The Root of Polygonum multiflorum Thunb. Alleviates Non-Alcoholic Steatosis and Insulin Resistance in High Fat Diet-Fed Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of PM Extract
2.2. High-Performance Liquid Chromatography (HPLC)
2.3. Animals and Treatments
2.4. Cell Culture and Treatments
2.5. Cytotoxicity of PM
2.6. Measurement of Blood Glucose Levels
2.7. Glucose Tolerance Test and Insulin Tolerance Test
2.8. Triglyceride Levels in Liver Tissues and HepG2 Cells
2.9. H&E and Oil Red O Staining of Liver Tissues
2.10. Western Blot Analysis
2.11. Statistical Analysis
3. Results
3.1. Phytochemical Contents in the PM Extract
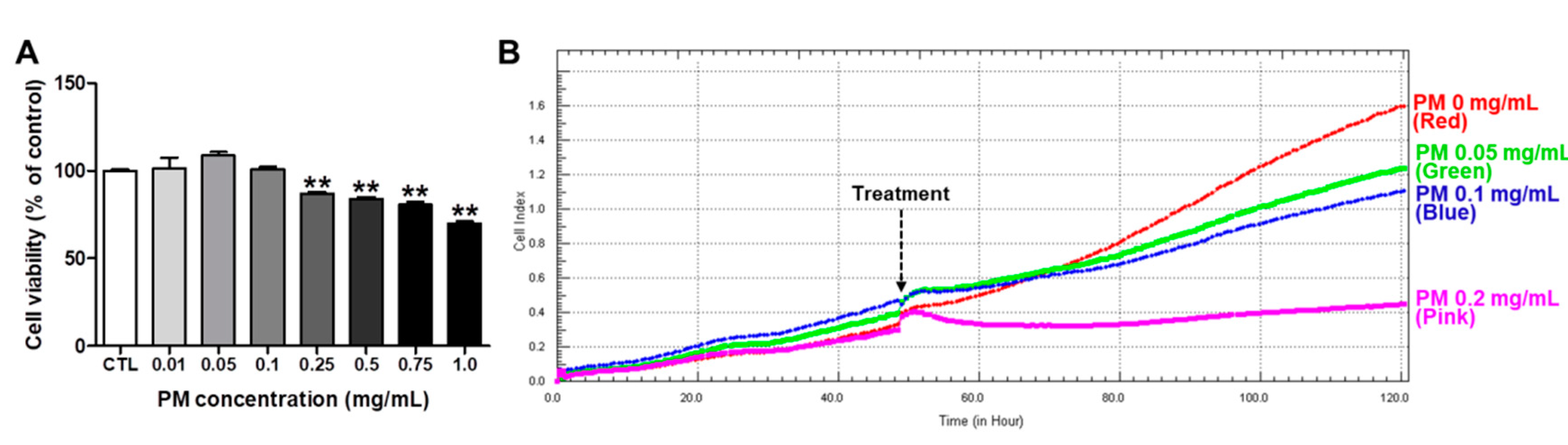
3.2. Cytotoxicity of PM in HepG2 Cells
3.3. PM Extract Attenuated the Increases of Lipid Accumulation and Intracellular TG Levels in FFA-Exposed HepG2 Cells
3.4. PM Extract Modulated Lipogenic and Lipolytic Protein Levels in FFA-Exposed HepG2 Cells
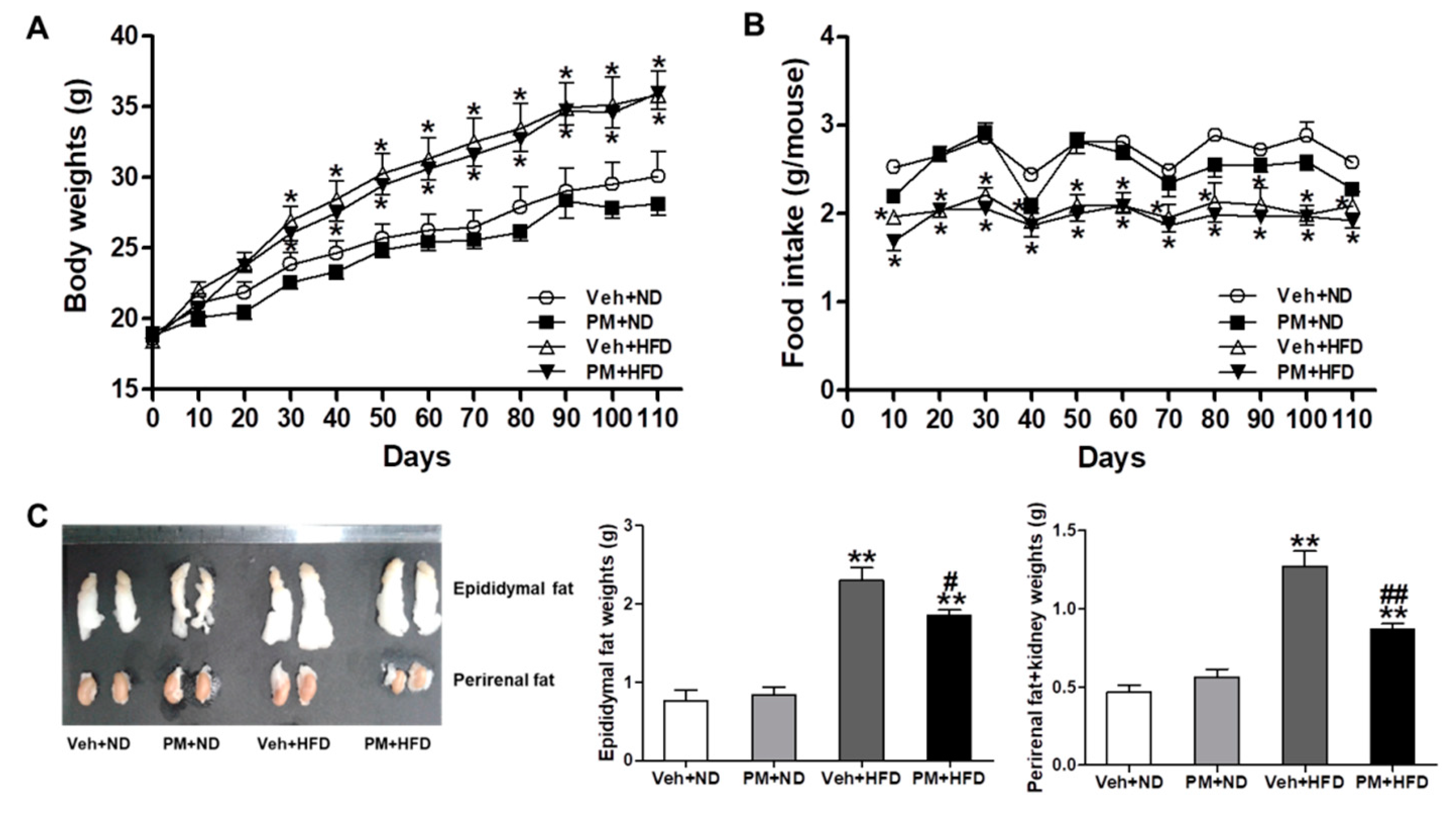
3.5. PM Extract Attenuated the Weight Increase of Adipose Tissues without Changing Body Weight or Food Intake in HFD-Fed Mice
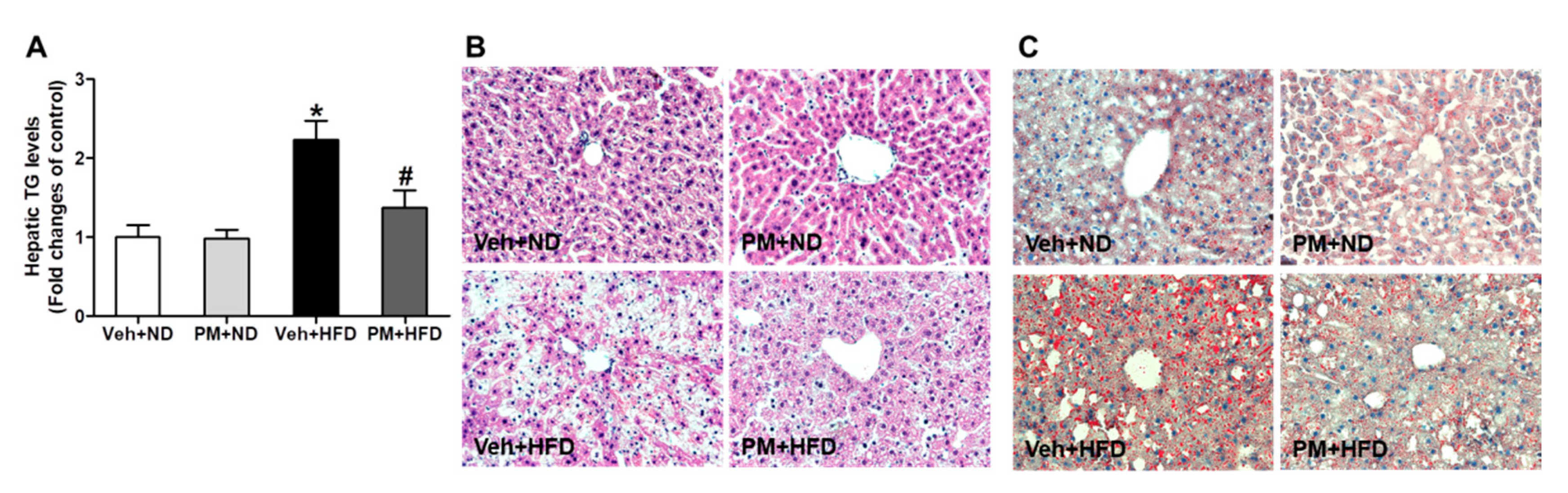
3.6. PM Extract Attenuated the Increases of Hepatic TG Levels and Hepatocellular Lipid Accumulation in HFD-Fed Mice
3.7. PM Extract Reduced Fasting Blood Glucose Levels, Improved Glucose Tolerance, and Insulin Sensitivity in HFD-Fed Mice
3.8. PM Extract Modulated Lipogenic and Lipolytic Protein Levels in HFD-Fed Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Osborn, O.; Olefsky, J.M. The cellular and signaling networks linking the immune system and metabolism in disease. Nat. Med. 2012, 18, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, S.; Loo, T.M.; Atarashi, K.; Kanda, H.; Sato, S.; Oyadomari, S.; Iwakura, Y.; Oshima, K.; Morita, H.; Hattori, M.; et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 2013, 499, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.A.; Angulo, P.; Lindor, K.D. Nonalcoholic fatty liver disease. CMAJ 2005, 172, 899–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef] [Green Version]
- Hudgins, L.C.; Hellerstein, M.K.; Seidman, C.E.; Neese, R.A.; Tremaroli, J.D.; Hirsch, J. Relationship between carbohydrate-induced hypertriglyceridemia and fatty acid synthesis in lean and obese subjects. J. Lipid Res. 2000, 41, 595–604. [Google Scholar]
