006, 16, 17–42. [Google Scholar] [CrossRef]
- Rock, P.L.; Roiser, J.P.; Riedel, W.J.; Blackwell, A.D. Cognitive impairment in depression: A systematic review and meta-analysis. Psychol. Med. 2014, 44, 2029. [Google Scholar] [CrossRef] [Green Version]
- Samamé, C.; Martino, D.J.; Strejilevich, S.A. Longitudinal course of cognitive deficits in bipolar disorder: A meta-analytic study. J. Affect. Disord. 2014, 164, 130–138. [Google Scholar] [CrossRef]
- Allison, D.J.; Ditor, D.S. The common inflammatory etiology of depression and cognitive impairment: A therapeutic target. J. Neuroinflammation 2014, 11, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Fourrier, C.; Singhal, G.; Baune, B.T. Neuroinflammation and cognition across psychiatric conditions. CNS Spectr. 2019, 24, 4–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldsmith, D.R.; Rapaport, M.H.; Miller, B.J. A meta-analysis of blood cytokine network alterations in psychiatric patients: Comparisons between schizophrenia, bipolar disorder and depression. Mol. Psychiatry 2016, 21, 1696. [Google Scholar] [CrossRef] [PubMed]
- Howren, M.B.; Lamkin, D.M.; Suls, J. Associations of depression with C-reactive protein, IL-1, and IL-6: A meta-analysis. Psychosom Med. 2009, 71, 171–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Queissner, R.; Pilz, R.; Dalkner, N.; Birner, A.; Bengesser, S.A.; Platzer, M.; Fellendorf, F.T.; Kainzbauer, N.; Herzog-Eberhard, S.; Hamm, S.; et al. The relationship between inflammatory state and quantity of affective episodes in Bipolar Disorder. Psychoneuroendocrinology 2019, 90, 61–67. [Google Scholar] [CrossRef]
- Valkanova, V.; Ebmeier, K.P.; Allan, C.L. CRP, IL-6 and depression: A systematic review and meta-analysis of longitudinal studies. J. Affect. Disord 2013, 150, 736–744. [Google Scholar] [CrossRef]
- Dixon, J.B. The effect of obesity on health outcomes. Mol Cell Endocrinol 2010, 316, 104–108. [Google Scholar] [CrossRef]
- Penninx, B.W.; Beekman, A.T.; Honig, A.; Deeg, D.J.; Schoevers, R.A.; van Eijk, J.T.; van Tilburg, W. Depression and cardiac mortality: Results from a community-based longitudinal study. Arch. Gen. Psychiatry 2001, 58, 221–227. [Google Scholar] [CrossRef] [Green Version]
- Opel, N.; Redlich, R.; Grotegerd, D.; Dohm, K.; Heindel, W.; Kugel, H.; Arolt, V.; Dannlowski, U. Obesity and major depression: Body-mass index (BMI) is associated with a severe course of disease and specific neurostructural alterations. Psychoneuroendocrinology 2015, 51, 219–226. [Google Scholar] [CrossRef]
- Soczynska, J.K.; Kennedy, S.H.; Woldeyohannes, H.O.; Liauw, S.S.; Alsuwaidan, M.; Yim, C.Y.; McIntyre, R.S. Mood disorders and obesity: Understanding inflammation as a pathophysiological nexus. Neuromol. Med. 2011, 13, 93–116. [Google Scholar] [CrossRef]
- Choi, J.; Joseph, L.; Pilote, L. Obesity and C-reactive protein in various populations: A systematic review and meta-analysis. Obes. Rev. 2013, 14, 232–244. [Google Scholar] [CrossRef]
- Santos, A.C.; Lopes, C.; Guimaraes, J.T.; Barros, H. Central obesity as a major determinant of increased high-sensitivity C-reactive protein in metabolic syndrome. Int. J. Obes. 2005, 29, 1452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A meta-analysis of cytokines in major depression. Biol Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Tegeler, C.; O’Sullivan, J.L.; Bucholtz, N.; Goldeck, D.; Pawelec, G.; Steinhagen-Thiessen, E.; Demuth, I. The inflammatory markers HSCRP, IL-6, and IL-10 are associated with cognitive function—Data from the Berlin Aging Study II. Neurobiol. Aging 2016, 38, 112–117. [Google Scholar] [CrossRef]
