Composition of Dietary Fatty Acids and Health Risks in Japanese Youths
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Cardiometabolic Risks
2.3. Dietary Assessment
2.4. Confounders
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Q.; Afshin, A.; Yakoob, M.Y.; Singh, G.M.; Rehm, C.D.; Khatibzadeh, S.; Micha, R.; Carukshi, A.; Mozaffarian, D.; Ezzati, M.; et al. Impact of Nonoptimal Intakes of Saturated, Polyunsaturated, and Trans Fat on Global Burdens of Coronary Heart Disease. J. Am. Heart Assoc. 2016, 5, 5. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.D.; Hu, F.B. Dietary Fat and Risk of Cardiovascular Disease: Recent Controversies and Advances. Annu. Rev. Nutr. 2017, 37, 423–446. [Google Scholar] [CrossRef]
- Morenga, L.T.; Montez, J.M. Health effects of saturated and trans-fatty acid intake in children and adolescents: Systematic review and meta-analysis. PLoS ONE 2017, 12, e0186672. [Google Scholar] [CrossRef] [Green Version]
- Mozaffarian, D.; Micha, R.; Wallace, S. Effects on Coronary Heart Disease of Increasing Polyunsaturated Fat in Place of Saturated Fat: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS Med. 2010, 7, e1000252. [Google Scholar] [CrossRef]
- Farvid, M.S.; Ding, M.; Pan, A.; Sun, Q.; Chiuve, S.E.; Steffen, L.M.; Willett, W.C.; Hu, F.B. Dietary Linoleic Acid and Risk of Coronary Heart Disease: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Circulationaha 2014, 130, 1568–1578. [Google Scholar] [CrossRef]
- Aitchison, J. The Statistical Analysis of Compositional Data. J. R. Stat. Soc. Ser. B 1982, 44, 139–160. [Google Scholar] [CrossRef]
- Chastin, S.; Palarea-Albaladejo, J.; Dontje, M.L.; Skelton, D.A. Combined Effects of Time Spent in Physical Activity, Sedentary Behaviors and Sleep on Obesity and Cardio-Metabolic Health Markers: A Novel Compositional Data Analysis Approach. PLoS ONE 2015, 10, e0139984. [Google Scholar] [CrossRef] [Green Version]
- Leite, M.L.C. Applying compositional data methodology to nutritional epidemiology. Stat. Methods Med. Res. 2016, 25, 3057–3065. [Google Scholar] [CrossRef]
- Micha, R.; Khatibzadeh, S.; Shi, P.; Fahimi, S.; Lim, S.; Andrews, K.G.; Engell, R.E.; Powles, J.; Ezzati, M.; Mozaffarian, D.; et al. Global, regional, and national consumption levels of dietary fats and oils in 1990 and 2010: A systematic analysis including 266 country-specific nutrition surveys. BMJ 2014, 348, g2272. [Google Scholar] [CrossRef] [Green Version]
- Alexander, D.D.; Miller, P.E.; Van Elswyk, M.E.; Kuratko, C.N.; Bylsma, L.C. A Meta-Analysis of Randomized Controlled Trials and Prospective Cohort Studies of Eicosapentaenoic and Docosahexaenoic Long-Chain Omega-3 Fatty Acids and Coronary Heart Disease Risk. Mayo Clin. Proc. 2017, 92, 15–29. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Hou, R.; Xi, Y.; Kowalski, A.; Wang, T.; Yu, Z.; Hu, Y.; Chandrasekar, E.K.; Sun, H.; Ali, M.K. The association and dose–response relationship between dietary intake of α-linolenic acid and risk of CHD: A systematic review and meta-analysis of cohort studies. Br. J. Nutr. 2018, 119, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Rizos, E.C.; Ntzani, E.E.; Bika, E.; Kostapanos, M.S.; Elisaf, M.S. Association Between Omega-3 Fatty Acid Supplementation and Risk of Major Cardiovascular Disease Events. JAMA 2012, 308, 1024–1033. [Google Scholar] [CrossRef]
