Ketogenic Dietary Therapies in Patients with Autism Spectrum Disorder: Facts or Fads? A Scoping Review and a Proposal for a Shared Protocol
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Study Selection
2.3. Data Charting
3. Results
3.1. Characteristics of Included Studies
3.2. Dietary Aspects
3.2.1. Ketogenic Dietary Therapies
3.2.2. Periods of Observation
3.2.3. Ketosis
3.2.4. Laboratory Findings and Changes
3.2.5. Side Effects
3.2.6. Retention Rate
3.3. Behavioral Outcomes
3.3.1. CARS Scoring
3.3.2. Other Instruments
4. Discussion
5. Limitations
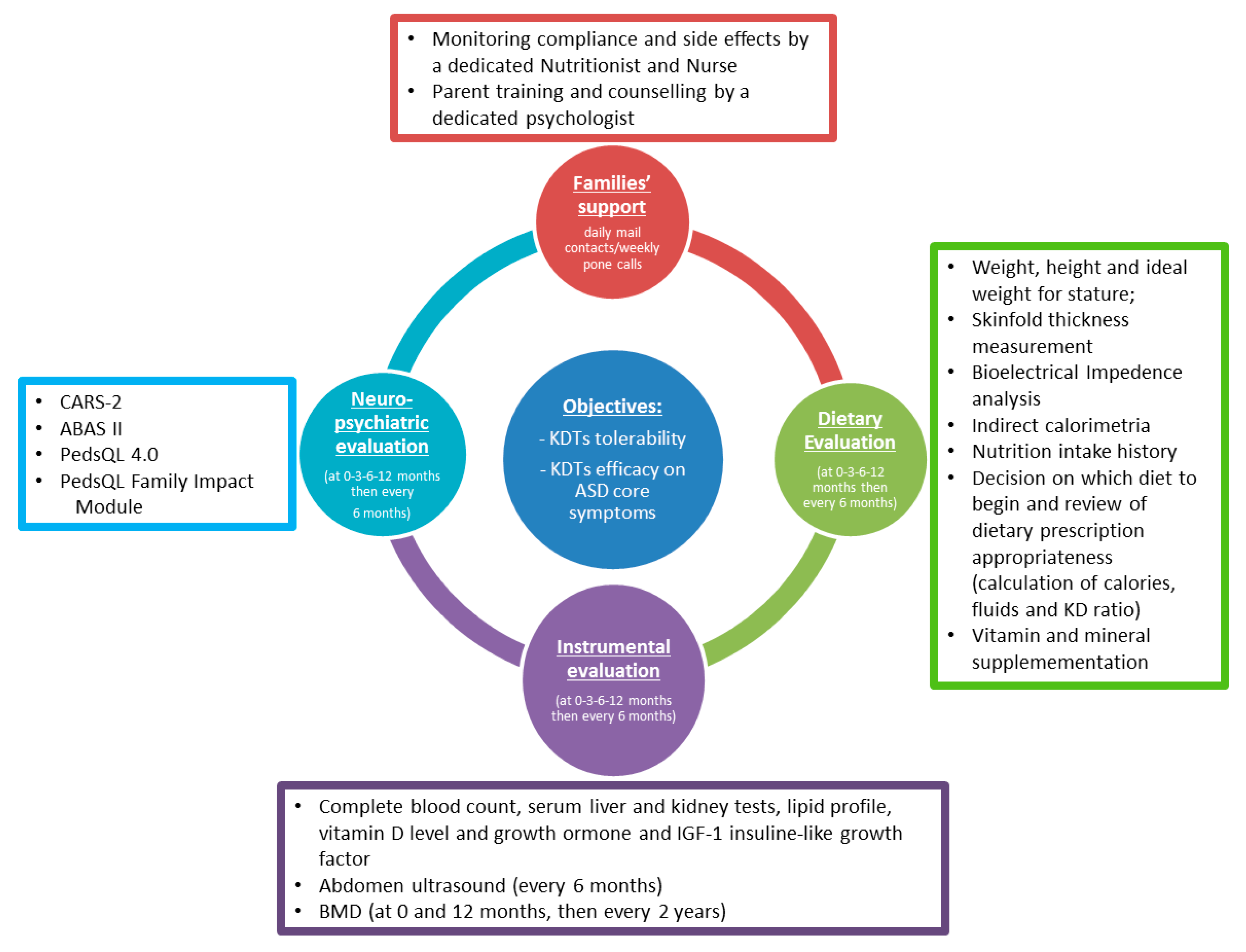
6. Proposal for a Shared Protocol (Figure 3)
Author Contributions
Funding
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Fombonne, E.; MacFarlane, H.; Salem, A.C. Epidemiological surveys of ASD: Advances and remaining challenges. J. Autism Dev. Disord. 2021. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Kodak, T.; Bergmann, S. Autism Spectrum Disorder: Characteristics, Associated Behaviors, and Early Intervention. Pediatr. Clin. N. Am. 2020, 67, 525–535. [Google Scholar] [CrossRef]
- Persico, A.M.; Ricciardello, A.; Lamberti, M.; Turriziani, L.; Cucinotta, F.; Brogna, C.; Vitiello, B.; Arango, C. The pediatric psychopharmacology of autism spectrum disorder: A systematic review—Part I: The past and the present. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 12, 110326. [Google Scholar] [CrossRef] [PubMed]
- Farmer, C.; Thurm, A.; Grant, P. Pharmacotherapy for the core symptoms in autistic disorder: Current status of the research. Drugs 2013, 73, 303–314. [Google Scholar] [CrossRef]
- Jobski, K.; Höfer, J.; Hoffmann, F.; Bachmann, C. Use of psychotropic drugs in patients with autism spectrum disorders: A systematic review. Acta Psychiatr. Scand. 2017, 135, 8–28. [Google Scholar] [CrossRef]
- Madra, M.; Ringel, R.; Margolis, K.G. Gastrointestinal Issues and Autism Spectrum Disorder. Psychiatr. Clin. N. Am. 2021, 44, 69–81. [Google Scholar] [CrossRef]
- Elder, J.H. The gluten-free, casein-free diet in autism: An overview with clinical implications. Nutr. Clin. Pract. Off. Publ. Am. Soc. Parenter. Enter. Nutr. 2008, 23, 583–588. [Google Scholar] [CrossRef]
- Marí-Bauset, S.; Zazpe, I.; Mari-Sanchis, A.; Llopis-González, A.; Morales-Suárez-Varela, M. Evidence of the gluten-free and casein-free diet in autism spectrum disorders: A systematic review. J. Child Neurol. 2014, 29, 1718–1727. [Google Scholar] [CrossRef]