- Bae, C.R.; Kwon, D.Y.; Cha, Y.S. Anti-obesity effects of traditional and standardized meju in high-fat diet-induced obese C57BL/6J mice. J. Clin. Biochem. Nutr. 2014, 54, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Boden, G. Obesity, insulin resistance and free fatty acids. Curr. Opin. Endocrinol. Diabetes Obes. 2011, 18, 139–143. [Google Scholar] [CrossRef] [Green Version]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nature reviews. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Xu, S.; Mihaylova, M.M.; Zheng, B.; Hou, X.; Jiang, B.; Park, O.; Luo, Z.; Lefai, E.; Shyy, J.Y.; et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011, 13, 376–388. [Google Scholar] [CrossRef] [Green Version]
- Ding, X.; Guo, L.; Zhang, Y.; Fan, S.; Gu, M.; Lu, Y.; Jiang, D.; Li, Y.; Huang, C.; Zhou, Z. Extracts of pomelo peels prevent high-fat diet-induced metabolic disorders in c57bl/6 mice through activating the PPARalpha and GLUT4 pathway. PLoS ONE 2013, 8, e77915. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-Penacarrillo, M.L.; Puente, J.; Redondo, A.; Clemente, F.; Valverde, I. Effect of GLP-1 treatment on GLUT2 and GLUT4 expression in type 1 and type 2 rat diabetic models. Endocrine 2001, 15, 241–248. [Google Scholar] [CrossRef]
- Wang, Y.; Kole, H.K.; Montrose-Rafizadeh, C.; Perfetti, R.; Bernier, M.; Egan, J.M. Regulation of glucose transporters and hexose uptake in 3T3-L1 adipocytes: Glucagon-like peptide-1 and insulin interactions. J. Mol. Endocrinol. 1997, 19, 241–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, R.A.; Pinkosky, S.L.; Filippov, S.; Hanselman, J.C.; Cramer, C.T.; Newton, R.S. AMP-activated protein kinase: An emerging drug target to regulate imbalances in lipid and carbohydrate metabolism to treat cardio-metabolic diseases. J. Lipid Res. 2012, 53, 2490–2514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bounda, G.A.; Feng, Y.U. Review of clinical studies of Polygonum multiflorum Thunb. and its isolated bioactive compounds. Pharmacognosy Res 2015, 7, 225–236. [Google Scholar] [CrossRef] [Green Version]
- Yue Liu, Y.; Wang, Q.; Yang, J.; Guo, X.; Liu, W.; Ma, S.; Li, S. Polygonum multiflorum Thunb.: A Review on Chemical Analysis, Processing Mechanism, Quality Evaluation, and Hepatotoxicity. Front Pharmacol. 2018, 9, 364. [Google Scholar] [CrossRef] [Green Version]
