Fructose Consumption by Adult Rats Exposed to Dexamethasone In Utero Changes the Phenotype of Intestinal Epithelial Cells and Exacerbates Intestinal Gluconeogenesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design and Diet
2.2. Pyruvate Tolerance Test (PTT)
2.3. Tissue Sampling and Preparation
2.4. Analysis of Blood Parameters
2.5. Enzymatic Activity
2.6. Molecular Analyses
2.7. Morphometric Analysis of the Jejunum Wall
2.8. Immunohistochemical Evaluation of Cell Proliferation
2.9. Statistical Analyses
3. Results
3.1. Consumption of Fructose by Rats Born to DEX-Treated Mothers Modifies Body Composition but Does Not Modulate Small Intestine Length
3.2. Biochemical Changes Detected in Rats Born to DEX-Treated Mothers That Consume Fructose during Adulthood Indicates Increased Intestinal Gluconeogenesis
3.3. Rats Born to DEX-Treated Mothers That Consume Fructose during Adulthood Display Increased Portal Glucose Levels after Challenge with Exogenous Pyruvate
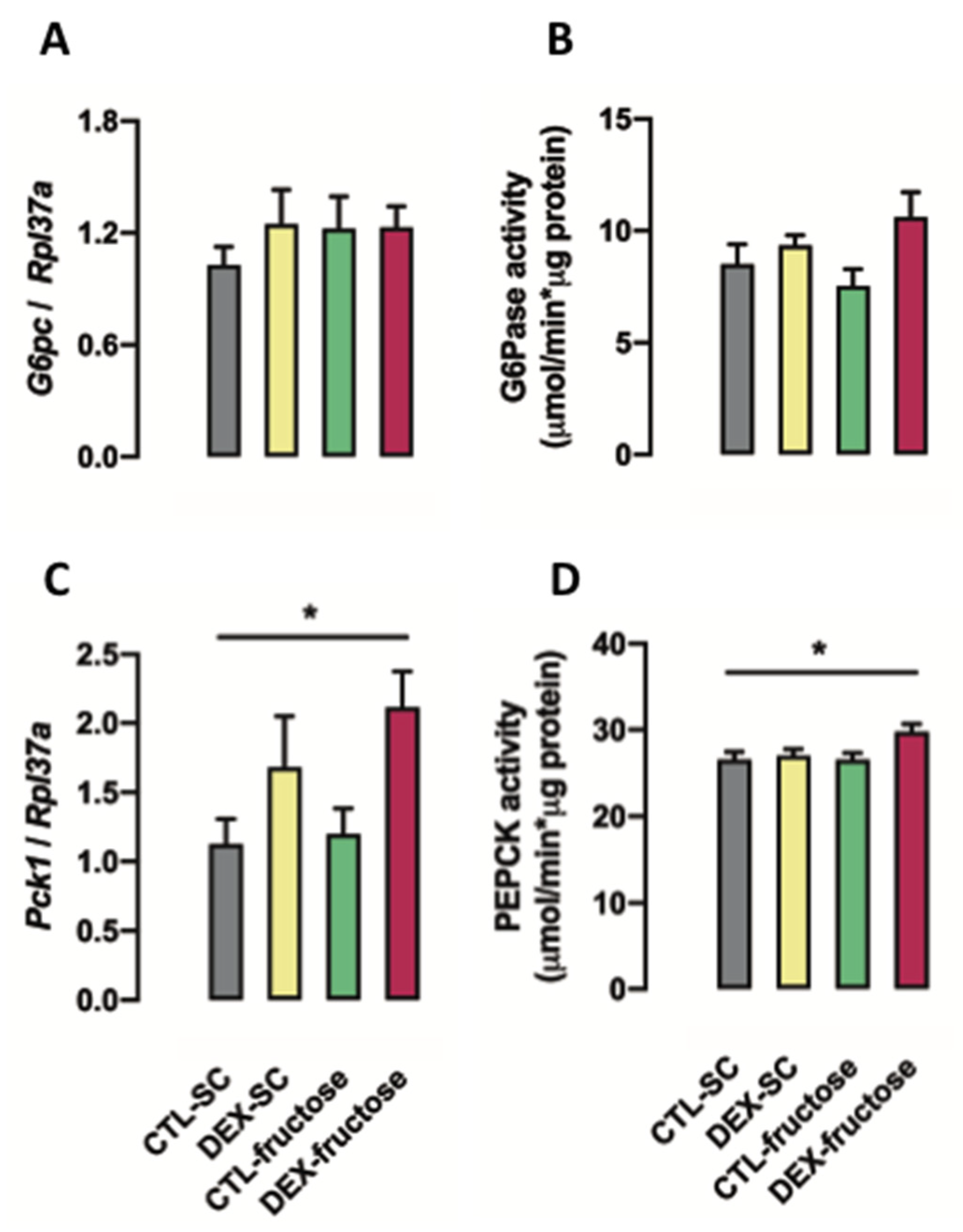
3.4. Rats Born to DEX-Treated Mothers That Consume Fructose during Adulthood Display Increased PEPCK Expression and Activity in the Jejunum
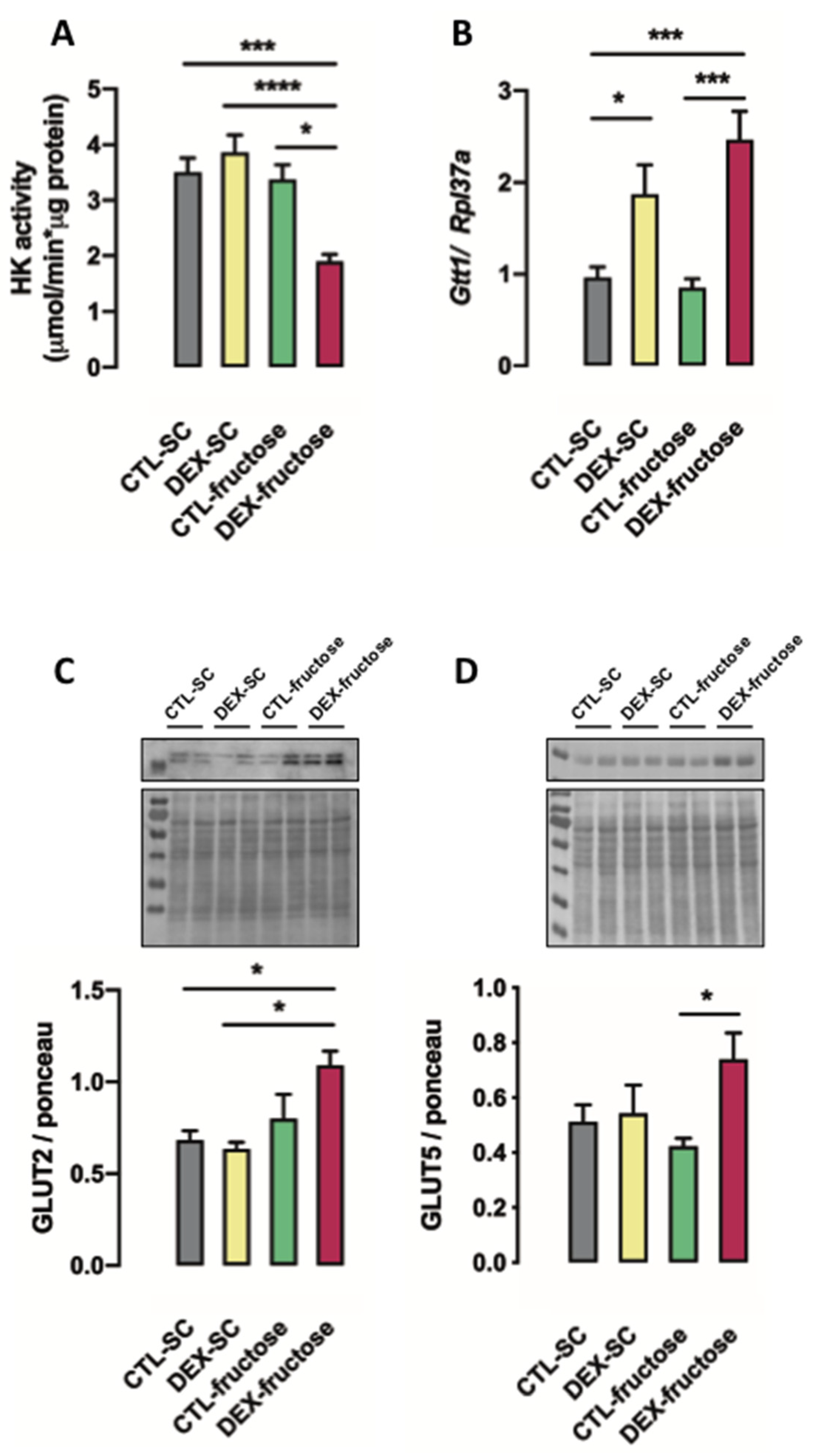
3.5. Rats Born to DEX-Treated Mothers That Consume Fructose during Adulthood Display Reduced HK Activity and Increased GLUT5 and GLUT2 Expression in the Jejunum
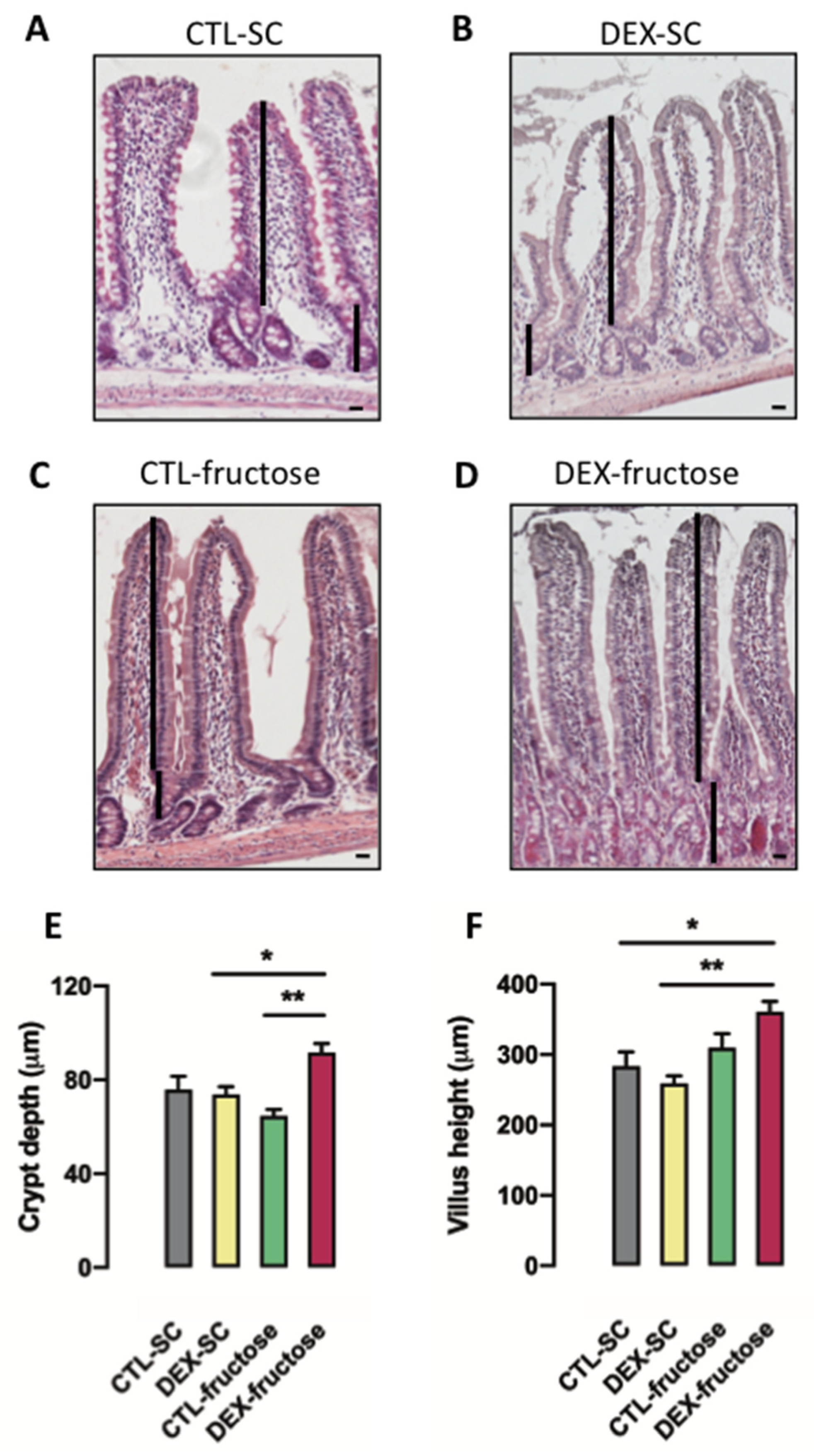
3.6. Rats Born to DEX-Treated Mothers That Consume Fructose during Adulthood Display Morphological Changes in the Jejunum Epithelium
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tappy, L.; Lê, K.A. Metabolic effects of fructose and the worldwide increase in obesity. Physiol. Rev. 2010, 90, 23–46. [Google Scholar] [CrossRef] [Green Version]
- Faria, J.A.; de Araújo, T.M.; Razolli, D.S.; Ignácio-Souza, L.M.; Souza, D.N.; Bordin, S.; Anhê, G.F. Metabolic impact of light phase-restricted fructose consumption is linked to changes in hypothalamic AMPK phosphorylation and melatonin production in rats. Nutrients 2017, 9, 332. [Google Scholar] [CrossRef] [Green Version]