- Teunissen, C.E.; van Boxtel, M.P.; Bosma, H.; Bosmans, E.; Delanghe, J.; De Bruijn, C.; Wauters, A.; Maes, M.; Jolles, J.; Steinbusch, H.W.M.; et al. Inflammation markers in relation to cognition in a healthy aging population. J. Neuroimmunol. 2003, 134, 142–150. [Google Scholar] [CrossRef]
- Karalis, K.P.; Giannogonas, P.; Kodela, E.; Koutmani, Y.; Zoumakis, M.; Teli, T. Mechanisms of obesity and related pathology: Linking immune responses to metabolic stress. Febs J. 2009, 276, 5747–5754. [Google Scholar] [CrossRef]
- Spyridaki, E.C.; Avgoustinaki, P.D.; Margioris, A.N. Obesity, inflammation and cognition. Curr. Opin. Behav. Sci. 2016, 9, 169–175. [Google Scholar] [CrossRef]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, L. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2017, 114, 1752–1761. [Google Scholar] [CrossRef]
- Fagundo, A.B.; de la Torre, R.; Jiménez-Murcia, S.; Agüera, Z.; Granero, R.; Tárrega, S.; Botella, C.; Baños, R.; Fernández-Real, J.M.; Rodríguez, R.; et al. Executive functions profile in extreme eating/weight conditions: From anorexia nervosa to obesity. PLoS ONE 2012, 7, e43382. [Google Scholar] [CrossRef] [Green Version]
- Fergenbaum, J.H.; Bruce, S.; Lou, W.; Hanley, A.J.; Greenwood, C.; Young, T.K. Obesity and lowered cognitive performance in a Canadian First Nations population. Obesity 2009, 17, 1957–1963. [Google Scholar] [CrossRef]
- Hall, P.A.; Fong, G.T.; Epp, L.J.; Elias, L.J. Executive function moderates the intention-behavior link for physical activity and dietary behavior. Psychol. Health 2008, 23, 309–326. [Google Scholar] [CrossRef]
- Riggs, N.; Chou, C.P.; Spruijt-Metz, D.; Pentz, M.A. Executive cognitive function as a correlate and predictor of child food intake and physical activity. Child. Neuropsychol. 2010, 16, 279–292. [Google Scholar] [CrossRef]
- Jaeger, J.; Berns, S.; Uzelac, S.; Davis-Conway, S. Neurocognitive deficits and disability in major depressive disorder. Psychiatry Res. 2006, 145, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Mangge, H.; Stelzer, I.; Reininghaus, E.Z.; Weghuber, D.; Postolache, T.T.; Fuchs, D. Disturbed tryptophan metabolism in cardiovascular disease. Curr Med. Chem 2014, 21, 1931–1937. [Google Scholar] [CrossRef] [PubMed]
- Capuron, L.; Schroecksnadel, S.; Féart, C.; Aubert, A.; Higueret, D.; Barberger-Gateau, P.; Layé, S.; Fuchs, D. Chronic low-grade inflammation in elderly persons is associated with altered tryptophan and tyrosine metabolism: Role in neuropsychiatric symptoms. Biol. Psychiatry 2011, 70, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Reininghaus, E.Z.; McIntyre, R.S.; Reininghaus, B.; Geisler, S.; Bengesser, S.A.; Lackner, N.; Hecht, K.; Birner, A.; Kattnig, F.; Unterweger, R.; et al. Tryptophan breakdown is increased in euthymic overweight individuals with bipolar disorder: A preliminary report. Bipolar Disord. 2014, 16, 432–440. [Google Scholar] [CrossRef]