- Hu, F.B.; Stampfer, M.J.; Rimm, E.; Ascherio, A.; Rosner, B.A.; Spiegelman, D.; Willett, W.C. Dietary Fat and Coronary Heart Disease: A Comparison of Approaches for Adjusting for Total Energy Intake and Modeling Repeated Dietary Measurements. Am. J. Epidemiol. 1999, 149, 531–540. [Google Scholar] [CrossRef] [Green Version]
- Shinozaki, K.; Okuda, M.; Kunitsugu, I.; Shigeta, M.; Sasaki, S. Dietary Fiber Consumption Decreases the Risks of Overweight and Hypercholesterolemia in Japanese Children. Ann. Nutr. Metab. 2015, 67, 58–64. [Google Scholar] [CrossRef]
- Okuda, M.; Fujiwara, A.; Sasaki, S. Added and Free Sugars Intake and Metabolic Biomarkers in Japanese Adolescents. Nutrients 2020, 12, 2046. [Google Scholar] [CrossRef]
- Kato, N.; Takimoto, H.; Sudo, N. The Cubic Functions for Spline Smoothed L, S and M Values for BMI Reference Data of Japanese Children. Clin. Pediatr. Endocrinol. 2011, 20, 47–49. [Google Scholar] [CrossRef] [Green Version]
- Okuda, M.; Sasaki, S.; Bando, N.; Hashimoto, M.; Kunitsugu, I.; Sugiyama, S.; Terao, J.; Hobara, T. Carotenoid, Tocopherol, and Fatty Acid Biomarkers and Dietary Intake Estimated by Using a Brief Self-Administered Diet History Questionnaire for Older Japanese Children and Adolescents. J. Nutr. Sci. Vitaminol. 2009, 55, 231–241. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, S.; Honda, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Both Comprehensive and Brief Self-Administered Diet History Questionnaires Satisfactorily Rank Nutrient Intakes in Japanese Adults. J. Epidemiol. 2012, 22, 151–159. [Google Scholar] [CrossRef] [Green Version]
- Okuda, M.; Asakura, K.; Sasaki, S. Protein Intake Estimated from Brief-Type Self-Administered Diet History Questionnaire and Urinary Urea Nitrogen Level in Adolescents. Nutrients 2019, 11, 319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fišerová, E.; Hron, K. On the Interpretation of Orthonormal Coordinates for Compositional Data. Math. Geol. 2011, 43, 455–468. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 10 November 2020).
- van den Boogaart, K.G.; Tolosana-Delgado, R.; Bren, M. Compositions: Compositional Data Analysis. R Package Version 2.0-0. Available online: https://CRAN.R-project.org/package=compositions (accessed on 24 November 2020).
- Hamilton, N.E.; Ferry, M. ggtern: Ternary Diagrams Using ggplot2. J. Stat. Softw. 2018, 87, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Ito, S.; Sasaki, S. (Eds.) Dietary Reference Intakes for Japanese 2020; Daiichi-Shuppan: Tokyo, Japan, 2020; ISBN 978-4-8041-1408-8. [Google Scholar]
- Harris, C.; Buyken, A.E.; Koletzko, S.; Filipiak-Pittroff, B.; Berdel, D.; Schikowski, T.; Koletzko, B.; Heinrich, J.; Standl, M. Dietary Fatty Acids and Changes in Blood Lipids during Adolescence: The Role of Substituting Nutrient Intakes. Nutrients 2017, 9, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, W.S.; Poston, W.C.; Haddock, C.K. Tissue n − 3 and n − 6 fatty acids and risk for coronary heart disease events. Atherosclerosis 2007, 193, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Iso, H.; Kobayashi, M.; Ishihara, J.; Sasaki, S.; Okada, K.; Kita, Y.; Kokubo, Y.; Tsugane, S. Intake of Fish and n3 Fatty Acids and Risk of Coronary Heart Disease Among Japanese. Circulationaha 2006, 113, 195–202. [Google Scholar] [CrossRef] [Green Version]
- Miyagawa, N.; Miura, K.; Okuda, N.; Kadowaki, T.; Takashima, N.; Nagasawa, S.-Y.; Nakamura, Y.; Matsumura, Y.; Hozawa, A.; Fujiyoshi, A.; et al. Long-chain n-3 polyunsaturated fatty acids intake and cardiovascular disease mortality risk in Japanese: A 24-year follow-up of NIPPON DATA80. Atherosclerosis 2014, 232, 384–389. [Google Scholar] [CrossRef]
