PNPLA3 Genotype, Arachidonic Acid Intake, and Unsaturated Fat Intake Influences Liver Fibrosis in Hispanic Youth with Obesity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Anthropometry, Adiposity, and Liver Fat and Fibrosis
2.3. Genotype
2.4. Dietary Intake
2.5. Statistical Analysis
3. Results
3.1. Description of Cohort
3.2. Associations between Biological Characteristics and Liver Fibrosis
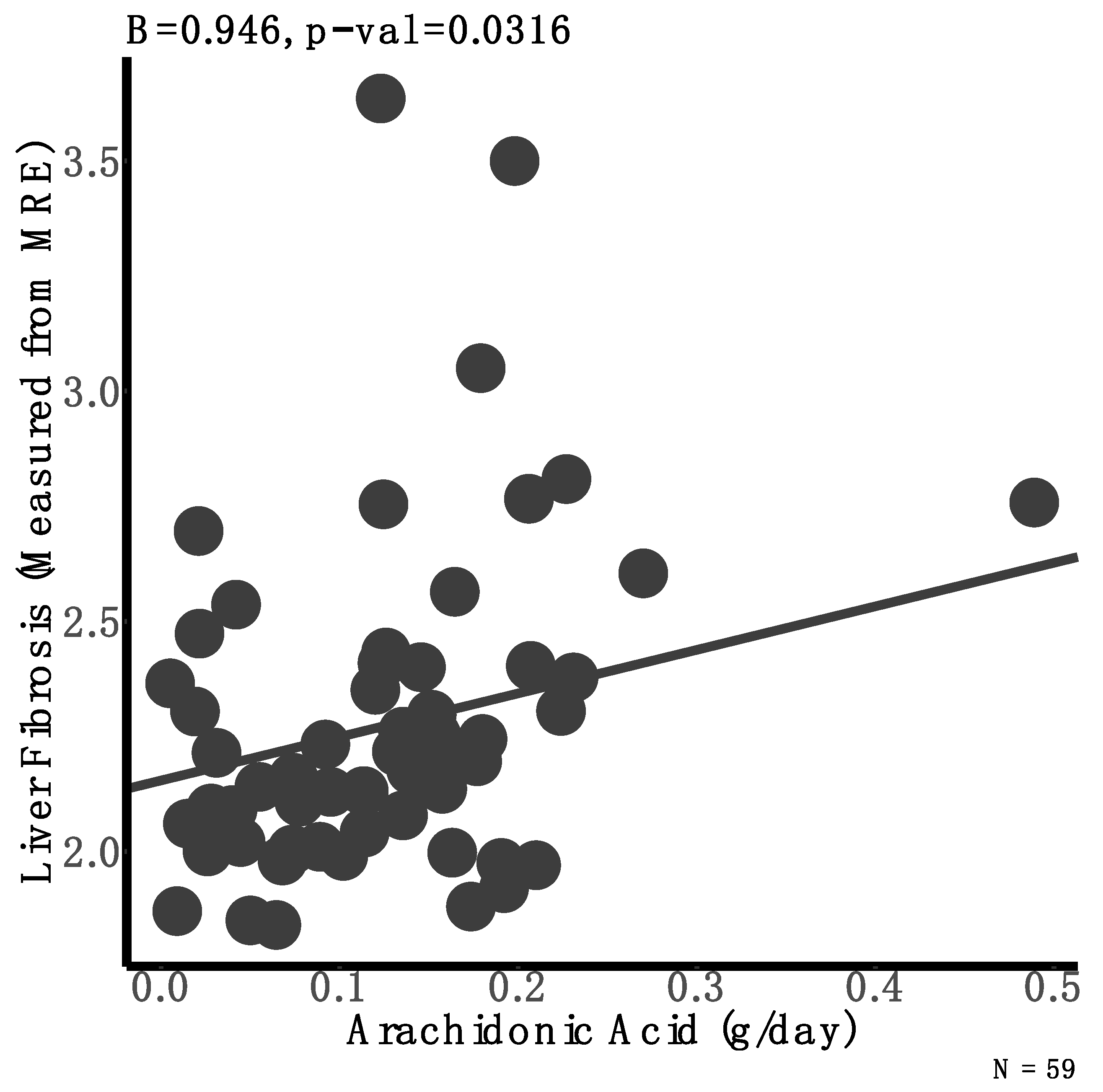
3.3. Associations between Dietary Intake and Liver Fibrosis
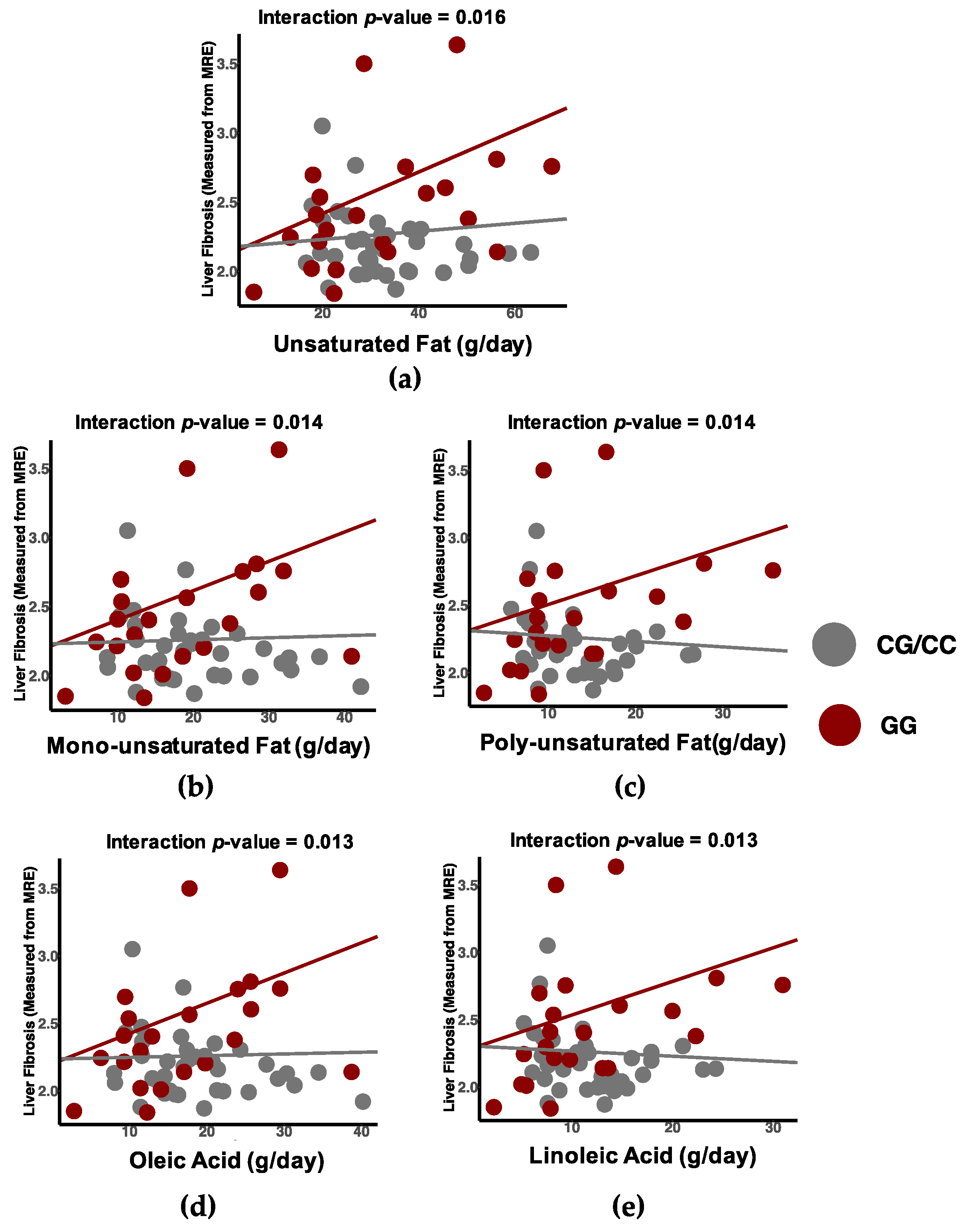
3.4. Differential Associations between Dietary Intake and Liver Fibrosis by Genotype
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, M.; Gong, S.; Ye, S.Q.; Lyman, B.; Geng, L.; Chen, P.; Li, D. Non-Alcoholic Fatty Liver Disease in Children: Focus on Nutritional Interventions. Nutrients 2014, 6, 4691–4705. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.L.; Howe, L.D.; Jones, H.E.; Higgins, J.P.T.; Lawlor, D.A.; Fraser, A. The Prevalence of Non-Alcoholic Fatty Liver Disease in Children and Adolescents: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0140908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welsh, J.A.; Karpen, S.; Vos, M.B. Increasing prevalence of nonalcoholic fatty liver disease among United States adolescents, 1988–1994 to 2007–2010. J. Pediatrics 2013, 162, 496–500.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Than, N.N.; Newsome, P.N. A concise review of non-alcoholic fatty liver disease. Atherosclerosis 2015, 239, 192–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vos, M.B.; Abrams, S.H.; Barlow, S.E.; Caprio, S.; Daniels, S.R.; Kohli, R.; Mouzaki, M.; Sathya, P.; Jeffrey, B.; Sundaram, S.S.; et al. NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children: Recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nu. J. Pediatric Gastroenterol. Nutr. 2017, 64, 319–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekstedt, M.; Hagström, H.; Nasr, P.; Fredrikson, M. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015, 61, 1547–1554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yki-järvinen, H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014, 2, 901–910. [Google Scholar] [CrossRef]
- Cook, L.T.; O’Reilly, G.A.; Goran, M.I.; Weigensberg, M.J.; Spruijt-Metz, D.; Davis, J.N. Vegetable Consumption Is Linked to Decreased Visceral and Liver Fat and Improved Insulin Resistance in Overweight Latino Youth. J. Acad. Nutr. Diet. 2014, 114, 1776–1783. [Google Scholar] [CrossRef] [Green Version]