- Sublette, M.E.; Galfalvy, H.C.; Fuchs, D.; Lapidus, M.; Grunebaum, M.F.; Oquendo, M.A.; Mann, J.J.; Postolache, T.T. Plasma kynurenine levels are elevated in suicide attempters with major depressive disorder. Brain Behav. Immun. 2011, 25, 1272–1278. [Google Scholar] [CrossRef] [Green Version]
- Birner, A.; Platzer, M.; Bengesser, S.A.; Dalkner, N.; Fellendorf, F.T.; Queissner, R.; Pilz, R.; Rauch, P.; Maget, A.; Hamm, C.; et al. Increased breakdown of kynurenine towards its neurotoxic branch in bipolar disorder. PLoS ONE 2017, 12, e0172699. [Google Scholar] [CrossRef]
- Kuo, H.K.; Yen, C.J.; Chang, C.H.; Kuo, C.K.; Chen, J.H.; Sorond, F. Relation of C-reactive protein to stroke, cognitive disorders, and depression in the general population: Systematic review and meta-analysis. Lancet Neurol. 2005, 4, 371–380. [Google Scholar] [CrossRef]
- Schram, M.T.; Euser, S.M.; de Craen, A.J.; Witteman, J.C.; Frölich, M.; Hofman, A.; Jolles, J.; Breteler, M.M.B.; Westendorp, R.G.J. Systemic markers of inflammation and cognitive decline in old age. J. Am. Geriatr. Soc. 2007, 55, 708–716. [Google Scholar] [CrossRef] [Green Version]
- Engelhart, M.J.; Geerlings, M.I.; Meijer, J.; Kiliaan, A.; Ruitenberg, A.; van Swieten, J.C.; Stijnen, T.; Hofman, A.; Witteman, J.C.M.; Breteler, M.M.B. Inflammatory proteins in plasma and the risk of dementia: The rotterdam study. Arch. Neurol. 2004, 61, 668–672. [Google Scholar] [CrossRef] [Green Version]
- Yaffe, K.; Kanaya, A.; Lindquist, K.; Simonsick, E.M.; Harris, T.; Shorr, R.I.; Tylavsky, F.A.; Newman, A.B. The metabolic syndrome, inflammation, and risk of cognitive decline. Jama 2004, 292, 2237–2242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sweat, V.; Starr, V.; Bruehl, H.; Arentoft, A.; Tirsi, A.; Javier, E.; Convit, A. C-reactive protein is linked to lower cognitive performance in overweight and obese women. Inflammation 2008, 31, 198–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misiak, B.; Stańczykiewicz, B.; Kotowicz, K.; Rybakowski, J.K.; Samochowiec, J.; Frydecka, D. Cytokines and C-reactive protein alterations with respect to cognitive impairment in schizophrenia and bipolar disorder: A systematic review. Schizophr. Res. 2017, 192, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Stenfors., C.U.; Jonsdottir, I.H.; Hanson, L.M.; Theorell, T. Associations between systemic pro-inflammatory markers, cognitive function and cognitive complaints in a population-based sample of working adults. J. Psychosom. Res. 2017, 96, 49–59. [Google Scholar] [CrossRef]
- Gimeno, D.; Kivimäki, M.; Brunner, E.J.; Elovainio, M.; De Vogli, R.; Steptoe, A.; Kumari, M.; Lowe, G.D.O.; Rumley, A.; Marmot, M.G.; et al. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychol. Med. 2009, 39, 413–423. [Google Scholar] [CrossRef]
- Dickerson, F.; Schroeder, J.; Stallings, C.; Origoni, A.; Katsafanas, E.; Schwienfurth, L.A.; Savage, C.L.G.; Khushalani, S.; Yolken, R. A longitudinal study of cognitive functioning in schizophrenia: Clinical and biological predictors. Schizophr Res. 2014, 156, 248–253. [Google Scholar] [CrossRef]
- Chang, H.H.; Lee, I.H.; Gean, P.W.; Lee, S.Y.; Chi, M.H.; Yang, Y.K.; Lu, R.-B.; Chen, P.S. Treatment response and cognitive impairment in major depression: Association with C-reactive protein. Brain Behav. Immun. 2012, 26, 90–95. [Google Scholar] [CrossRef]
