Omega-3 Eicosapentaenoic Acid Is Related to Happiness and a Sense of Fulfillment—A Study among Female Nursing Workers
Abstract
1. Introduction
2. Materials and Methods
2.1. About This Study
2.2. Participants and Procedures
2.3. Questionnaire
2.4. Assays
2.4.1. Blood Collection
2.4.2. Lipid and PUFA Assays
2.4.3. Statistical Analysis
3. Results
3.1. Participant Characteristics and PUFA
3.2. Correlation between Psychological Factors an Levels of Serum PUFA
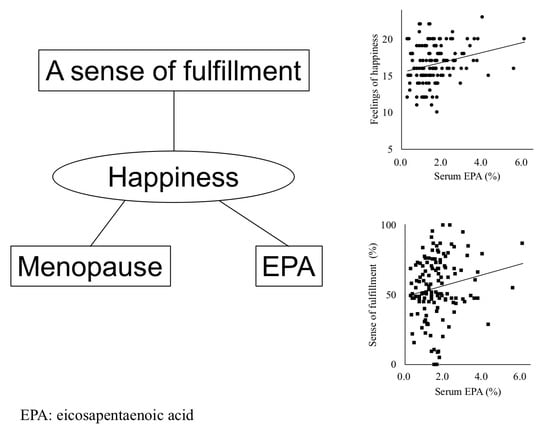
3.3. Regression Models to Explain Happiness
4. Discussion
4.1. ALA and Emotion
4.2. EPA and Happiness
4.3. Speculation about the Mediation between a Sense of Fulfillment and Happiness
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, B.; Lin, L.; Bazinet, R.P.; Chien, Y.C.; Chang, J.P.; Satyanarayanan, S.K.; Su, H.; Su, K.P. Clinical efficacy and biological regulations of ω-3 PUFA-derived endocannabinoids in major depressive disorder. Psychother. Psychosom. 2019, 88, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Deacon, G.; Kettle, C.; Hayes, D.; Dennis, C.; Tucci, J. Omega 3 polyunsaturated fatty acids and the treatment of depression. Crit. Rev. Food Sci. Nutr. 2017, 57, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Peet, M.; Stokes, C. Omega-3 fatty acids in the treatment of psychiatric disorders. Drugs 2005, 65, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.T.; Bazinet, R.P. β-oxidation and rapid metabolism, but not uptake regulate brain eicosapentaenoic acid levels. Prostaglandins Leukot Essent Fat. Acids 2015, 92, 33–40. [Google Scholar] [CrossRef]
- Horrobin, D.F.; Jenkins, K.; Bennett, C.N.; Christie, W.W. Eicosapentaenoic acid and arachidonic acid: Collaboration and not antagonism is the key to biological understanding. Prostaglandins Leukot Essent Fat. Acids 2002, 66, 83–90. [Google Scholar] [CrossRef]
- Dong, Y.; Xu, M.; Kalueff, A.V.; Song, C. Dietary eicosapentaenoic acid normalizes hippocampal omega-3 and 6 polyunsaturated fatty acid profile, attenuates glial activation and regulates BDNF function in a rodent model of neuroinflammation induced by central interleukin-1β administration. Eur. J. Nutr. 2018, 57, 1781–1791. [Google Scholar] [CrossRef]
- Peng, Z.; Zhang, C.; Yan, L.; Zhang, Y.; Yang, Z.; Wang, J.; Song, C. EPA is more effective than DHA to improve depression-like behavior, glia cell dysfunction and hippcampal apoptosis signaling in a chronic stress-induced rat model of depression. Int. J. Mol. Sci. 2020, 21, 1769. [Google Scholar] [CrossRef]
- Lucas, M.; Mirzaei, F.; O’Reilly, E.J.; Pan, A.; Willett, W.C.; Kawachi, I.; Koenen, K.; Ascherio, A. Dietary intake of n-3 and n-6 fatty acids and the risk of clinical depression in women: A 10-y prospective follow-up study. Am. J. Clin. Nutr. 2011, 93, 1337–1343. [Google Scholar] [CrossRef]
- Baker, E.J.; Miles, E.A.; Burdge, G.C.; Yaqoob, P.; Calder, P.C. Metabolism and functional effects of plant-derived omega-3 fatty acids in humans. Prog. Lipid Res. 2016, 64, 30–56. [Google Scholar] [CrossRef]
- Arterburn, L.M.; Hall, E.B.; Oken, H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am. J. Clin. Nutr. 2006, 83, 1467s–1476s. [Google Scholar] [CrossRef]
- Innis, S.M. The role of dietary n-6 and n-3 fatty acids in the developing brain. Dev. Neurosci. 2000, 22, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Spinhoven, P.; Elzinga, B.M.; Giltay, E.; Penninx, B.W. Anxious or depressed and still happy? PLoS ONE 2015, 10, e0139912. [Google Scholar] [CrossRef] [PubMed]