- Oron-Herman, M.; Kamari, Y.; Grossman, E.; Yeger, G.; Peleg, E.; Shabtay, Z.; Shamiss, A.; Sharabi, Y. Metabolic syndrome: Comparison of the two commonly used animal models. Am. J. Hypertens. 2008, 21, 1018–1022. [Google Scholar] [CrossRef] [Green Version]
- Bizeau, M.E.; Thresher, J.S.; Pagliassotti, M.J. A high-sucrose diet increases gluconeogenic capacity in isolated periportal and perivenous rat hepatocytes. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E695–E702. [Google Scholar] [CrossRef]
- Merino, B.; Fernández-Díaz, C.M.; Cózar-Castellano, I.; Perdomo, G. Intestinal fructose and glucose metabolism in health and disease. Nutrients 2019, 12, 94. [Google Scholar] [CrossRef] [Green Version]
- Douard, V.; Ferraris, R.P. Regulation of the fructose transporter GLUT5 in health and disease. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E227–E237. [Google Scholar] [CrossRef] [Green Version]
- Jang, C.; Hui, S.; Lu, W.; Cowan, A.J.; Morscher, R.J.; Lee, G.; Liu, W.; Tesz, G.J.; Birnbaum, M.J.; Rabinowitz, J.D. The small intestine converts dietary fructose into glucose and organic acids. Cell Metab. 2018, 27, 351–361.e3. [Google Scholar] [CrossRef]
- Theytaz, F.; de Giorgi, S.; Hodson, L.; Stefanoni, N.; Rey, V.; Schneiter, P.; Giusti, V.; Tappy, L. Metabolic fate of fructose ingested with and without glucose in a mixed meal. Nutrients 2014, 6, 2632–2649. [Google Scholar] [CrossRef] [Green Version]
- Oh, A.R.; Sohn, S.; Lee, J.; Park, J.M.; Nam, K.T.; Hahm, K.B.; Kim, Y.B.; Lee, H.J.; Cha, J.Y. ChREBP deficiency leads to diarrhea-predominant irritable bowel syndrome. Metabolism 2018, 85, 286–297. [Google Scholar] [CrossRef]
- Iizuka, K. The Role of carbohydrate response element binding protein in intestinal and hepatic fructose metabolism. Nutrients 2017, 9, 181. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.S.; Krawczyk, S.A.; Doridot, L.; Fowler, A.J.; Wang, J.X.; Trauger, S.A.; Noh, H.L.; Kang, H.J.; Meissen, J.K.; Blatnik, M.; et al. ChREBP regulates fructose-induced glucose production independently of insulin signaling. J. Clin. Investig. 2016, 126, 4372–4386. [Google Scholar] [CrossRef] [Green Version]