- Estes, C.; Anstee, Q.M.; Arias-Loste, M.T.; Bantel, H.; Bellentani, S.; Caballeria, J.; Colombo, M.; Craxì, A.; Crespo, J.; Day, C.P.; et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J. Hepatol. 2018, 69, 896–904. [Google Scholar] [CrossRef]
- Tsuruta, G.; Tanaka, N.; Hongo, M.; Komatsu, M.; Horiuchi, A.; Hamamoto, K.; Iguchi, C.; Nakayama, Y.; Umemura, T.; Ichijo, T.; et al. Nonalcoholic fatty liver disease in Japanese junior high school students: Its prevalence and relationship to lifestyle habits. J. Gastroenterol. 2010, 45, 666–672. [Google Scholar] [CrossRef]
- Chen, L.-H.; Wang, Y.-F.; Xu, Q.-H.; Chen, S.-S. Omega-3 fatty acids as a treatment for non-alcoholic fatty liver disease in children: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 2018, 37, 516–521. [Google Scholar] [CrossRef]
- Parker, H.M.; Johnson, N.A.; Burdon, C.A.; Cohn, J.S.; O’Connor, H.T.; George, J. Omega-3 supplementation and non-alcoholic fatty liver disease: A systematic review and meta-analysis. J. Hepatol. 2012, 56, 944–951. [Google Scholar] [CrossRef] [Green Version]
- He, X.-X.; Wu, X.-L.; Chen, R.-P.; Chen, C.; Liu, X.-G.; Wu, B.-J.; Huang, Z.-M. Effectiveness of Omega-3 Polyunsaturated Fatty Acids in Non-Alcoholic Fatty Liver Disease: A Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2016, 11, e0162368. [Google Scholar] [CrossRef]
- Newton, K.P.; LaVine, J.E.; Wilson, L.; Behling, C.; Vos, M.B.; Molleston, J.P.; Rosenthal, P.; Miloh, T.; Fishbein, M.H.; Jain, A.K.; et al. Alanine Aminotransferase and Gamma-Glutamyl Transpeptidase Predict Histologic Improvement in Pediatric Nonalcoholic Steatohepatitis. Hepatology 2020, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Nobili, V.; Alisi, A.; Della Corte, C.; Risé, P.; Galli, C.; Agostoni, C.; Bedogni, G. Docosahexaenoic acid for the treatment of fatty liver: Randomised controlled trial in children. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 1066–1070. [Google Scholar] [CrossRef] [PubMed]
- Janczyk, W.; Lebensztejn, D.; Wierzbicka-Rucińska, A.; Mazur, A.; Neuhoff-Murawska, J.; Matusik, P.; Socha, P. Omega-3 Fatty Acids Therapy in Children with Nonalcoholic Fatty Liver Disease: A Randomized Controlled Trial. J. Pediatr. 2015, 166, 1358–1363.e3. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, J.; Xu, H.; Dong, G.; Huang, K.; Wu, W.; Ye, J.; Fu, J. Ferritin as a key risk factor for nonalcoholic fatty liver disease in children with obesity. J. Clin. Lab. Anal. 2020, e23602, (online ahead of print). [Google Scholar] [CrossRef]
- Mörwald, K.; Aigner, E.; Bergsten, P.; Brunner, S.M.; Forslund, A.; Kullberg, J.; Ahlström, H.; Manell, H.; Roomp, K.; Schütz, S.; et al. Serum Ferritin Correlates with Liver Fat in Male Adolescents with Obesity. Front. Endocrinol. 2020, 11, 340. [Google Scholar] [CrossRef]
- Okuda, M.; Sasaki, S.; Kunitsugu, I.; Sakurai, R.; Yoshitake, N.; Hobara, T. Iron Load and Liver Enzymes in 10- and 13-year-olds. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 333–338. [Google Scholar] [CrossRef]
- Fernandes, J.C.; The GBD 2015 Obesity Collaborators. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef]
- World Health Organization. The Global Health Observatory; World Health Organization: Geneva, Switzerland, 2017; Available online: https://www.who.int/data/gh (accessed on 24 January 2021).