- Peterman, M.G. The ketogenic diet in epilepsy. J. Am. Med. Assoc. 1925, 84, 1979–1983. [Google Scholar] [CrossRef]
- Kossoff, E.H.; Zupec-Kania, B.A.; Auvin, S.; Ballaban-Gil, K.R.; Christina Bergqvist, A.G.; Blackford, R.; Buchhalter, J.R.; Caraballo, R.H.; Cross, J.H.; Dahlin, M.G.; et al. Optimal clinical management of children receiving dietary therapies for epilepsy: Updated recommendations of the International Ketogenic Diet Study Group. Epilepsia Open 2018, 21, 175–192. [Google Scholar] [CrossRef]
- Decampo, D.M.; Kossoff, E.H. Ketogenic dietary therapies for epilepsy and beyond. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 264–268. [Google Scholar] [CrossRef]
- Cross, J.H. Dietary therapies--an old idea with a new lease of life. Seizure 2010, 19, 671–674. [Google Scholar] [CrossRef]
- Augustin, K.; Khabbush, A.; Williams, S.; Eaton, S.; Orford, M.; Cross, J.H.; Heales, S.J.R.; Walker, M.C.; Williams, R.S.B. Mechanisms of action for the medium-chain triglyceride ketogenic diet in neurological and metabolic disorders. Lancet Neurol. 2018, 17, 84–93. [Google Scholar] [CrossRef]
- Zarnowska, I.M. Therapeutic Use of the Ketogenic Diet in Refractory Epilepsy: What We Know and What Still Needs to Be Learned. Nutrients 2020, 12, 2616. [Google Scholar] [CrossRef]
- Lee, M. The use of ketogenic diet in special situations: Expanding use in intractable epilepsy and other neurologic disorders. Korean J. Pediatr. 2012, 55, 316–321. [Google Scholar] [CrossRef]
- Rawat, K.; Singh, N.; Kumari, P.; Saha, L. A review on preventive role of ketogenic diet (KD) in CNS disorders from the gut microbiota perspective. Rev. Neurosci. 2020, 19, 143–157. [Google Scholar] [CrossRef]
- Ruskin, D.N.; Svedova, J.; Cote, J.L.; Sandau, U.; Rho, J.M.; Kawamura, M., Jr.; Boison, D.; Masino, S.A. Ketogenic diet improves core symptoms of autism in BTBR mice. PLoS ONE 2013, 5, e65021. [Google Scholar] [CrossRef]
- Castro, K.; Baronio, D.; Perry, I.S.; Riesgo, R.D.S.; Gottfried, C. The effect of ketogenic diet in an animal model of autism induced by prenatal exposure to valproic acid. Nutr. Neurosci. 2017, 20, 343–350. [Google Scholar] [CrossRef]
- Ahn, Y.; Narous, M.; Tobias, R.; Rho, J.M.; Mychasiuk, R. The ketogenic diet modifies social and metabolic alterations identified in the prenatal valproic acid model of autism spectrum disorder. Dev. Neurosci. 2014, 36, 371–380. [Google Scholar] [CrossRef]