- Seo, M.S.; Kim, J.H.; Kim, H.J.; Chang, K.C.; Park, S.W. Honokiol activates the LKB1-AMPK signaling pathway and attenuates the lipid accumulation in hepatocytes. Toxicol. Appl. Pharm. 2015, 284, 113–124. [Google Scholar] [CrossRef]
- Jeon, B.T.; Jeong, E.A.; Shin, H.J.; Lee, Y.; Lee, D.H.; Kim, H.J.; Kang, S.S.; Cho, G.J.; Choi, W.S.; Roh, G.S. Resveratrol attenuates obesity-associated peripheral and central inflammation and improves memory deficit in mice fed a high-fat diet. Diabetes 2012, 61, 1444–1454. [Google Scholar] [CrossRef] [Green Version]
- Banarova, A.; Koller, T.; Payer, J. Toxic hepatitis induced by Polygonum multiflorum. Vnitrni Lekarstvi 2012, 58, 958–962. [Google Scholar]
- Jung, K.A.; Min, H.J.; Yoo, S.S.; Kim, H.J.; Choi, S.N.; Ha, C.Y.; Kim, H.J.; Kim, T.H.; Jung, W.T.; Lee, O.J.; et al. Drug-Induced Liver Injury: Twenty Five Cases of Acute Hepatitis Following Ingestion of Polygonum multiflorum Thunb. Gut Liver 2011, 5, 493–499. [Google Scholar] [CrossRef] [Green Version]
- Lei, X.; Chen, J.; Ren, J.; Li, Y.; Zhai, J.; Mu, W.; Zhang, L.; Zheng, W.; Tian, G.; Shang, H. Liver Damage Associated with Polygonum multiflorum Thunb.: A Systematic Review of Case Reports and Case Series. Evid.-Based Complement. Altern. Med. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ipsen, D.H.; Lykkesfeldt, J.; Tveden-Nyborg, P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell. Mol. Life Sci. 2018, 75, 3313–3327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.B.; Zhou, G.; Li, C. AMPK: An emerging drug target for diabetes and the metabolic syndrome. Cell Metab. 2009, 9, 407–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qatanani, M.; Lazar, M.A. Mechanisms of obesity-associated insulin resistance: Many choices on the menu. Genes Dev. 2007, 21, 1443–1455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.; Czech, M.P. The GLUT4 glucose transporter. Cell Metab. 2007, 5, 237–252. [Google Scholar] [CrossRef] [Green Version]
- Shaw, L.M. The insulin receptor substrate (IRS) proteins: At the intersection of metabolism and cancer. Cell Cycle 2011, 10, 1750–1756. [Google Scholar] [CrossRef] [Green Version]