- OECD. The Heavy Burden of Obesity; Organisation for Economic Co-Operation and Development (OECD): Paris, France, 2019. [Google Scholar]
- Ndrepepa, G.; Colleran, R.; Kastrati, A. Gamma-glutamyl transferase and the risk of atherosclerosis and coronary heart disease. Clin. Chim. Acta 2018, 476, 130–138. [Google Scholar] [CrossRef]
- Leite, M.L.C. Compositional data analysis as an alternative paradigm for nutritional studies. Clin. Nutr. ESPEN 2019, 33, 207–212. [Google Scholar] [CrossRef]
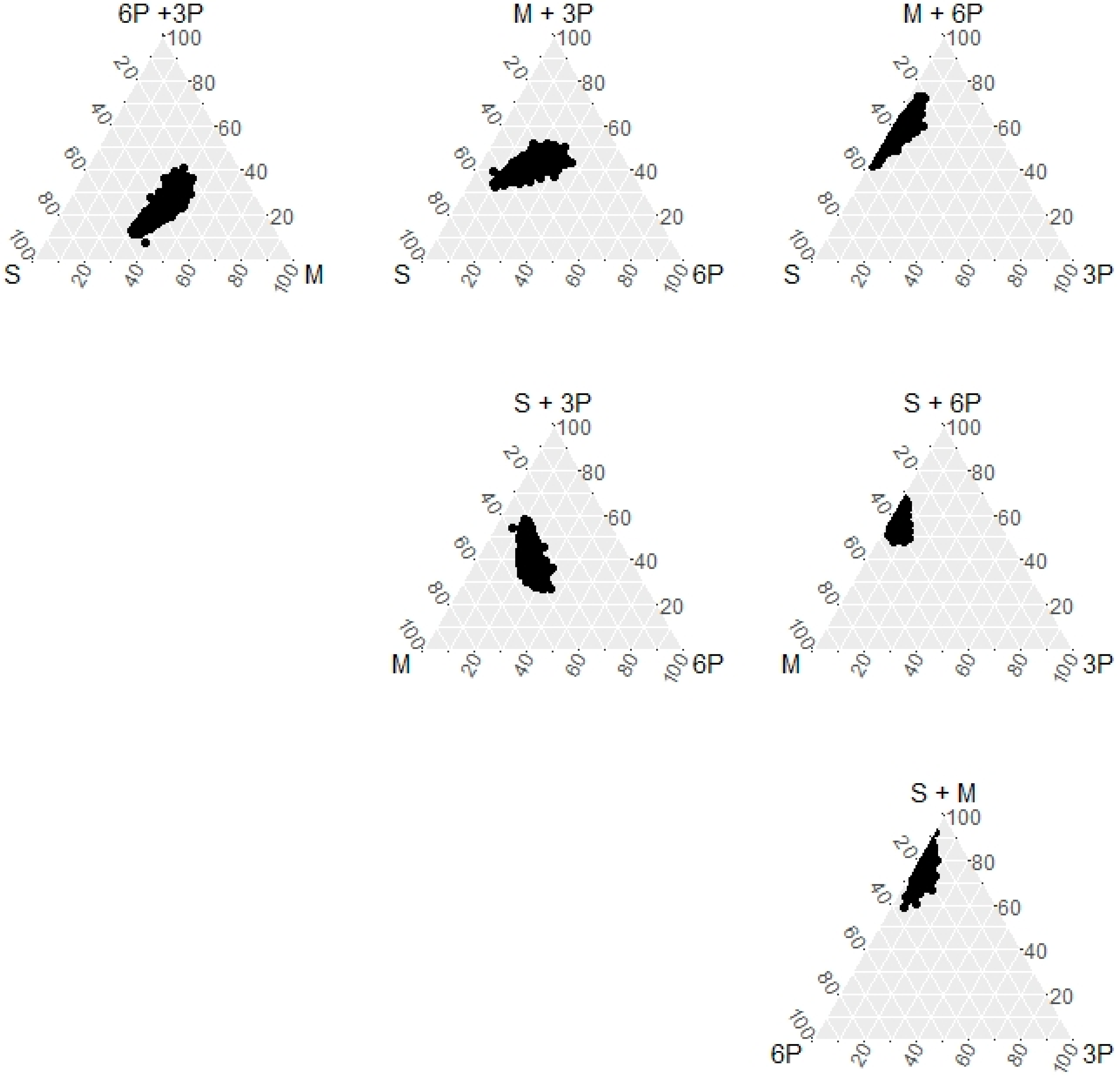
| n | Count (%) or Mean ± SD | |
|---|---|---|
| Sex, male | 2858 (52.1) | |
| Age, years | 13.56 ± 0.29 | |
| Height, cm | 156.8 ± 7.1 | |
| Weight, kg | 47.2 ± 8.6 | |
| BMI, kg/m2 | 19.1 ± 2.7 | |
| LDL-C, mg/dL | 4927 | 86.9 ± 19 |
| HDL-C, mg/dL | 4927 | 67.2 ± 13.8 |
| SBP, mmHg | 4944 | 114.5 ± 11.5 |
| DBP, mmHg | 4944 | 67.7 ± 8.6 |
| AST, IU/L | 4927 | 21.1 ± 5.9 |
| ALT, IU/L | 4927 | 13.5 ± 6.9 |
| GGT, IU/L | 4927 | 15.4 ± 5 |
| Dietary energy, kcal | 2238 ± 640 | |
| Protein, %E | 14.2 ± 2.4 | |
| Fat, %E | 30.1 ± 5.6 | |
| Carbohydrate, %E | 54.2 ± 6.7 | |
| SFA, %E | 9.6 ± 2.4 | |
| MUFA, %E | 10.3 ± 2.1 | |
| PUFA, %E | 6.3 ± 1.4 | |
| omega-6 PUFA, %E | 5.3 ± 1.2 | |
| omega-3 PUFA, %E | 1.1 ± 0.3 | |
| Sodium, mg/1000 kcal | 1848 ± 429 |
| Mean | SFA | MUFA | Omega-6 PUFAs | Omega-3 PUFAs | |
|---|---|---|---|---|---|