- Krogh, J.; Benros, M.E.; Jørgensen, M.B.; Vesterager, L.; Elfving, B.; Nordentoft, M. The association between depressive symptoms, cognitive function, and inflammation in major depression. Brain Behav. Immun. 2014, 35, 70–76. [Google Scholar] [CrossRef]
- Munkholm, K.; Braüner, J.V.; Kessing, L.V.; Vinberg, M. Cytokines in bipolar disorder vs. healthy control subjects: A systematic review and meta-analysis. J. Psychiatr Res. 2013, 47, 1119–1133. [Google Scholar] [CrossRef]
- Platzer, M.; Dalkner, N.; Fellendorf, F.T.; Birner, A.; Bengesser, S.A.; Queissner, R.; Kainzbauer, N.; Pilz, R.; Herzog-Eberhard, S.; Hamm, C.; et al. Tryptophan breakdown and cognition in bipolar disorder. Psychoneuroendocrinology 2017, 81, 144–150. [Google Scholar] [CrossRef]
- Forrest, C.M.; Mackay, G.M.; Oxford, L.; Millar, K.; Darlington, L.G.; Higgins, M.J.; Stone, T.W. Kynurenine metabolism predicts cognitive function in patients following cardiac bypass and thoracic surgery. J. Neurochem. 2011, 119, 136–152. [Google Scholar] [CrossRef] [PubMed]
- Dilling, H.; Mombour, W.; Schmidt, M.H. World Health Organization. Kapitel V (F). In Internationale Klassifikation Psychischer Störungen: ICD-10, Klinisch-Diagnostische Leitlinien [The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic guidelines], 9th ed.; Verlag Hans Huber: Bern, Switzerland, 1991. [Google Scholar]
- Bäumler, G.; Stroop, J.R. Farbe-Wort-Interferenztest Nach JR Stroop (FWIT) [Color-Word-Interference-Test after Stroop]; Hogrefe, Verlag für Psychologie: Göttingen, Germany, 1985. [Google Scholar]
- Reitan, R.M. TMT, Trail Making Test. A & B.; AR: Reitan Neuropsychology Laboratory: South Tucson, AZ, USA, 1992. [Google Scholar]
- Lehrl, S.; Triebig, G.; Fischer, B. Multiple choice vocabulary test MWT as a valid and short test to estimate premorbid intelligence. Acta Neurol. Scand. 1995, 91, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Hautzinger, M.; Keller, F.; Kühner, C. Beck Depressions-Inventar (BDI-II) [Beck Depression Inventory BDI-II]; Harcourt Test Services: Frankfurt, Germany, 2006. [Google Scholar]
- Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guy, W. (Ed.) Clinical Global Impressions. In ECDEU Assessment Manual for Psychopharmacology, Revised; National Institute of Mental Health: Rockville, MD, USA, 1976. [Google Scholar]
- Widner, B.; Werner, E.R.; Schennach, H.; Wachter, H.; Fuchs, D. Simultaneous measurement of serum tryptophan and kynurenine by HPLC. Clin. Chem. 1997, 43, 2424–2426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Shibuya-Tayoshi, S.; Sumitani, S.; Kikuchi, K.; Tanaka, T.; Tayoshi, S.Y.; Ueno, S.I.; Ohmori, T. Activation of the prefrontal cortex during the Trail-Making Test detected with multichannel near-infrared spectroscopy. Psychiatry Clin. Neurosci. 2007, 61, 616–621. [Google Scholar] [CrossRef]
- Kortte, K.B.; Horner, M.D.; Windham, W.K. The trail making test, part B: Cognitive flexibility or ability to maintain set? Appl. Neuropsychol. 2002, 9, 106–109. [Google Scholar] [CrossRef]
- Demakis, G.J. Frontal lobe damage and tests of executive processing: A meta-analysis of the category test, stroop test, and trail-making test. J. Clin. Exp. Neuropsychol. 2004, 26, 441–450. [Google Scholar] [CrossRef]
- Quak, J.; Doornbos, B.; Roest, A.M.; Duivis, H.E.; Vogelzangs, N.; Nolen, W.A.; Penninx, B.W.J.H.; Kema, I.P.; de Jonge, P. Does tryptophan degradation along the kynurenine pathway mediate the association between pro-inflammatory immune activity and depressive symptoms? Psychoneuroendocrinology 2014, 45, 202–210. [Google Scholar] [CrossRef]