- Park, S.H.; Jeon, W.O.O.K.; Kim, S.H.; Kim, H.J.; Park, D.I. Prevalence and risk factors of non-alcoholic fatty liver disease among Korean adults. J. Gastroenterol. Hepatol. 2006, 21, 138–143. [Google Scholar] [CrossRef]
- Romeo, S.; Kozlitina, J.; Xing, C.; Pertsemlidis, A.; Cox, D.; Pennacchio, L.A.; Boerwinkle, E.; Cohen, J.C.; Hobbs, H.H. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 2008, 40, 1461–1465. [Google Scholar] [CrossRef] [Green Version]
- Browning, J.D.; Szczepaniak, L.S.; Dobbins, R.; Nuremberg, P.; Horton, J.D.; Cohen, J.C.; Grundy, S.M.; Hobbs, H.H. Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatology 2004, 40, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Martínez, L.A.; Larrieta, E.; Calva, J.J.; Kershenobich, D.; Torre, A. The Expression of PNPLA3 Polymorphism could be the Key for Severe Liver Disease in NAFLD in Hispanic Population. Ann. Hepatol. 2019, 16, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.R.; Raskin, S. The eicosapentaenoic acid: Arachidonic acid ratio and its clinical utility in cardiovascular disease. Postgrad. Med. 2019, 131, 268–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simopoulos, A.P. The Importance of the Omega-6/Omega-3 Fatty Acid Ratio in Cardiovascular Disease and Other Chronic Diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Tomita, K.; Teratani, T.; Suzuki, T.; Shimizu, M.; Sato, H.; Narimatsu, K.; Okada, Y.; Kurihara, C.; Irie, R.; Yokoyama, H.; et al. Free Cholesterol Accumulation in Hepatic Stellate Cells: Mechanism of Liver Fibrosis Aggravation in Nonalcoholic Steatohepatitis in Mice. Hepatology 2014, 59, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Kisseleva, T.; Diego, S.; Jolla, L. Reversibility of liver fibrosis. Clin. Res. Hepatol. Gastroenterol. 2015, 39, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Bataller, R.; Brenner, D.A. Liver fibrosis. J. Clin. Investig. 2005, 115, 209–218. [Google Scholar] [CrossRef]
- Davis, J.N.; Lê, K.-A.; Walker, R.W.; Vikman, S.; Spruijt-Metz, D.; Weigensberg, M.J.; Allayee, H.; Goran, M.I. Increased hepatic fat in overweight Hispanic youth influenced by interaction between genetic variation in PNPLA3 and high dietary carbohydrate and sugar consumption. Am. J. Clin. Nutr. 2010, 92, 1522–1527. [Google Scholar] [CrossRef] [Green Version]
- Jones, R.B.; Alderete, T.L.; Martin, A.A.; Geary, B.A.; Hwang, D.H.; Palmer, S.L.; Goran, M.I. Probiotic supplementation increases obesity with no detectable effects on liver fat or gut microbiota in obese Hispanic adolescents: A 16-week, randomized, placebo-controlled trial. Pediatric Obes. 2018, 13, 705–714. [Google Scholar] [CrossRef]