| SFA | 36.1% | 0 | 0.031 | 0.077 | 0.112 |
| MUFA | 38.9% | 0.031 | 0 | 0.018 | 0.046 |
| Omega-6 PUFAs | 20.0% | 0.077 | 0.018 | 0 | 0.038 |
| Omega-3 PUFAs | 4.2% | 0.112 | 0.046 | 0.038 | 0 |
| SFA | MUFA | Omega-6 PUFAs | Omega-3 PUFAs | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R-Squared Statistics | p | β (SE) | p | Β (SE) | p | β (SE) | p | β (SE) | p | |
| Height, cm | 0.0030 | <0.001 | −0.22 (0.76) | 0.773 | 0.78 (1.68) | 0.642 | −0.32 (1.07) | 0.762 | −0.06 (0.63) | 0.920 |
| Weight, kg | 0.0003 | 0.179 | −0.83 (0.98) | 0.393 | 2.55 (2.15) | 0.234 | −1.52 (1.37) | 0.266 | 0.92 (0.80) | 0.253 |
| zBMI | 0.0009 | 0.043 | 0.01 (0.11) | 0.896 | 0.10 (0.24) | 0.664 | −0.17 (0.15) | 0.272 | 0.17 (0.09) | 0.0495 |
| Log (LDL-C, mg/dL) | 0.0033 | <0.001 | 0.02 (0.03) | 0.496 | 0.08 (0.06) | 0.161 | −0.11 (0.04) | 0.003 | 0.00 (0.02) | 0.961 |
| Log (HDL-C, mg/dL) | 0.0016 | 0.012 | 0.03 (0.02) | 0.215 | −0.03 (0.05) | 0.581 | 0.02 (0.03) | 0.633 | −0.02 (0.02) | 0.323 |
| SBP, mmHg | 0.0001 | 0.330 | −0.85 (1.34) | 0.523 | 1.44 (2.95) | 0.625 | 1.32 (1.86) | 0.480 | −2.35 (1.11) | 0.034 |
| DBP, mmHg | 0.0010 | 0.046 | −0.47 (1.04) | 0.653 | 0.57 (2.28) | 0.804 | 1.68 (1.44) | 0.245 | −2.41 (0.86) | 0.005 |
| Log (AST, IU/L) | 0.0036 | <0.001 | 0.01 (0.03) | 0.638 | −0.01 (0.06) | 0.900 | −0.01 (0.04) | 0.710 | 0.02 (0.02) | 0.249 |