- Sayem, A.S.M.; Arya, A.; Karimian, H.; Krishnasamy, N.; Ashok Hasamnis, A.; Hossain, C.F. Action of Phytochemicals on Insulin Signaling Pathways Accelerating Glucose Transporter (GLUT4) Protein Translocation. Molecules 2018, 23, 258. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Luo, G.; Chen, D.; Xiang, Z. A Comprehensive and System Review for the Pharmacological Mechanism of Action of Rhein, an Active Anthraquinone Ingredient. Front. Pharm. 2016, 7, 247. [Google Scholar] [CrossRef] [Green Version]
- Duan, J.; Han, X.; Ling, S.; Gan, W.; Sun, L.; Ni, R.Z.; Xu, J.W. Aortic Remodelling Is Improved by 2,3,5,4′-Tetrahydroxystilbene-2-O-beta-D-glucoside Involving the Smad3 Pathway in Spontaneously Hypertensive Rats. Evid.-Based Complement. Altern. Med. 2015, 2015, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Alipour, M.; Malihi, R.; Hosseini, S.A.; Abbasnezhad, A.; Ghavami, A.; Shahmohammadi, H.A.; Ghanavati, M. The effects of catechins on related risk factors with Type 2 diabetes: A review. Prog. Nutr. 2018, 20, 12–20. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Y.; Xie, Z.; Zhou, Y.; Zhang, Y.; Wan, X. The anti-obesity effects of green tea in human intervention and basic molecular studies. Eur. J. Clin. Nutr. 2014, 68, 1075–1087. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.M.; Cao, S.Y.; Wei, X.L.; Gan, R.Y.; Wang, Y.F.; Cai, S.X.; Xu, X.Y.; Zhang, P.Z.; Li, H.B. Effects and Mechanisms of Tea for the Prevention and Management of Diabetes Mellitus and Diabetic Complications: An Updated Review. Antioxidants 2019, 8, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, J.; Zhao, Y.; Suo, S.; Liu, Y.; Zhao, B. Green tea catechins ameliorate adipose insulin resistance by improving oxidative stress. Free Radic. Biol. Med. 2012, 52, 1648–1657. [Google Scholar] [CrossRef]
- Qian, J.; Hou, M.; Wu, X.; Dai, C.; Sun, J.; Dong, L. A review on the extraction, purification, detection, and pharmacological effects of 2,3,5,4′-tetrahydroxystilbene-2-O-beta-d-glucoside from Polygonum multiflorum. Biomed. Pharm. 2020, 124, 109923. [Google Scholar] [CrossRef]
- Tang, W.; Li, S.; Liu, Y.; Wu, J.C.; Pan, M.H.; Huang, M.T.; Ho, C.T. Anti-diabetic activities of cis- and trans-2,3,5,4′-tetrahydroxystilbene 2-O-beta-glucopyranoside from Polygonum multiflorum. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Hekmatdoost, A.; Shamsipour, A.; Meibodi, M.; Gheibizadeh, N.; Eslamparast, T.; Poustchi, H. Adherence to the Dietary Approaches to Stop Hypertension (DASH) and risk of Nonalcoholic Fatty Liver Disease. Int J. Food Sci. Nutr. 2016, 67, 1024–1029. [Google Scholar] [CrossRef]