- Koo, H.Y.; Wallig, M.A.; Chung, B.H.; Nara, T.Y.; Cho, B.H.; Nakamura, M.T. Dietary fructose induces a wide range of genes with distinct shift in carbohydrate and lipid metabolism in fed and fasted rat liver. Biochim. Biophys. Acta 2008, 1782, 341–348. [Google Scholar] [CrossRef] [Green Version]
- Geidl-Flueck, B.; Gerber, P.A. Insights into the hexose liver metabolism-glucose versus fructose. Nutrients 2017, 9, 1026. [Google Scholar] [CrossRef] [Green Version]
- Balakumar, M.; Raji, L.; Prabhu, D.; Sathishkumar, C.; Prabu, P.; Mohan, V.; Balasubramanyam, M. High-fructose diet is as detrimental as high-fat diet in the induction of insulin resistance and diabetes mediated by hepatic/pancreatic endoplasmic reticulum (ER) stress. Mol. Cell. Biochem. 2016, 423, 93–104. [Google Scholar] [CrossRef]
- Chan, S.M.; Sun, R.Q.; Zeng, X.Y.; Choong, Z.H.; Wang, H.; Watt, M.J.; Ye, J.M. Activation of PPARα ameliorates hepatic insulin resistance and steatosis in high fructose-fed mice despite increased endoplasmic reticulum stress. Diabetes 2013, 62, 2095–2105. [Google Scholar] [CrossRef] [Green Version]
- Payolla, T.B.; Teixeira, C.J.; Sato, F.T.; Murata, G.M.; Zonta, G.A.; Sodré, F.S.; Campos, C.V.; Mesquita, F.N.; Anhê, G.F.; Bordin, S. In Utero dexamethasone exposure exacerbates hepatic steatosis in rats that consume fructose during adulthood. Nutrients 2019, 11, 2114. [Google Scholar] [CrossRef]
- Nyirenda, M.J.; Lindsay, R.S.; Kenyon, C.J.; Burchell, A.; Seckl, J.R. Glucocorticoid exposure in late gestation permanently programs rat hepatic phosphoenolpyruvate carboxykinase and glucocorticoid receptor expression and causes glucose intolerance in adult offspring. J. Clin. Investig. 1998, 101, 2174–2181. [Google Scholar] [CrossRef] [Green Version]
- Santos-Silva, J.C.; da Silva, P.M.R.; de Souza, D.N.; Teixeira, C.J.; Bordin, S.; Anhê, G.F. In utero exposure to dexamethasone programs the development of the pancreatic β- and α-cells during early postnatal life. Life Sci. 2020, 255, 117810. [Google Scholar] [CrossRef]