| Log (ALT, IU/L) | 0.0048 | <0.001 | 0.05 (0.04) | 0.225 | −0.10 (0.09) | 0.266 | −0.01 (0.06) | 0.823 | 0.10 (0.03) | 0.003 |
| Log (GGT, IU/L) | 0.0072 | <0.001 | 0.07 (0.03) | 0.019 | −0.08 (0.07) | 0.235 | −0.01 (0.04) | 0.735 | 0.03 (0.02) | 0.182 |
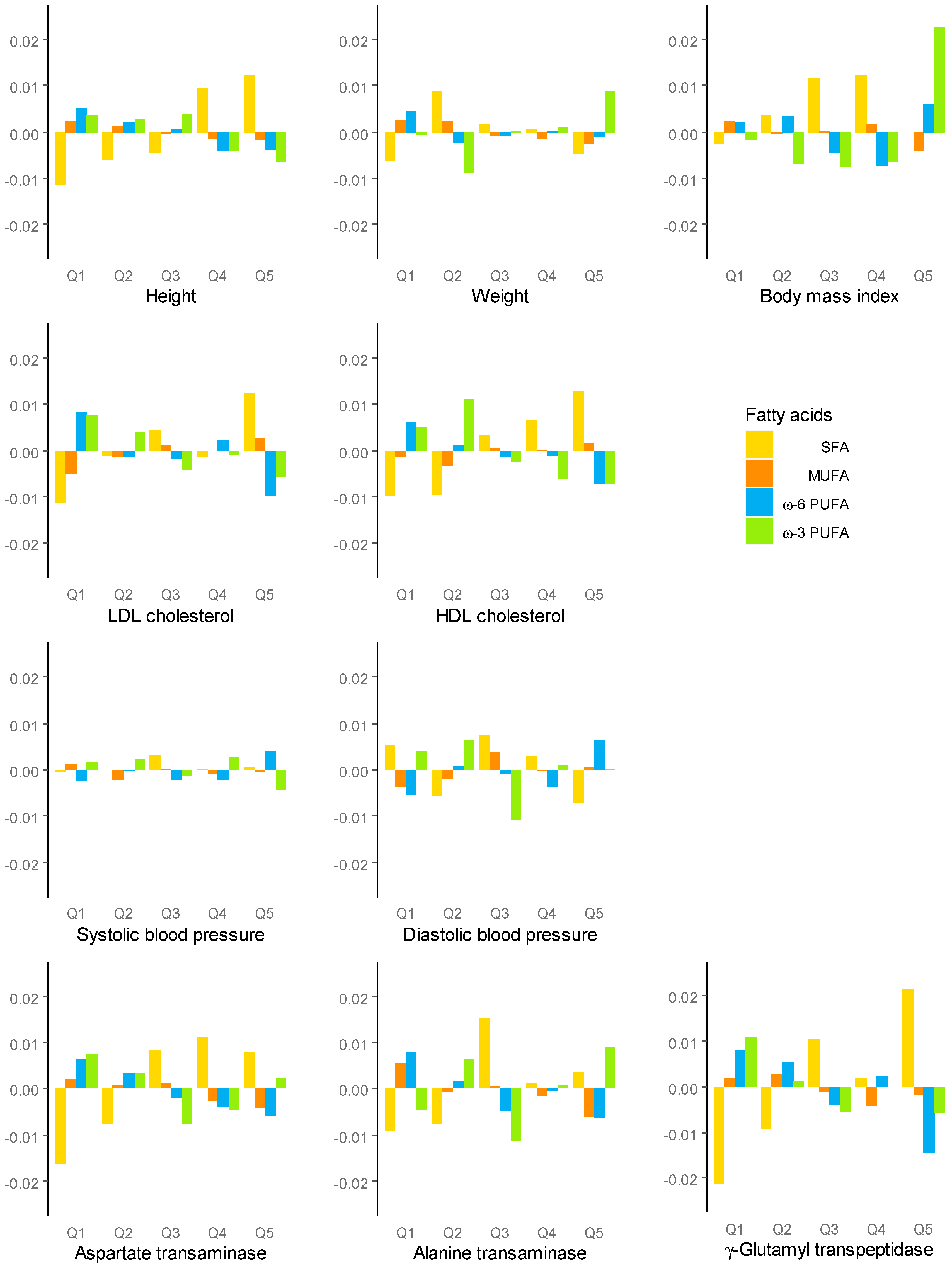
| SFA ← Omega-6 PUFA | MUFA ← SFA | Omega-6 PUFAs ← SFA | Omega-3 PUFAs ← SFA | |||||
|---|---|---|---|---|---|---|---|---|
| β (SE) | p | β (SE) | p | β (SE) | p | β (SE) | p | |
| Height, cm | −0.045 (0.084) | 0.595 | −0.007 (0.136) | 0.959 | −0.032 (0.158) | 0.839 | −0.216 (0.493) | 0.662 |
| Weight, kg | −0.270 (0.107) | 0.012 | −0.355 (0.174) | 0.042 | −0.090 (0.203) | 0.657 | 0.405 (0.631) | 0.521 |
| zBMI | −0.022 (0.012) | 0.069 | −0.054 (0.019) | 0.005 | −0.015 (0.022) | 0.517 | 0.079 (0.070) | 0.259 |
| Log (LDL-C, mg/dL) | 0.011 (0.003) | <0.001 | 0.012 (0.005) | 0.011 | −0.021 (0.006) | <0.001 | −0.002 (0.018) | 0.931 |
| Log (HDL-C, mg/dL) | 0.000 (0.003) | 0.891 | 0.000 (0.004) | 0.924 | 0.001 (0.005) | 0.907 | −0.029 (0.016) | 0.063 |
| SBP, mmHg | 0.110 (0.147) | 0.454 | 0.462 (0.239) | 0.053 | −0.012 (0.277) | 0.965 | −0.873 (0.866) | 0.314 |
| DBP, mmHg | 0.178 (0.114) | 0.118 | 0.385 (0.185) | 0.038 | 0.105 (0.214) | 0.625 | −0.828 (0.671) | 0.217 |
| Log (AST, IU/L) | −0.004 (0.003) | 0.213 | −0.011 (0.005) | 0.019 | 0.001 (0.005) | 0.800 | 0.006 (0.017) | 0.710 |