- Oddy, W.H.; Herbison, C.E.; Jacoby, P.; Ambrosini, G.L.; O’Sullivan, T.A.; Ayonrinde, O.T.; Olynyk, J.K.; Black, L.J.; Beilin, L.J.; Mori, T.A.; et al. The Western dietary pattern is prospectively associated with nonalcoholic fatty liver disease in adolescence. Am. J. Gastroenterol. 2013, 108, 778–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, A.B.; Godin, J.P.; Minehira, K.; Kirwan, J.P. Increasing whole grain intake as part of prevention and treatment of nonalcoholic Fatty liver disease. Int. J. Endocrinol. 2013, 2013, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Salomone, F.; Godos, J.; Zelber-Sagi, S. Natural antioxidants for non-alcoholic fatty liver disease: Molecular targets and clinical perspectives. Liver Int. 2016, 36, 5–20. [Google Scholar] [CrossRef]
- Eslamparast, T.; Eghtesad, S.; Poustchi, H.; Hekmatdoost, A. Recent advances in dietary supplementation, in treating non-alcoholic fatty liver disease. World J. Hepatol. 2015, 7, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Markowiak, P.; Slizewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef] [PubMed]




| Phytochemical | Content 1 (mg/g) |
|---|---|
| Catechin | 1.51 ± 0.07 |
| 2,3,5,4′-tetrahydroxystilbene-2-O-α-glucoside (TSG) | 36.68 ± 1.83 |
| Rhein | 0.3 ± 0.02 |
| Emodin | 0.05 ± 0.00 |
| Chrysophenol | ND 2 |
| Total | 38.54 ± 1.93 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, S.; Son, H.; Hwang, C.E.; Cho, K.M.; Park, S.W.; Kim, H.; Kim, H.J. The Root of Polygonum multiflorum Thunb. Alleviates Non-Alcoholic Steatosis and Insulin Resistance in High Fat Diet-Fed Mice. Nutrients 2020, 12, 2353. https://doi.org/10.3390/nu12082353
Jung S, Son H, Hwang CE, Cho KM, Park SW, Kim H, Kim HJ. The Root of Polygonum multiflorum Thunb. Alleviates Non-Alcoholic Steatosis and Insulin Resistance in High Fat Diet-Fed Mice. Nutrients. 2020; 12(8):2353. https://doi.org/10.3390/nu12082353
Chicago/Turabian StyleJung, Soonwoong, Hyeonwi Son, Chung Eun Hwang, Kye Man Cho, Sang Won Park, Hwajin Kim, and Hyun Joon Kim. 2020. "The Root of Polygonum multiflorum Thunb. Alleviates Non-Alcoholic Steatosis and Insulin Resistance in High Fat Diet-Fed Mice" Nutrients 12, no. 8: 2353. https://doi.org/10.3390/nu12082353