- Peiró, J.M.; Kozusznik, M.W.; Soriano, A. From happiness orientations to work performance: The mediating role of hedonic and eudaimonic experiences. Int. J. Env. Res. Public Health 2019, 16, 5002. [Google Scholar] [CrossRef] [PubMed]
- Hou, B.; Ji, L.; Chen, Z.; An, L.; Zhang, N.; Ren, D.; Yuan, F.; Liu, L.; Bi, Y.; Guo, Z.; et al. Role of rs454214 in personality mediated depression and subjective well-being. Sci. Rep. 2020, 10, 5702. [Google Scholar] [CrossRef]
- Colangelo, L.A.; He, K.; Whooley, M.A.; Daviglus, M.L.; Liu, K. Higher dietary intake of long-chain omega-3 polyunsaturated fatty acids is inversely associated with depressive symptoms in women. Nutrition 2009, 25, 1011–1019. [Google Scholar] [CrossRef]
- Henriksson, A.; Carlander, I.; Årestedt, K. Feelings of rewards among family caregivers during ongoing palliative care. Palliat. Supportive Care 2015, 13, 1509–1517. [Google Scholar] [CrossRef]
- Tsuboi, H.; Sakakibara, H.; Tatsumi, A.; Yamakawa-Kobayashi, K.; Matsunaga, M.; Kaneko, H.; Shimoi, K. Serum IL-6 levels and oxidation rate of LDL cholesterol were related to depressive symptoms independent of omega-3 fatty acids among female hospital and nursing home workers in Japan. J. Affect. Disord. 2019, 249, 385–393. [Google Scholar] [CrossRef]
- Shimai, S.; Otake, K.; Utsuki, N.; Ikemi, A.; Lyubomirsky, S. Development of a Japanese version of the subjective happiness scale (SHS), and examination of its validity and reliability. [Nihon Koshu Eisei Zasshi] Jpn. J. Public Health 2004, 51, 845–853. [Google Scholar]
- Lyubomirsky, S.; Lepper, H.S. A measure of subjective happiness: Preliminary reliability and construct validation. Soc. Indic. Res. 1999, 46, 137–155. [Google Scholar] [CrossRef]
- Lesage, F.X.; Berjot, S. Validity of occupational stress assessment using a visual analogue scale. Occup. Med. (Lond.) 2011, 61, 434–436. [Google Scholar] [CrossRef]
- Hayes, A.F. The PROCESS Macro for SPSS, SAS, and R. Available online: https://www.processmacro.org/index.html (accessed on 6 November 2020).
- Picard, M.; McEwen, B.S. Psychological stress and mitochondria: A systematic review. Psychosom. Med. 2018, 80, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.T.; Domenichiello, A.F.; Trépanier, M.O.; Liu, Z.; Masoodi, M.; Bazinet, R.P. The low levels of eicosapentaenoic acid in rat brain phospholipids are maintained via multiple redundant mechanisms. J. Lipid Res. 2013, 54, 2410–2422. [Google Scholar] [CrossRef] [PubMed]
- Bazinet, R.P.; Layé, S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 2014, 15, 771–785. [Google Scholar] [CrossRef]
- Zehr, K.R.; Walker, M.K. Omega-3 polyunsaturated fatty acids improve endothelial function in humans at risk for atherosclerosis: A review. Prostaglandins Other Lipid Mediat. 2018, 134, 131–140. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Lemaitre, R.N.; King, I.B.; Song, X.; Spiegelman, D.; Sacks, F.M.; Rimm, E.B.; Siscovick, D.S. Circulating long-chain ω-3 fatty acids and incidence of congestive heart failure in older adults: The cardiovascular health study: A cohort study. Ann. Intern. Med. 2011, 155, 160–170. [Google Scholar] [CrossRef]
- Steffen, B.T.; Steffen, L.M.; Tracy, R.; Siscovick, D.; Hanson, N.Q.; Nettleton, J.; Tsai, M.Y. Obesity modifies the association between plasma phospholipid polyunsaturated fatty acids and markers of inflammation: The multi-ethnic study of atherosclerosis. Int. J. Obesity 2012, 36, 797–804. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, N.; Chen, D.; Meng, L.; Zheng, Y.; Hui, R. Effects of n-3 PUFA supplementation on plasma soluble adhesion molecules: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2012, 95, 972–980. [Google Scholar] [CrossRef]