- Chen, Y.T.; Hu, Y.; Yang, Q.Y.; Son, J.S.; Liu, X.D.; de Avila, J.M.; Zhu, M.J.; Du, M. Excessive Glucocorticoids During Pregnancy Impair Fetal Brown Fat Development and Predispose Offspring to Metabolic Dysfunctions. Diabetes 2020, 9, 1662–1674. [Google Scholar] [CrossRef]
- Mithieux, G.; Vidal, H.; Zitoun, C.; Bruni, N.; Daniele, N.; Minassian, C. Glucose-6-phosphatase mRNA and activity are increased to the same extent in kidney and liver of diabetic rats. Diabetes 1996, 45, 891–896. [Google Scholar] [CrossRef]
- Rajas, F.; Bruni, N.; Montano, S.; Zitoun, C.; Mithieux, G. The glucose-6 phosphatase gene is expressed in human and rat small intestine: Regulation of expression in fasted and diabetic rats. Gastroenterology 1999, 117, 132–139. [Google Scholar] [CrossRef]
- Rajas, F.; Croset, M.; Zitoun, C.; Montano, S.; Mithieux, G. Induction of PEPCK gene expression in insulinopenia in rat small intestine. Diabetes 2000, 49, 1165–1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croset, M.; Rajas, F.; Zitoun, C.; Hurot, J.M.; Montano, S.; Mithieux, G. Rat small intestine is an insulin-sensitive gluconeogenic organ. Diabetes 2001, 50, 740–746. [Google Scholar] [CrossRef] [Green Version]
- Mithieux, G.; Bady, I.; Gautier, A.; Croset, M.; Rajas, F.; Zitoun, C. Induction of control genes in intestinal gluconeogenesis is sequential during fasting and maximal in diabetes. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E370–E375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penhoat, A.; Fayard, L.; Stefanutti, A.; Mithieux, G.; Rajas, F. Intestinal gluconeogenesis is crucial to maintain a physiological fasting glycemia in the absence of hepatic glucose production in mice. Metabolism 2014, 63, 104–111. [Google Scholar] [CrossRef]
- Berg, B.; Harmison, C.R. Growth, disease, and aging in the rat. J. Geront. 1957, 13, 370–377. [Google Scholar] [CrossRef] [Green Version]