- Sawh, M.C.; Newton, K.P.; Goyal, N.P.; Angeles, J.E.; Harlow, K.; Bross, C. Normal Range for MR Elastography Measured Liver Stiffness in Children Without Liver Disease. J. Magn. Reson Imaging 2019, 51, 26905. [Google Scholar] [CrossRef]
- Shen, J.; Wong, G.L.-H.; Chan, H.L.-Y.; Chan, H.-Y.; Yeung, D.K.-W.; Chan, R.S.-M.; Chim, A.M.-L.; Chan, A.W.-H.; Choi, P.C.-L.; Woo, J.; et al. PNPLA3 gene polymorphism accounts for fatty liver in community subjects without metabolic syndrome. Aliment. Pharm. 2014, 39, 532–539. [Google Scholar] [CrossRef]
- Goran, M.I.; Walker, R.; Le, K.; Mahurkar, S.; Vikman, S.; Davis, J.N.; Spruijt-metz, D.; Weigensberg, M.J.; Allayee, H. Effects of PNPLA3 on Liver Fat and Metabolic Profile in Hispanic Children and Adolescents. Diabetes 2010, 59, 3127–3130. [Google Scholar] [CrossRef] [Green Version]
- Wagenknecht, L.E.; Palmer, N.D.; Bowden, D.W.; Rotter, J.I.; Norris, M.; Ziegler, J.; Chen, Y.I.; Haffner, S.; Scherzinger, A.; Carl, D. NIH Public Access. Liver Int. 2011, 31, 412–416. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Qu, H.; Rentfro, A.R.; Grove, M.L.; Lu, Y.; Hanis, C.L.; Fallon, M.B.; Boerwinkle, E.; Fisher-hoch, S.P.; Mccormick, J.B. NIH Public Access. Clin. Investig. Med. 2012, 35, E237. [Google Scholar] [CrossRef] [Green Version]
- Valenti, L.; Al-serri, A.; Daly, A.K.; Galmozzi, E.; Rametta, R.; Dongiovanni, P.; Nobili, V.; Mozzi, E.; Roviaro, G.; Vanni, E.; et al. Homozygosity for the Patatin-Like Phospholipase-3/Adiponutrin I148M Polymorphism Influences Liver Fibrosis in Patients with Nonalcoholic Fatty Liver Disease. Hepatology 2010, 2008, 1209–1217. [Google Scholar] [CrossRef]
- Allemand, D.; Reinehr, T.; Widhalm, K.; Holl, R.W. Obese boys at increased risk for nonalcoholic liver disease: Evaluation of 16 390 overweight or obese children and adolescents. Int. J. Obes. 2010, 34, 1468–1474. [Google Scholar] [CrossRef] [Green Version]
- Ballestri, S.; Nascimbeni, F.; Baldelli, E.; Marrazzo, A.; Romagnoli, D.; Lonardo, A. NAFLD as a Sexual Dimorphic Disease: Role of Gender and Reproductive Status in the Development and Progression of Nonalcoholic Fatty Liver Disease and Inherent Cardiovascular Risk. Adv. Ther. 2017, 34, 1291–1326. [Google Scholar] [CrossRef]