- Ruskin, D.N.; Fortin, J.A.; Bisnauth, S.N.; Masino, S.A. Ketogenic diets improve behaviors associated with autism spectrum disorder in a sex-specific manner in the EL mouse. Physiol. Behav. 2017, 1, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Peters, M.D.J.; Godfrey, C.M.; Khalil, H.; McInerney, P.; Parker, D.; Soares, C.B. Guidance for conducting systematic scoping reviews. JBI Evid. Implement. 2015, 13, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Herbert, M.R.; Buckley, J.A. Autism and Dietary Therapy: Case Report and Review of the Literature. J. Child Neurol. 2013, 28, 975–982. [Google Scholar] [CrossRef]
- Żarnowska, I.; Chrapko, B.; Gwizda, G.; Nocuń, A.; Mitosek-Szewczyk, K.; Gasior, M. Therapeutic use of carbohydrate-restricted diets in an autistic child; a case report of clinical and 18FDG PET findings. Metab. Brain Dis. 2018, 33, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Frye, R.; Sreenivasula, E.S.; Adams, J.B. Traditional and non-traditional treatments for autism spectrum disorder with seizures: An on-line survey. BMC Pediatr. 2011, 18, 37. [Google Scholar] [CrossRef]
- Lee, R.W.Y.; Corley, M.J.; Pang, A.; Arakaki, G.; Abbott, L.; Nishimoto, M.; Miyamoto, R.; Lee, E.; Yamamoto, S.; Maunakea, A.K.; et al. A modified ketogenic gluten-free diet with MCT improves behavior in children with autism spectrum disorder. Physiol. Behav. 2018, 1, 205–211. [Google Scholar] [CrossRef]
- Mu, C.; Corley, M.J.; Lee, R.W.Y.; Wong, M.; Pang, A.; Arakaki, G.; Miyamoto, R.; Rho, J.M.; Mickiewicz, B.; Dowlatabadi, R.; et al. Metabolic Framework for the Improvement of Autism Spectrum Disorders by a Modified Ketogenic Diet: A Pilot Study. J. Proteome Res. 2020, 3, 382–390. [Google Scholar] [CrossRef] [PubMed]
- El-Rashidy, O.; El-Baz, F.; El-Gendy, Y.; Khalaf, R.; Reda, D.; Saad, K. Ketogenic diet versus gluten free casein free diet in autistic children: A case-control study. Metab. Brain Dis. 2017, 32, 1935–1941. [Google Scholar] [CrossRef]
- Evangeliou, A.; Vlachonikolis, I.; Mihailidou, H.; Spilioti, M.; Skarpalezou, A.; Makaronas, N.; Prokopiou, A.; Christodoulou, P.; Liapi-Adamidou, G.; Helidonis, E.; et al. Application of a ketogenic diet in children with autistic behavior: Pilot study. J. Child Neurol. 2003, 18, 113–118. [Google Scholar] [CrossRef]
- (CARSTM-2) Childhood Autism Rating ScaleTM, Second Edition, WPS. Available online: http://www.wpspublish.com/cars-2-childhood-autism-rating-scale-second-edition (accessed on 7 April 2021).