- Mocking, R.J.; Harmsen, I.; Assies, J.; Koeter, M.W.; Ruhé, H.G.; Schene, A.H. Meta-analysis and meta-regression of omega-3 polyunsaturated fatty acid supplementation for major depressive disorder. Transl. Psychiatry 2016, 6, e756. [Google Scholar] [CrossRef]
- Sadhasivam, S.; Alankar, S.; Maturi, R.; Vishnubhotla, R.V.; Mudigonda, M.; Pawale, D.; Narayanan, S.; Hariri, S.; Ram, C.; Chang, T.; et al. Inner engineering practices and advanced 4-day isha yoga retreat are associated with cannabimimetic effects with increased endocannabinoids and short-term and sustained improvement in mental health: A prospective observational study of meditators. Evid. Based Complementary Altern. Med. Ecam. 2020, 2020, 8438272. [Google Scholar] [CrossRef]
- Bossong, M.G.; van Hell, H.H.; Jager, G.; Kahn, R.S.; Ramsey, N.F.; Jansma, J.M. The endocannabinoid system and emotional processing: A pharmacological fMRI study with ∆9-tetrahydrocannabinol. Eur. Neuropsychopharmacol. 2013, 23, 1687–1697. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Chen, M.; Guo, L.; Yun, Y.; Li, G.; Sang, N. Endocannabinoid 2-arachidonoylglycerol protects inflammatory insults from sulfur dioxide inhalation via cannabinoid receptors in the brain. J. Environ. Sci. 2017, 51, 265–274. [Google Scholar] [CrossRef] [PubMed]
- McDougle, D.R.; Watson, J.E.; Abdeen, A.A.; Adili, R.; Caputo, M.P.; Krapf, J.E.; Johnson, R.W.; Kilian, K.A.; Holinstat, M.; Das, A. Anti-inflammatory ω-3 endocannabinoid epoxides. Proc. Natl. Acad. Sci. USA 2017, 114, e6034–e6043. [Google Scholar] [CrossRef] [PubMed]
- MacKinnon, D.P.; Fairchild, A.J.; Fritz, M.S. Mediation analysis. Annu. Rev. Psychol. 2007, 58, 593–614. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.F. Introduction to Mediation, Moderation, and Conditional Process Analysis, 2nd ed.; Guilford Press: New York, NY, USA, 2018; p. 692. [Google Scholar]
- Janssen, C.I.; Kiliaan, A.J. Long-chain polyunsaturated fatty acids (LCPUFA) from genesis to senescence: The influence of LCPUFA on neural development, aging, and neurodegeneration. Prog. Lipid. Res. 2014, 53, 1–17. [Google Scholar] [CrossRef] [PubMed]


| Variable | Mean ± SD | Range |
|---|---|---|
| Age (years) | 45.4 ± 13.24 | 19–75 |
| BMI (kg/m2) | 21.7 ± 3.35 | 15.8–32.8 |
| SHS scores * | 16.7 ± 2.82 | 10–28 |
| Sense of fulfillment (%) | 55.6 ± 22.00 | 0–100 |
| Occupational status | n | (%) |
| Licensed nurse or caregiver | 90 | 67.7 |
| Others without license | 43 | 32.3 |
| Physical and lifestyle variables | n | (%) |
| Menopause | ||
| No | 71 | 53.4 |
| Yes | 62 | 46.6 |
| Smoking status | ||
| Ex- or non-smoker | 103 | 77.4 |
| Current smoker | 30 | 22.6 |
| Current alcohol consumption | ||
| (Almost) Never | 112 | 84.2 |
| ≥Twice per week | 21 | 15.8 |
| Eating Breakfast | ||
| No or sometimes | 16 | 12.0 |
| Yes | 117 | 88.0 |
| Eating between meals | ||
| No or seldom | 57 | 42.9 |
| Yes | 76 | 57.1 |
| Leisure-time physical activities | ||
| <A few per month | 104 | 78.2 |
| ≥once per week | 29 | 21.8 |
| Serum profile of lipids | Mean ± SD | Range |
| Triglycerides (mMol/L) | 0.984 ± 0.4784 | 0.33–2.38 |
| Total cholesterol (mMol/L) | 5.51 ± 1.128 | 3.39–8.28 |
| Non-esterified fatty acid (mEq/L) | 0.65 ± 0.239 | 0.20–1.27 |
| FA concentrations (µmol/L) | Mean ± SD | Range |