- Tricò, D.; Caprio, S.; Umano, G.R.; Pierpont, B.; Nouws, J.; Galderisi, A.; Kim, G.; Mata, M.M.; Santoro, N. Metabolic Features of Nonalcoholic Fatty Liver (NAFL) in Obese Adolescents: Findings From a Multiethnic Cohort. Hepatology 2018, 68. [Google Scholar] [CrossRef] [Green Version]
- Sonnweber, T.; Pizzini, A.; Nairz, M.; Weiss, G.; Tancevski, I. Arachidonic Acid Metabolites in Cardiovascular and Metabolic Diseases. Int. J. Mol. Sci. 2018, 19, 3285. [Google Scholar] [CrossRef] [Green Version]
- Burns, J.L.; Nakamura, M.T.; Ma, D.W.L. Differentiating the biological effects of linoleic acid from arachidonic acid in health and disease. Prostaglandins Leukot. Essent. Fat. Acids 2018, 135, 1–4. [Google Scholar] [CrossRef]
- Innes, J.K.; Calder, P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 41–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scorletti, E.; Byrne, C.D. Omega-3 Fatty Acids, Hepatic Lipid Metabolism, and Nonalcoholic Fatty Liver Disease. Annu. Rev. Nutr. 2013, 33, 231–258. [Google Scholar] [CrossRef]
- López-Vicario, C.; González-Périz, A.; Rius, B.; Morán-Salvador, E.; García-Alonso, V.; Lozano, J.J.; Bataller, R.; Kang, J.X.; Arroyo, V.; Clària, J.; et al. Molecular interplay between Δ5/Δ6 desaturases and long-chain fatty acids in the pathogenesis of non-alcoholic steatohepatitis. Gut 2014, 63, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Araya, J.; Rodrigo, R.; Pettinelli, P.; Araya, A.V.; Poniachik, J.; Videla, L.A. Decreased Liver Fatty Acid Δ-6 and Δ-5 Desaturase Activity in Obese Patients. Obesity 2010, 18, 1460–1463. [Google Scholar] [CrossRef] [PubMed]
- Adam, O.; Beringer, C.; Kless, T.; Lemmen, C.; Adam, A.; Wiseman, M.; Adam, P.; Klimmek, R.; Forth, W. Anti-inflammatory effects of a low arachidonic acid diet and fish oil in patients with rheumatoid arthritis. Rheumatol. Int. 2003, 23, 27–36. [Google Scholar] [CrossRef]
- Ariyoshi, K.; Okuya, S.; Kunitsugu, I.; Matsunaga, K.; Nagao, Y.; Nomiyama, R.; Takeda, K.; Tanizawa, Y. Ultrasound analysis of gray-scale median value of carotid plaques is a useful reference index for cerebro-cardiovascular events in patients with type 2 diabetes. J. Diabetes Investig. 2015, 6, 91–97. [Google Scholar] [CrossRef]
- Ishitobi, T.; Hyogo, H.; Kan, H.; Hiramatsu, A.; Arihiro, K.; Aikata, H.; Chayama, K. Eicosapentaenoic acid/arachidonic acid ratio as a possible link between non-alcoholic fatty liver disease and cardiovascular disease. Hepatol. Res. 2015, 45, 533–539. [Google Scholar] [CrossRef]
- Molendi-coste, O.; Legry, V.; Leclercq, I.A. Why and How Meet n-3 PUFA Dietary Recommendations? Gastroenterol. Res. Pract. 2011. [Google Scholar] [CrossRef] [Green Version]