- Page, S.D.; Souders, M.C.; Kral, T.V.E.; Chao, A.M.; Pinto-Martin, J. Correlates of Feeding Difficulties Among Children with Autism Spectrum Disorder: A Systematic Review. J. Autism Dev. Disord. 2021. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Jurek, L.; Balthazar, M.; Gulati, S.; Novakovic, N.; Núñez, M.; Oakley, J.; O’Hagan, A. Response (minimum clinically relevant change) in ASD symptoms after an intervention according to CARS-2: Consensus from an expert elicitation procedure. Eur. Child Adolesc. Psychiatry 2021, 7, 1–10. [Google Scholar]
- Grzadzinski, R.; Janvier, D.; Kim, S.H. Recent Developments in Treatment Outcome Measures for Young Children with Autism Spectrum Disorder (ASD). Semin. Pediatr. Neurol. 2020, 34, 100806. [Google Scholar] [CrossRef]
- Mayes, S.D.; Zickgraf, H. Atypical eating behaviors in children and adolescents with autism, ADHD, other disorders, and typical development. Res. Autism Spectr. Disord. 2019, 64, 76–83. [Google Scholar] [CrossRef]
- Sucharew, H.; Macaluso, M. Progress Notes: Methods for Research Evidence Synthesis: The Scoping Review Approach. J. Hosp. Med. 2019, 14, 416–418. [Google Scholar] [CrossRef]
- Pham, M.T.; Rajić, A.; Greig, J.D.; Sargeant, J.M.; Papadopoulos, A.; McEwen, S.A. A scoping review of scoping reviews: Advancing the approach and enhancing the consistency. Res. Synth. Methods 2014, 5, 371–385. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, C.; Guglielmetti, M.; Tamagni, E.; Trentani, C.; De Giorgis, V.; Pasca, L.; Varesio, C.; Ferraro, O.E.; Tagliabue, A. Use of Remote Monitoring by E-mail for Long-Term Management of the Classic Ketogenic Diet. Nutrients 2020, 19, 1833. [Google Scholar] [CrossRef] [PubMed]
- De Giorgis, V.; Veggiotti, P. GLUT1 deficiency syndrome 2013: Current state of the art. Seizure 2013, 22, 803–811. [Google Scholar] [CrossRef]
- Dupuis, A.; Moon, M.J.; Brian, J.; Georgiades, S.; Levy, T.; Anagnostou, E.; Nicolson, R.; Schachar, R.; Crosbie, J. Concurrent Validity of the ABAS-II Questionnaire with the Vineland II Interview for Adaptive Behavior in a Pediatric ASD Sample: High Correspondence Despite Systematically Lower Scores. J. Autism Dev. Disord. 2020. Epub ahead of print. [Google Scholar] [CrossRef]
- Varni, J.W.; Seid, M.; Kurtin, P.S. PedsQL 4.0: Reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med. Care 2001, 39, 800–812. [Google Scholar] [CrossRef] [PubMed]
- Varni, J.; Limbers, C. The Pediatric Quality of Life Inventory: Measuring Pediatric Health-Related Quality of Life from the Perspective of Children and Their Parents. Pediatr. Clin. N. Am. 2009, 56, 843–863. [Google Scholar] [CrossRef] [PubMed]
- Varni, J.W.; Sherman, S.A.; Burwinkle, T.M.; Dickinson, P.E.; Dixon, P. The PedsQL Family Impact Module: Preliminary reliability and validity. Health Qual. Life Outcomes 2004, 27, 55. [Google Scholar] [CrossRef] [PubMed]


| Study | Country | Study Design | Comorbidity | Sample Size | Females (%) | Mean Age (Y) (Range) | Ketogenic Dietary Therapies | Laboratory Outcomes | Behavioral Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| El-Rashidy et al., 2017 | Egypt | RCT | NA | 15 | 26.7 | 5.29 (3–8) | MAD with approximately 60% of the calories from fat sources, 30% from proteins, and 10% from carbohydrates | Complete blood count, serum electrolytes, carnitine, and β-hydroxybutyric acid | Changes in CARS and ATECafter 6 months |
| Evangeliou et al., 2003 | Greece | Prospective follow-up | NA | 30 | 46.6 | 7 (4–10) | John Radcliffe diet: 30% of energy as medium-chain triglyceride oil, 30% as fresh cream, 11% as saturated fat, 19% as carbohydrates, and 10% as protein. | NA | Changes in CARS after 6 months |