| log(ALT, IU/L) | −0.007 (0.005) | 0.114 | −0.026 (0.007) | 0.000 | 0.001 (0.009) | 0.950 | 0.036 (0.027) | 0.177 |
| log(GGT, IU/L) | 0.002 (0.003) | 0.598 | −0.009 (0.005) | 0.088 | −0.006 (0.006) | 0.348 | −0.002 (0.019) | 0.911 |
| SFA | MUFA | ω6-PUFAs | ω3-PUFAs | |||
|---|---|---|---|---|---|---|
| Height, cm | diff. | SFA | 0 | −0.075 | 0.042 | 0.135 |
| MUFA | 0.072 | 0 | 0.112 | 0.205 | ||
| ω6-PUFAs | −0.029 | −0.106 | 0 | 0.104 | ||
| ω3-PUFAs | −0.014 | −0.091 | 0.026 | 0 | ||
| Weight, kg | diff. | SFA | 0 | −0.192 | 0.215 | −2.426 |
| MUFA | 0.189 | 0 | 0.395 | −2.246 | ||
| ω6-PUFAs | −0.155 | −0.356 | 0 | −2.589 | ||
| ω3-PUFAs | 0.619 | 0.418 | 0.825 | 0 | ||
| zBMI | diff. | SFA | 0 | 0.005 | 0.033 | −0.442 |
| MUFA | −0.005 | 0 | 0.028 | −0.447 | ||
| ω6-PUFAs | −0.028 | −0.022 | 0 | −0.470 | ||
| ω3-PUFAs | 0.100 | 0.105 | 0.133 | 0 | ||
| LDL-C, mg/dL | ratio | SFA | 1 | 0.993 | 1.023 | 1.004 |
| MUFA | 1.006 | 1 | 1.030 | 1.011 | ||
| ω6-PUFAs | 0.981 | 0.974 | 1 | 0.985 | ||
| ω3-PUFAs | 0.997 | 0.991 | 1.021 | 1 | ||
| HDL-C, mg/dL | ratio | SFA | 1 | 1.005 | 1.000 | 1.054 |
| MUFA | 0.995 | 1 | 0.995 | 1.049 | ||
| ω6-PUFAs | 0.999 | 1.005 | 1 | 1.053 | ||
| ω3-PUFAs | 0.986 | 0.991 | 0.986 | 1 | ||
| SBP, mmHg | diff. | SFA | 0 | −0.244 | −0.334 | 5.813 |
| MUFA | 0.236 | 0 | −0.106 | 6.041 | ||
| ω6-PUFAs | 0.295 | 0.043 | 0 | 6.100 | ||