- Hodson, L.; Bhatia, L.; Scorletti, E.; Smith, D.E.; Jackson, N.C.; Umpleby, M.; Calder, P.C.; Byrne, C.D. Docosahexaenoic acid enrichment in NAFLD is associated with improvements in hepatic metabolism and hepatic insulin sensitivity: A pilot study This article has been corrected since Advance Online Publication and a corrigendum is also printed in this issue. Eur. J. Clin. Nutr. 2017, 71, 973–979. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Guan, B.; Gao, H.; Peng, X. Omega-3 polyunsaturated fatty acid supplementation and non-alcoholic fatty liver disease. Med. Baltim. 2018, 97, 1–10. [Google Scholar] [CrossRef]
- Janczyk, W.; Lebensztejn, D.; Wierzbicka-ruci, A. Omega-3 Fatty Acids Therapy in Children with Nonalcoholic Fatty Liver Disease: A Randomized Controlled Trial. J. Pediatrics 2015, 166, 1358–1365. [Google Scholar] [CrossRef]
- Scorletti, E.; Bhatia, L.; Mccormick, K.G.; Clough, G.F.; Nash, K.; Hodson, L.; Moyses, H.E.; Calder, P.C.; Byrne, C.D. Effects of Purified Eicosapentaenoic and Docosahexaenoic Acids in Nonalcoholic Fatty Liver Disease: Results From the WELCOME* Study. Hepatology 2014, 60, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Romeo, S.; Huang-Doran, I.; Baroni, M.G.; Kotronen, A. Unravelling the pathogenesis of fatty liver disease: Patatin-like phospholipase domain-containing 3 protein. Curr. Opin. Lipidol. 2010, 21, 247–252. [Google Scholar] [CrossRef]
- He, S.; McPhaul, C.; Li, J.Z.; Garuti, R.; Kinch, L.; Grishin, N.V.; Cohen, J.C.; Hobbs, H.H. A Sequence Variation (I148M) in PNPLA3 Associated with Nonalcoholic Fatty Liver Disease Disrupts Triglyceride Hydrolysis. J. Biol. Chem. 2010, 285, 6706–6715. [Google Scholar] [CrossRef] [Green Version]
- Anstee, Q.M.; Day, C.P. The Genetics of Nonalcoholic Fatty Liver Disease: Spotlight on PNPLA3 and TM6SF2. Semin. Liver Dis. 2015, 35, 270–290. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; He, S.; Li, J.Z.; Seo, Y.-K.; Osborne, T.F.; Cohen, J.C.; Hobbs, H.H. A feed-forward loop amplifies nutritional regulation of PNPLA3. Proc. Natl. Acad. Sci. USA 2010, 107, 7892–7897. [Google Scholar] [CrossRef] [Green Version]
- Li, J.Z.; Huang, Y.; Karaman, R.; Ivanova, P.T.; Brown, H.A.; Roddy, T.; Castro-Perez, J.; Cohen, J.C.; Hobbs, H.H. Chronic overexpression of PNPLA3I148M in mouse liver causes hepatic steatosis. J. Clin. Investig. 2012, 122, 4130–4144. [Google Scholar] [CrossRef] [Green Version]