- Opie, L.H.; Newsholme, E.A. The activities of fructose 1,6-diphosphatase, phosphofructokinase and phosphoenolpyruvate carboxykinase in white muscle and red muscle. Biochem. J. 1967, 103, 391–399. [Google Scholar] [CrossRef] [Green Version]
- Trinder, P. Determination of blood glucose using an oxidase-peroxidase system with a non-carcinogenic chromogen. J. Clin. Pathol. 1969, 22, 158–161. [Google Scholar] [CrossRef] [Green Version]
- Zammit, V.A.; Newsholme, E.A. The maximum activities of hexokinase, phosphorylase, phosphofructokinase, glycerol phosphate dehydrogenases, lactate dehydrogenase, octopine dehydrogenase, phosphoenolpyruvate carboxykinase, nucleoside diphosphatekinase, glutamate-oxaloacetate transaminase and arginine kinase in relation to carbohydrate utilization in muscles from marine invertebrates. Biochem. J. 1976, 160, 447–462. [Google Scholar]
- Romero-Calvo, I.; Ocón, B.; Martínez-Moya, P.; Suárez, M.D.; Zarzuelo, A.; Martínez-Augustin, O.; de Medina, F.S. Reversible Ponceau staining as a loading control alternative to actin in Western blots. Anal. Biochem. 2010, 401, 318–320. [Google Scholar] [CrossRef]
- Prakatur, I.; Miskulin, M.; Pavic, M.; Marjanovic, K.; Blazicevic, V.; Miskulin, I.; Domacinovic, M. Intestinal morphology in broiler chickens supplemented with propolis and bee pollen. Animals 2019, 9, 301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uni, Z.; Geyra, A.; Ben-Hur, H.; Sklan, D. Small intestinal development in the young chick: Crypt formation and enterocyte proliferation and migration. Br. Poult. Sci. 2000, 41, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Pantaleão, L.C.; Murata, G.; Teixeira, C.J.; Payolla, T.B.; Santos-Silva, J.C.; Duque-Guimaraes, D.E.; Sodré, F.S.; Lellis-Santos, C.; Vieira, J.C.; de Souza, D.N.; et al. Prolonged fasting elicits increased hepatic triglyceride accumulation in rats born to dexamethasone-treated mothers. Sci. Rep. 2017, 7, 10367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyirenda, M.J.; Welberg, L.A.; Seckl, J.R. Programming hyperglycaemia in the rat through prenatal exposure to glucocorticoids-fetal effect or maternal influence? J. Endocrinol. 