| ω3-PUFAs | −1.268 | −1.521 | −1.610 | 0 | ||
| DBP, mmHg | diff. | SFA | 0 | −0.153 | −0.364 | 6.009 |
| MUFA | 0.147 | 0 | −0.222 | 6.151 | ||
| ω6-PUFAs | 0.310 | 0.153 | 0 | 6.315 | ||
| ω3-PUFAs | −1.342 | −1.500 | −1.711 | 0 | ||
| AST, IU/L | ratio | SFA | 1 | 1.003 | 1.004 | 0.941 |
| MUFA | 0.997 | 1 | 1.001 | 0.938 | ||
| ω6-PUFAs | 0.997 | 1.000 | 1 | 0.938 | ||
| ω3-PUFAs | 1.013 | 1.017 | 1.017 | 1 | ||
| ALT, IU/L | ratio | SFA | 1 | 1.018 | 1.007 | 0.781 |
| MUFA | 0.983 | 1 | 0.991 | 0.768 | ||
| ω6-PUFAs | 0.993 | 1.011 | 1 | 0.776 | ||
| ω3-PUFAs | 1.054 | 1.073 | 1.062 | 1 | ||
| GGT, IU/L | ratio | SFA | 1 | 1.015 | 1.009 | 0.926 |
| MUFA | 0.985 | 1 | 0.995 | 0.913 | ||
| ω6-PUFAs | 0.991 | 1.006 | 1 | 0.918 | ||
| ω3-PUFAs | 1.012 | 1.028 | 1.022 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okuda, M.; Fujiwara, A.; Sasaki, S. Composition of Dietary Fatty Acids and Health Risks in Japanese Youths. Nutrients 2021, 13, 426. https://doi.org/10.3390/nu13020426
Okuda M, Fujiwara A, Sasaki S. Composition of Dietary Fatty Acids and Health Risks in Japanese Youths. Nutrients. 2021; 13(2):426. https://doi.org/10.3390/nu13020426
Chicago/Turabian StyleOkuda, Masayuki, Aya Fujiwara, and Satoshi Sasaki. 2021. "Composition of Dietary Fatty Acids and Health Risks in Japanese Youths" Nutrients 13, no. 2: 426. https://doi.org/10.3390/nu13020426