- Nanji, A.A.; French, S.W. Dietary linoleic acid is required for development of experimentally induced alcoholic liver injury. Life Sci. 1989, 44, 223–227. [Google Scholar] [CrossRef]
- Jeyapal, S.; Kona, S.R.; Mullapudi, S.V.; Putcha, U.K.; Gurumurthy, P.; Ibrahim, A. Substitution of linoleic acid with α-linolenic acid or long chain n-3 polyunsaturated fatty acid prevents Western diet induced nonalcoholic steatohepatitis. Sci. Rep. 2018, 8, 10953. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, Y.; Tanaka, S.; Haga, Y. Enhanced GLUT2 gene expression in an oleic acid-induced in vitro fatty liver model. Hepatol. Res. 2002. [Google Scholar] [CrossRef]
- Janorkar, A.V.; King, K.R.; Megeed, Z.; Yarmush, M.L. Development of an in vitro cell culture model of hepatic steatosis using hepatocyte-derived reporter cells. Biotechnol. Bioeng. 2009, 102, 1466–1474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cansanção, K.; Silva Monteiro, L.; Carvalho Leite, N.; Dávalos, A.; Tavares do Carmo, M.D.G.; Arantes Ferreira Peres, W. Advanced Liver Fibrosis Is Independently Associated with Palmitic Acid and Insulin Levels in Patients with Non-Alcoholic Fatty Liver Disease. Nutrients 2018, 10, 1586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| N | CC (n = 13) | N | CG (n = 24) | GG (n = 22) | p-Value | |
|---|---|---|---|---|---|---|
| Biological Characteristics | ||||||
| Age (years) | 13 | 14.2 (2) | 24 | 14.4 (2) | 14.1 (2) | 0.85 |
| Females (N (%)) | 13 | 8 (61.5) | 24 | 13 (54.2) | 9 (40.9) | 0.46 |
| BMI (kg/m2) | 13 | 35.7 (8.8) | 24 | 33.7 (5) | 32.8 (5.6) | 0.19 |
| Waist circumference (cm) | 13 | 107 (16) | 24 | 104 (12) | 103 (11) | 0.43 |
| Body fat (%) | 13 | 42.6 (5.7) | 24 | 42.8 (4.8) | 41.7 (5.4) | 0.59 |
| Trunk fat (%) | 13 | 43 (6.1) | 24 | 43.8 (5.3) | 42.6 (5.4) | 0.84 |
| Subcutaneous adipose tissue (L) | 13 | 7.7 (2.5) | 24 | 8.5 (3.7) | 7 (2.2) | 0.53 |
| Visceral adipose tissue (L) | 13 | 1.8 (0.7) | 24 | 1.8 (0.7) | 2.2 (1) | 0.29 |
| Liver fat content (%) | 13 | 6.8 (5.2) | 24 | 8.8 (5.8) | 17.9 (11.3) | <0.001 |
| Liver stiffness (kPa) | 13 | 2.3 (0.2) | 24 | 2.4 (0.3) | 2.7 (0.5) | 0.01 |
| Liver fibrosis (N, %) | 13 | 1 (7.7) | 24 | 2 (8.3) | 7 (31.8) | 0.06 |
| Dietary Intake | ||||||
| Energy intake (kcal/day) | 13 | 1856 (779) | 24 | 1479 (384) | 1639 (607) | 0.28 |
| Fat (g/day) | 13 | 68 (30) | 24 | 55 (17) | 57 (28) | 0.20 |
| Protein (g/day) | 13 | 69 (28) | 24 | 63 (20) | 72 (28) | 0.72 |
| Carbohydrates (g/day) | 13 | 248 (113) | 24 | 186 (69) | 214 (82) | 0.25 |