2001, 170, 653–660. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, K.; Takagi, T.; Fujii, T.; Matsubara, T.; Hase, K.; Taketani, Y.; Oka, T.; Minami, H.; Nakabou, Y. Role of liver-type glucose transporter (GLUT2) in transport across the basolateral membrane in rat jejunum. FEBS Lett. 1992, 314, 466–470. [Google Scholar] [CrossRef] [Green Version]
- Hahn, P.; Wei-Ning, H. Gluconeogenesis from lactate in the small intestinal mucosa of suckling rats. Pediatr. Res. 1986, 20, 1321–1323. [Google Scholar] [CrossRef] [Green Version]
- Douard, V.; Cui, X.L.; Soteropoulos, P.; Ferraris, R.P. Dexamethasone sensitizes the neonatal intestine to fructose induction of intestinal fructose transporter (Slc2A5) function. Endocrinology 2008, 149, 409–423. [Google Scholar] [CrossRef] [Green Version]
- Drozdowski, L.A.; Iordache, C.; Clandinin, M.T.; Todd, Z.S.C.; Gonnet, M.; Wild, G.; Uwiera, R.R.E.; Thomson, A.B.R. Dexamethasone and GLP-2 administered to rat dams during pregnancy and lactation have late effects on intestinal sugar transport in their postweaning offspring. J. Nutr. Biochem. 2008, 19, 49–60. [Google Scholar] [CrossRef]
- Drozdowski, L.A.; Iordache, C.; Clandinin, M.T.; Wild, G.; Todd, Z.; Thomson, A.B.R. A combination of dexamethasone and glucagon-like peptide-2 increase intestinal morphology and glucose uptake in suckling rats. J. Pediatr. Gastroenterol. Nutr. 2006, 42, 32–39. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Li, Q.; Zhang, J.; Duan, X.-L. Morphological changes of cell proliferation and apoptosis in rat jejunal mucosa at different ages. World J. Gastroenterol. 2003, 9, 2060–2064. [Google Scholar] [CrossRef]
- Uchida, H.; Nakajima, Y.; Ohtake, K.; Ito, J.; Morita, M.; Kamimura, A.; Kobayashi, J. Protective effects of oral glutathione on fasting-induced intestinal atrophy through oxidative stress. World J. Gastroenterol. 2017, 23, 6650–6664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghavami, S.; Mutawe, M.M.; Schaafsma, D.; Yeganeh, B.; Unruh, H.; Klonisch, T.; Halayko, A.J. Geranylgeranyl transferase 1 modulates autophagy and apoptosis in human airway smooth muscle. Am. J. Physiol. Lung Cell Mol. Physiol. 