- Fabricatore, A.N.; Wadden, T.A.; Higginbotham, A.J.; Faulconbridge, L.F.; Nguyen, A.M.; Heymsfield, S.B.; Faith, M.S. Intentional weight loss and changes in symptoms of depression: A systematic review and meta-analysis. Int. J. Obes. 2011, 35, 1363–1376. [Google Scholar] [CrossRef] [Green Version]
- Siervo, M.; Arnold, R.; Wells, J.C.K.; Tagliabue, A.; Colantuoni, A.; Albanese, E.; Brayne, C.; Stephan, B.C.M. Intentional weight loss in overweight and obese individuals and cognitive function: A systematic review and meta-analysis. Obes. Rev. 2011, 12, 968–983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Pinilla, F. Brain foods: The effects of nutrients on brain function. Nat. Rev. Neurosci. 2008, 9, 568–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutliffe, J.T.; Wilson, L.D.; de Heer, H.D.; Foster, R.L.; Carnot, M.J. C-reactive protein response to a vegan lifestyle intervention. Complementary Ther. Med. 2015, 23, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.S.C.; Redoblado-Hodge, M.A.; Naismith, S.L.; Hermens, D.F.; Porter, M.A.; Hickie, I.B. Cognitive remediation improves memory and psychosocial functioning in first-episode psychiatric out-patients. Psychol. Med. 2013, 43, 1161–1173. [Google Scholar] [CrossRef] [Green Version]
| N | M | SD | Min | Max | |
|---|---|---|---|---|---|
| Demographics and clinical characteristics | |||||
| Age [years] | 364 | 52.4 | 7.5 | 22.8 | 67.3 |
| Verbal IQ | 346 | 112.4 | 14.3 | 86 | 145 |
| Illness duration [years] | 364 | 12.4 | 12.1 | 0.1 | 55.5 |
| Psychopathology | |||||
| HAMD | 327 | 10.8 | 6.6 | 0 | 31 |
| BDI-2 | 358 | 20.1 | 9.8 | 0 | 51 |
| CGI | 357 | 3.6 | 0.9 | 0 | 6 |
| Metabolic risk variables | |||||
| Systolic blood pressure [mmHg] | 361 | 141.4 | 18.6 | 98 | 198 |
| Diastolic blood pressure [mmHg] | 361 | 88.0 | 11.6 | 55 | 129 |
| BMI [kg/m2] | 360 | 26.6 | 4.7 | 18.5 | 46.5 |
| Biomarkers | |||||
| hsCRP [mg/L] | 362 | 3.34 | 2.5 | 0.01 | 16.6 |
| TRP [ng/mL] | 355 | 65.5 | 8.4 | 38.9 | 92.7 |
| KYN [ng/mL] | 355 | 1.9 | 0.46 | 0.94 | 4.4 |
| KYN/TRP | 355 | 0.03 | 0.01 | 0.02 | 0.07 |
| LDL [mg/dL] | 363 | 144.4 | 38.1 | 45 | 250 |
| HDL [mg/dL] | 363 | 54.6 | 15.4 | 26 | 104 |
| Triglycerides [mg/dL] | 364 | 141.9 | 76.0 | 38 | 537 |
| N | M | SD | Min | Max | ||
|---|---|---|---|---|---|---|
| Cognitive tasks | ||||||
| Verbal IQ | 364 | 112.4 | 14.3 | 86 | 145 | |
| Attention | ||||||
| TMT A [s] | 364 | 33.4 | 14.0 | 9.9 | 120.4 | |
| Stroop word reading [s] | 364 | 31.3 | 8.3 | 18 | 138 | |
| Stroop color naming [s] | 364 | 47.2 | 10.0 | 26 | 111 | |
| Cognitive flexibility/Executive function | ||||||
| TMT B [s] | 364 | 70.0 | 27.4 | 15.9 | 223 | |
| Stroop interference [s] | 364 | 112.4 | 14.3 | 41 | 185 | |
| TMT A | TMT B | |||||
|---|---|---|---|---|---|---|
| β | t | p | β | t | p | |
| hsCRP a | 0.1 | 1.79 | 0.074 | 0.14 | 2.52 | 0.012 * |
| hsCRP b | 0.06 | 1.27 | 0.207 | 0.1 | 2.09 | 0.037 |
| Age | 0.37 | 7.07 | <0.001 * | 0.4 | 7.78 | <0.001 * |
| IQ | −0.18 | −3.52 | <0.001 * | −0.23 | −4.55 | <0.001 * |
| Sex | 0.01 | 0.172 | 0.836 | 0.12 | 2.38 | 0.018 |
| Illness duration | −0.04 | −0.84 | 0.006 * | 0.03 | 0.667 | 0.505 |