| Total sugars (g/day) | 13 | 86 (38) | 24 | 80 (39) | 94 (49) | 0.57 |
| Added sugars (g/day) | 13 | 54 (39) | 24 | 44 (28) | 45 (39) | 0.49 |
| Sucrose(g/day) | 13 | 30 (17) | 24 | 36 (27) | 35 (26) | 0.57 |
| Fiber (g/day) | 13 | 18 (8) | 24 | 12 (5) | 16 (5) | 0.22 |
| Dietary fatty acids profile | ||||||
| Saturated fatty acids (g/day) | 13 | 22 (9) | 19 (7) | 18 (9) | 0.20 | |
| MUFA (g/day) | 13 | 23 (10) | 19 (7) | 19 (9) | 0.12 | |
| PUFA (g/day) | 13 | 18 (12) | 13 (5) | 13 (8) | 0.13 | |
| Omega-3 fatty acids (g/day) | 13 | 1.8 (1.4) | 1.2 (0.4) | 1.4 (0.9) | 0.25 | |
| EPA (g/day) | 13 | 0.01 (0.03) | 0.02 (0.04) | 0.01 (0.01) | 0.84 | |
| DHA (g/day) | 13 | 0.02 (0.03) | 0.05 (0.08) | 0.03 (0.04) | 0.55 | |
| Omega-6 fatty acids (g/day) | 13 | 15.9 (10.5) | 11.7 (4.8) | 11.9 (7.1) | 0.12 | |
| Arachidonic acid (g/day) | 13 | 0.1 (0.07) | 0.1 (0.07) | 0.15 (0.1) | 0.08 | |
| Linoleic acid (g/day) | 13 | 15.8 (10.4) | 11.5 (4.8) | 11.7 (7.0) | 0.11 | |
| Saturated fatty acids (g/day) | 13 | 22 (9) | 24 | 19 (7) | 18 (9) | 0.20 |
| β | p-Value | |
|---|---|---|
| Age (year) | −0.03 | 0.18 |
| Sex * | - | 0.008 |
| PNPLA Genotype (GG vs. CG/CC) * | - | 0.004 |
| BMI (kg/m2) | −0.0003 | 0.97 |
| Waist circumference (cm) | 0.003 | 0.44 |
| Body fat percent (%) | −0.004 | 0.64 |
| Trunk fat percent (%) | −0.004 | 0.69 |
| Subcutaneous adipose tissue (L) | −0.009 | 0.59 |
| Visceral adipose tissue (L) | 0.04 | 0.47 |
| Liver fat (%) | 0.01 | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, R.B.; Arenaza, L.; Rios, C.; Plows, J.F.; Berger, P.K.; Alderete, T.L.; Fogel, J.L.; Nayak, K.; Mohamed, P.; Hwang, D.; et al. PNPLA3 Genotype, Arachidonic Acid Intake, and Unsaturated Fat Intake Influences Liver Fibrosis in Hispanic Youth with Obesity. Nutrients 2021, 13, 1621. https://doi.org/10.3390/nu13051621
Jones RB, Arenaza L, Rios C, Plows JF, Berger PK, Alderete TL, Fogel JL, Nayak K, Mohamed P, Hwang D, et al. PNPLA3 Genotype, Arachidonic Acid Intake, and Unsaturated Fat Intake Influences Liver Fibrosis in Hispanic Youth with Obesity. Nutrients. 2021; 13(5):1621. https://doi.org/10.3390/nu13051621
Chicago/Turabian StyleJones, Roshonda B., Lide Arenaza, Claudia Rios, Jasmine F. Plows, Paige K. Berger, Tanya L. Alderete, Jennifer L. Fogel, Krishna Nayak, Passant Mohamed, Darryl Hwang, and et al. 2021. "PNPLA3 Genotype, Arachidonic Acid Intake, and Unsaturated Fat Intake Influences Liver Fibrosis in Hispanic Youth with Obesity" Nutrients 13, no. 5: 1621. https://doi.org/10.3390/nu13051621