2012, 302, L420–L428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ockner, R.K.; Hughes, F.B.; Isselbacher, K.J. Very low density lipoproteins in intestinal lymph: Origin, composition, and role in lipid transport in the fasting state. J. Clin. Investig. 1969, 48, 2079–2088. [Google Scholar] [CrossRef] [PubMed]
- Haidari, M.; Leung, N.; Mahbub, F.; Uffelman, K.D.; Kohen-Avramoglu, R.; Lewis, G.F.; Adeli, K. Fasting and postprandial overproduction of intestinally derived lipoproteins in an animal model of insulin resistance. Evidence that chronic fructose feeding in the hamster is accompanied by enhanced intestinal de novo lipogenesis and ApoB48-containing lipoprotein overproduction. J. Biol. Chem. 2002, 277, 31646–31655. [Google Scholar] [PubMed] [Green Version]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, G.A.; Sodré, F.S.; Murata, G.M.; Amaral, A.G.; Payolla, T.B.; Campos, C.V.; Sato, F.T.; Anhê, G.F.; Bordin, S. Fructose Consumption by Adult Rats Exposed to Dexamethasone In Utero Changes the Phenotype of Intestinal Epithelial Cells and Exacerbates Intestinal Gluconeogenesis. Nutrients 2020, 12, 3062. https://doi.org/10.3390/nu12103062
Pereira GA, Sodré FS, Murata GM, Amaral AG, Payolla TB, Campos CV, Sato FT, Anhê GF, Bordin S. Fructose Consumption by Adult Rats Exposed to Dexamethasone In Utero Changes the Phenotype of Intestinal Epithelial Cells and Exacerbates Intestinal Gluconeogenesis. Nutrients. 2020; 12(10):3062. https://doi.org/10.3390/nu12103062
Chicago/Turabian StylePereira, Gizela A., Frhancielly S. Sodré, Gilson M. Murata, Andressa G. Amaral, Tanyara B. Payolla, Carolina V. Campos, Fabio T. Sato, Gabriel F. Anhê, and Silvana Bordin. 2020. "Fructose Consumption by Adult Rats Exposed to Dexamethasone In Utero Changes the Phenotype of Intestinal Epithelial Cells and Exacerbates Intestinal Gluconeogenesis" Nutrients 12, no. 10: 3062. https://doi.org/10.3390/nu12103062