| BDI-2 | 0.14 | 2.67 | 0.008 * | 0.06 | 1.19 | 0.234 |
| hsCRP c | 0.13 | 2.19 | 0.029 | 0.12 | 2.15 | 0.033 |
| Age | 0.4 | 7.32 | <0.001 * | 0.41 | 7.76 | <0.001 * |
| IQ | −0.20 | −3.79 | <0.001 * | −0.24 | −4.70 | <0.001 * |
| Sex | 0 | 0 | 0.999 | 0.12 | 2.1 | 0.037 |
| Illness duration | −0.05 | −0.87 | 0.387 | 0.04 | 1.18 | 0.495 |
| BDI-2 | 0.14 | 2.77 | 0.006 * | 0.06 | 0.68 | 0.238 |
| BMI | −0.17 | −2.65 | 0.008 * | −0.07 | −1.04 | 0.301 |
| DBP | 0 | 0.07 | 0.945 | 0.02 | 0.28 | 0.783 |
| Triglycerides | 0.03 | 0.44 | 0.66 | 0.07 | 1.11 | 0.267 |
| HDL | −0.05 | −0.73 | 0.467 | 0.03 | 0.38 | 0.706 |
| Diabetes | −0.01 | −0.09 | 0.926 | −0.06 | −1.1 | 0.263 |
| Stroop Word Reading | Stroop Color Naming | Stroop Interference | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β | t | p | β | t | p | β | t | p | |
| hsCRP a | 0.08 | 1.62 | 0.107 | 0.07 | 1.21 | 0.229 | 0.08 | 1.4 | 0.162 |
| hsCRP b | 0.06 | 1.22 | 0.224 | 0.04 | 0.82 | 0.412 | 0.04 | 1.44 | 0.413 |
| Age | 0.21 | −0.26 | <0.001 * | 0.17 | 3.01 | 0.003 * | 0.25 | 4.77 | <0.001 * |
| IQ | −0.14 | −2.62 | 0.009 * | −0.15 | −2.79 | 0.006 * | −0.27 | −4.71 | <0.001 * |
| Sex | 0.02 | 0.27 | 0.787 | 0.09 | 1.61 | 0.107 | 0.07 | 1.6 | 0.18 |
| Illness duration | −0.11 | −2.06 | 0.04 | −0.06 | −1.03 | 0.303 | −0.12 | −2.35 | 0.029 |
| BDI−2 | 0.11 | 1.98 | 0.049 | 0.12 | 2.18 | 0.03 | 0.2 | 3.65 | <0.001 * |
| hsCRP c | 0.09 | 1.45 | 0.146 | −0.00 | −0.01 | 0.989 | −0.01 | 0.16 | 0.371 |
| Age | 0.22 | 3.87 | <0.001 * | 0.17 | 3.04 | 0.003 * | 0.25 | 4.62 | <0.001 * |
| IQ | −0.14 | −2.53 | 0.012 | −0.14 | −2.54 | 0.012 | −0.26 | −4.52 | <0.001 * |
| Sex | 0.04 | 0.653 | 0.514 | 0.08 | 1.25 | 0.211 | 0.02 | 0.95 | 0.501 |
| Illness duration | −0.11 | −2.00 | 0.047 | −0.05 | −0.91 | 0.366 | −0.12 | −2.26 | 0.037 |
| BDI−2 | 0.12 | 2.13 | 0.034 | 0.12 | 2.17 | 0.031 | 0.19 | 3.48 | <0.001 * |
| BMI | −0.00 | −0.00 | 0.972 | 0.11 | 1.69 | 0.093 | 0.15 | 1.74 | 0.012 |
| DBP | −0.04 | −0.04 | 0.537 | 0.02 | 0.38 | 0.704 | 0.08 | 1.1 | 0.155 |
| Triglycerides | −0.04 | −0.04 | 0.593 | −0.07 | −1.02 | 0.311 | −0.01 | −0.46 | 0.878 |
| HDL | 0.01 | 0.01 | 0.935 | −0.05 | −0.64 | 0.521 | −0.06 | −0.51 | 0.613 |
| Diabetes | −0.07 | −0.07 | 0.181 | −0.10 | −1.79 | 0.074 | −0.06 | −1.09 | 0.278 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalkner, N.; Reininghaus, E.; Schwalsberger, K.; Rieger, A.; Hamm, C.; Pilz, R.; Lenger, M.; Queissner, R.; Falzberger, V.S.; Platzer, M.; et al. C-Reactive Protein as a Possible Predictor of Trail-Making Performance in Individuals with Psychiatric Disorders. Nutrients 2020, 12, 3019. https://doi.org/10.3390/nu12103019
Dalkner N, Reininghaus E, Schwalsberger K, Rieger A, Hamm C, Pilz R, Lenger M, Queissner R, Falzberger VS, Platzer M, et al. C-Reactive Protein as a Possible Predictor of Trail-Making Performance in Individuals with Psychiatric Disorders. Nutrients. 2020; 12(10):3019. https://doi.org/10.3390/nu12103019
Chicago/Turabian StyleDalkner, Nina, Eva Reininghaus, Karin Schwalsberger, Alexandra Rieger, Carlo Hamm, René Pilz, Melanie Lenger, Robert Queissner, Valerie S. Falzberger, Martina Platzer, and et al. 2020. "C-Reactive Protein as a Possible Predictor of Trail-Making Performance in Individuals with Psychiatric Disorders" Nutrients 12, no. 10: 3019. https://doi.org/10.3390/nu12103019