| Frye et al., 2011 | USA | Cross-sectional | Clinical seizures, subclinical epileptiform discharges | 733 | 33 | 12 | MAD | NA | Perceived effect on seizures, sleep, language, verbal and non-verbal communication, stereotyped/repetitive movements, rigidity, hyperactivity, attention, and mood. |
| Herbert & Buckley, 2013 | USA | Case report | Epilepsy | 1 | 100 | 12 | Gluten-free casein-free ketogenic 1.5:1 ratio | Cholesterol | Clinical improvements after 14 months |
| Lee et al., 2018 | USA | Open-label, observer-blinded clinical trial | NA | 15 | 13.3 | 3.3 (3–13) | Modified ketogenic gluten-free diet regimen with supplemental MCT | High-density and Low-density lipoprotein; Cholesterol; Eosinophil blood cell percent | Changes in CARS after 3 months and changes in ADOS after 3 and 6 months |
| Mu et al., 2020 | USA and Canada | Open-label, observer-blinded clinical trial | NA | 17 | 11.76 | 9 (2–17) | Modified KD regimen consisted of a gluten-free diet incorporating MCT oil | Metabolic changes | Changes in ADOS after 3 months |
| Żarnowska et al., 2018 | Poland | Case report | ADHD | 1 | 0 | 6 | CKD with a 2:1 ratio. After 1 month on this classic KD, the diet was switched per the parents’ request to a MAD. After five months on the MAD, patient was placed on the LGIT. | Blood parameters | Changes in CARS after 16 months |
| Laboratory Assessment |
|---|
| Capillary Ketonemia * |
| Complete blood count with platelets |
| Serum Electrolytes |
| Sodium |
| Potassium |
| Chlorine |
| Calcium |
| Phosphorus |
| Magnesium |
| Zinc |
| Selenium |
| Serum Metabolic parameters |
| Blood glucose |
| Triacylglycerols ** |
| Total cholesterol ** |
| Low-density lipoprotein cholesterol ** |
| High-density lipoprotein cholesterol ** |
| Total lipids ** |
| Uric acid *** |
| Serum parameters of nutritional status |
| Total protein concentration |
| Prealbumin |
| Albumin |
| Serum Liver and Kidney profile |
| AST |
| ALT |
| Blood urea nitrogen |
| Creatinine |
| Total bilirubin |
| Gamma-GT |
| Pseudocholinesterase |
| Serum Vitamins |
| Folic Acid |
| Vitamin B12 |
| 25-hydroxyvitamin D |
| Serum hormonal profile |
| Insulin **** |
| IGF1 *** |
| Growth hormone **** |
| Serum Iron profile |
| Iron |
| Ferritin |
| Transferrin |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varesio, C.; Grumi, S.; Zanaboni, M.P.; Mensi, M.M.; Chiappedi, M.; Pasca, L.; Ferraris, C.; Tagliabue, A.; Borgatti, R.; De Giorgis, V. Ketogenic Dietary Therapies in Patients with Autism Spectrum Disorder: Facts or Fads? A Scoping Review and a Proposal for a Shared Protocol. Nutrients 2021, 13, 2057. https://doi.org/10.3390/nu13062057
Varesio C, Grumi S, Zanaboni MP, Mensi MM, Chiappedi M, Pasca L, Ferraris C, Tagliabue A, Borgatti R, De Giorgis V. Ketogenic Dietary Therapies in Patients with Autism Spectrum Disorder: Facts or Fads? A Scoping Review and a Proposal for a Shared Protocol. Nutrients. 2021; 13(6):2057. https://doi.org/10.3390/nu13062057
Chicago/Turabian StyleVaresio, Costanza, Serena Grumi, Martina Paola Zanaboni, Martina Maria Mensi, Matteo Chiappedi, Ludovica Pasca, Cinzia Ferraris, Anna Tagliabue, Renato Borgatti, and Valentina De Giorgis. 2021. "Ketogenic Dietary Therapies in Patients with Autism Spectrum Disorder: Facts or Fads? A Scoping Review and a Proposal for a Shared Protocol" Nutrients 13, no. 6: 2057. https://doi.org/10.3390/nu13062057
APA StyleVaresio, C., Grumi, S., Zanaboni, M. P., Mensi, M. M., Chiappedi, M., Pasca, L., Ferraris, C., Tagliabue, A., Borgatti, R., & De Giorgis, V. (2021). Ketogenic Dietary Therapies in Patients with Autism Spectrum Disorder: Facts or Fads? A Scoping Review and a Proposal for a Shared Protocol. Nutrients, 13(6), 2057. https://doi.org/10.3390/nu13062057