| ω6 PUFA | 4165 ± 754.9 | 2763–6937 |
| Linoleic acid (LA) * | 3392 ± 623.0 | 2068–4806 |
| Arachidonic acid (AA) | 570.71 ± 125.79 | 296–1003 |
| Dihomo-γ-linolenic acid | 115.1 ± 37.87 | 40.5–204.2 |
| γ-Linolenic acid * | 32.77± 19.734 | 3.6–94.1 |
| Eicosadienoic acid | 18.46 ± 4.320 | 9.7–29.8 |
| Docosatetraenoic acid | 13.59 ± 4.187 | 0.0–24.1 |
| ω3 PUFA | 741.8 ± 296.6 | 265–1836 |
| Docosahexaenoic acid (DHA) | 414.7 ± 150.50 | 152–889 |
| Eicosapentaenoic acid (EPA) * | 184.5 ± 113.12 | 25.0–549.0 |
| α-Linolenic acid (ALA) | 81.77 ± 30.809 | 28.0–183.2 |
| Docosapentaenoic acid | 56.39 ± 21.077 | 19.4–121.9 |
| Correlation Coefficient | SHS Score | Fulfillment | LA (%) | AA (%) | DGLA (%) | LNA (%) | EDA (%) | DTA (%) | DHA (%) | EPA (%) a | ALA (%) | DPA (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SHS score | ||||||||||||
| Fulfillment | 0.24 * | |||||||||||
| LA (%) | −0.17 | −0.07 | ||||||||||
| AA (%) | 0.02 | 0.12 | −0.11 | |||||||||
| DGLA (%) | 0.01 | −0.04 | −0.25 ** | 0.13 | ||||||||
| LNA (%) | 0.01 | 0.03 | −0.45 *** | 0.27 ** | 0.56 *** | |||||||
| EDA (%) | −0.1 | −0.25 | 0.05 | −0.16 | 0.40 *** | −0.08 | ||||||
| DTA (%) | −0.03 | 0.01 | −0.16 | 0.32 *** | 0.54 *** | 0.50 *** | 0.25 * | |||||
| DHA (%) | 0.20 * | 0.21 * | −0.35 *** | 0.09 | −0.19 * | −0.17 | −0.06 | −0.30 ** | ||||
| EPA (%) a | 0.27 ** | 0.20 * | −0.32 *** | 0.095 | −0.28 ** | −0.09 | −0.28 ** | −0.35 *** | 0.78 *** | |||
| ALA (%) | −0.01 | −0.30 ** | −0.05 | −0.44 *** | −0.06 | −0.07 | 0.40 *** | −0.14 | 0.01 | −0.06 | ||
| DPA (%) | 0.14 | 0.15 | −0.50 *** | 0.03 | −0.03 | 0.09 | 0.04 | −0.09 | 0.81 *** | 0.66 *** | 0.10 |
| Models | Model 1 | Model 2 | Model 1 + 2 * | |||
|---|---|---|---|---|---|---|
| Adjusted R2 | 0.08 | 0.10 | 0.10 | |||
| f-value | 2.80 (6, 124) | 3.44 (6, 124) | 3.00 (7, 123) | |||
| p-value | 0.014 | 0.004 | 0.006 | |||
| Independent variables | B | p | B | p | B | p |
| Sense of fulfillment | 0.20 | 0.023 | 0.19 | 0.036 | 0.19 | 0.035 |
| DHA | 0.13 | 0.187 | −0.09 | 0.573 | ||
| EPA | 0.25 | 0.025 | 0.32 | 0.058 | ||
| Age | 0.23 | 0.09 | 0.19 | 0.154 | 0.18 | 0.193 |
| BMI | −0.04 | 0.633 | −0.02 | 0.858 | −0.01 | 0.953 |
| Menopause | −0.29 | 0.046 | −0.30 | 0.035 | −0.27 | 0.064 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsuboi, H.; Sakakibara, H.; Matsunaga, M.; Tatsumi, A.; Yamakawa-Kobayashi, K.; Yoshida, N.; Shimoi, K. Omega-3 Eicosapentaenoic Acid Is Related to Happiness and a Sense of Fulfillment—A Study among Female Nursing Workers. Nutrients 2020, 12, 3462. https://doi.org/10.3390/nu12113462
Tsuboi H, Sakakibara H, Matsunaga M, Tatsumi A, Yamakawa-Kobayashi K, Yoshida N, Shimoi K. Omega-3 Eicosapentaenoic Acid Is Related to Happiness and a Sense of Fulfillment—A Study among Female Nursing Workers. Nutrients. 2020; 12(11):3462. https://doi.org/10.3390/nu12113462
Chicago/Turabian StyleTsuboi, Hirohito, Hiroyuki Sakakibara, Masahiro Matsunaga, Asami Tatsumi, Kimiko Yamakawa-Kobayashi, Naoko Yoshida, and Kayoko Shimoi. 2020. "Omega-3 Eicosapentaenoic Acid Is Related to Happiness and a Sense of Fulfillment—A Study among Female Nursing Workers" Nutrients 12, no. 11: 3462. https://doi.org/10.3390/nu12113462
APA StyleTsuboi, H., Sakakibara, H., Matsunaga, M., Tatsumi, A., Yamakawa-Kobayashi, K., Yoshida, N., & Shimoi, K. (2020). Omega-3 Eicosapentaenoic Acid Is Related to Happiness and a Sense of Fulfillment—A Study among Female Nursing Workers. Nutrients, 12(11), 3462. https://doi.org/10.3390/nu12113462