Broussonetia papyrifera Polysaccharide Alleviated Acetaminophen-Induced Liver Injury by Regulating the Intestinal Flora
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Extraction, Separation and Purification of BPP
2.3. Structural Characterization of BPP
2.3.1. Polysaccharide Content and Purity of BPP
2.3.2. FT-IR Analysis
2.3.3. Monosaccharide Composition
2.4. Gut Microbiota Depletion and Fecal Microbiota Transplantation (FMT)
2.4.1. Gut Microbiota Depletion
2.4.2. Fecal Microbiota Transplantation (FMT)
2.5. Animals and Experimental Design
2.6. Serum Biochemical Detection
2.7. Histological Analysis
2.8. Determination of Liver Oxidative Stress Indicators
2.9. Determination of Cytokines, Metabolic Enzymes and Apoptosis-Related Proteins in Liver Tissue
2.10. Hoechst 33258 Staining
2.11. RNA Extraction and qRT-PCR Analysis
2.12. 16S-rDNA Sequencing of Cecal Content
2.13. Data Analysis
3. Results
3.1. Structural Characterization of BPP
3.2. BPP Improved APAP Induced Liver Dysfunction
3.3. BPP Inhibited APAP-Induced Liver Oxidative Stress
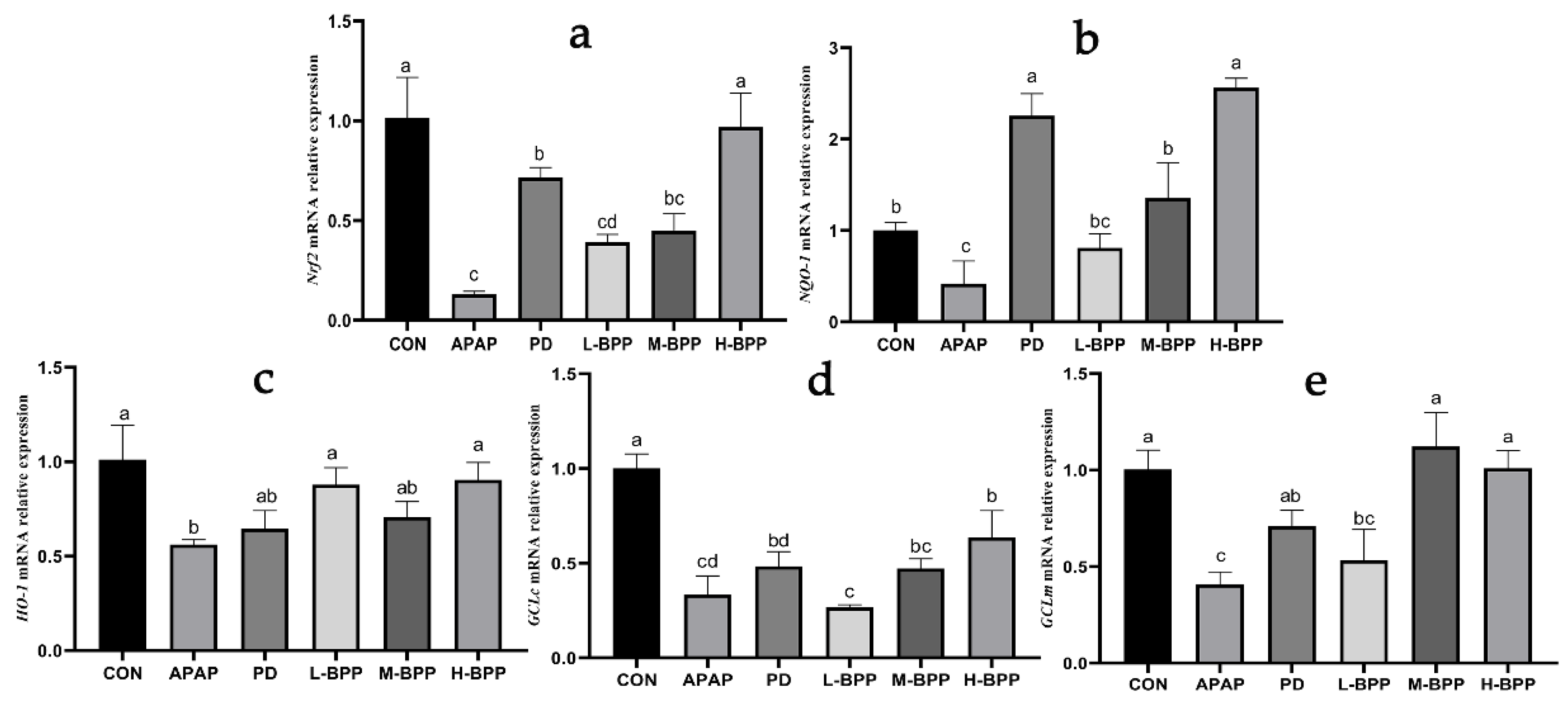
3.4. BPP Enhanced Expression of Nrf2 and Its Target Genes
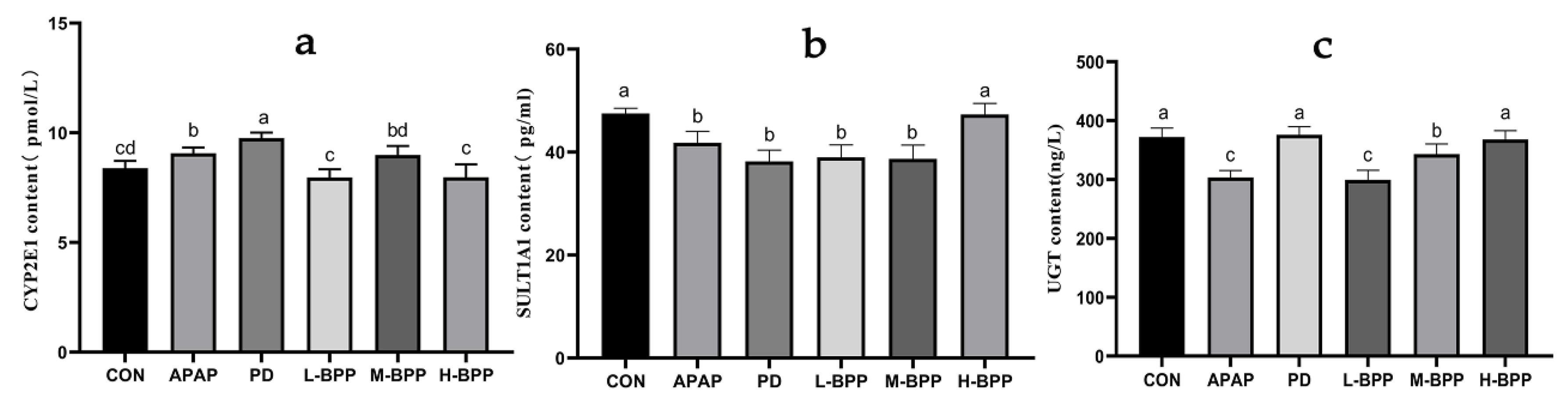
3.5. Effects of BPP on Major Metabolic Enzymes in Liver of Mice with Liver Injury
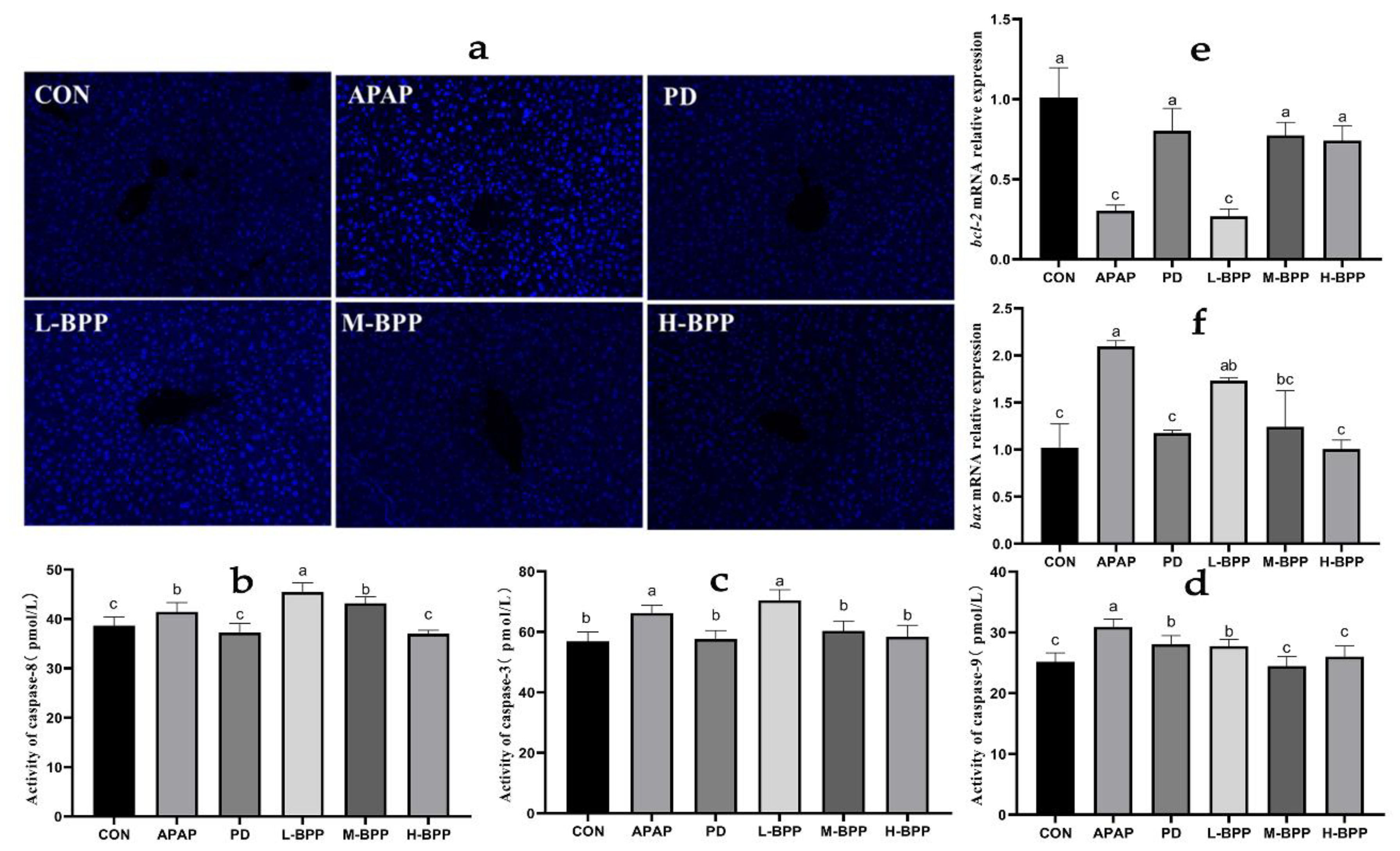
3.6. BPP Alleviated Liver Cell Apoptosis Induced by APAP
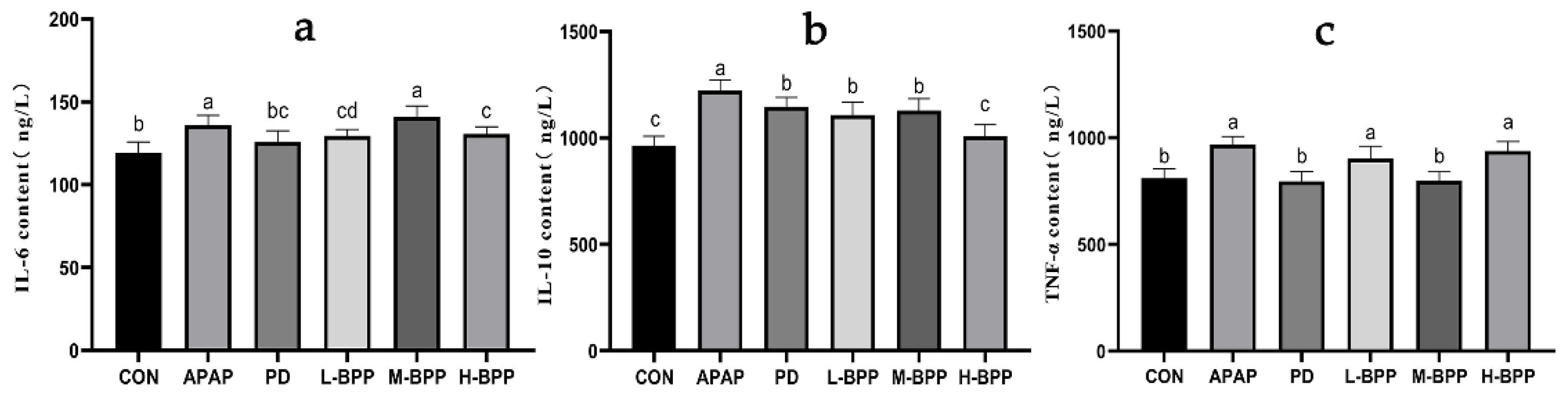
3.7. BPP Reduced Inflammatory Factors in the Liver
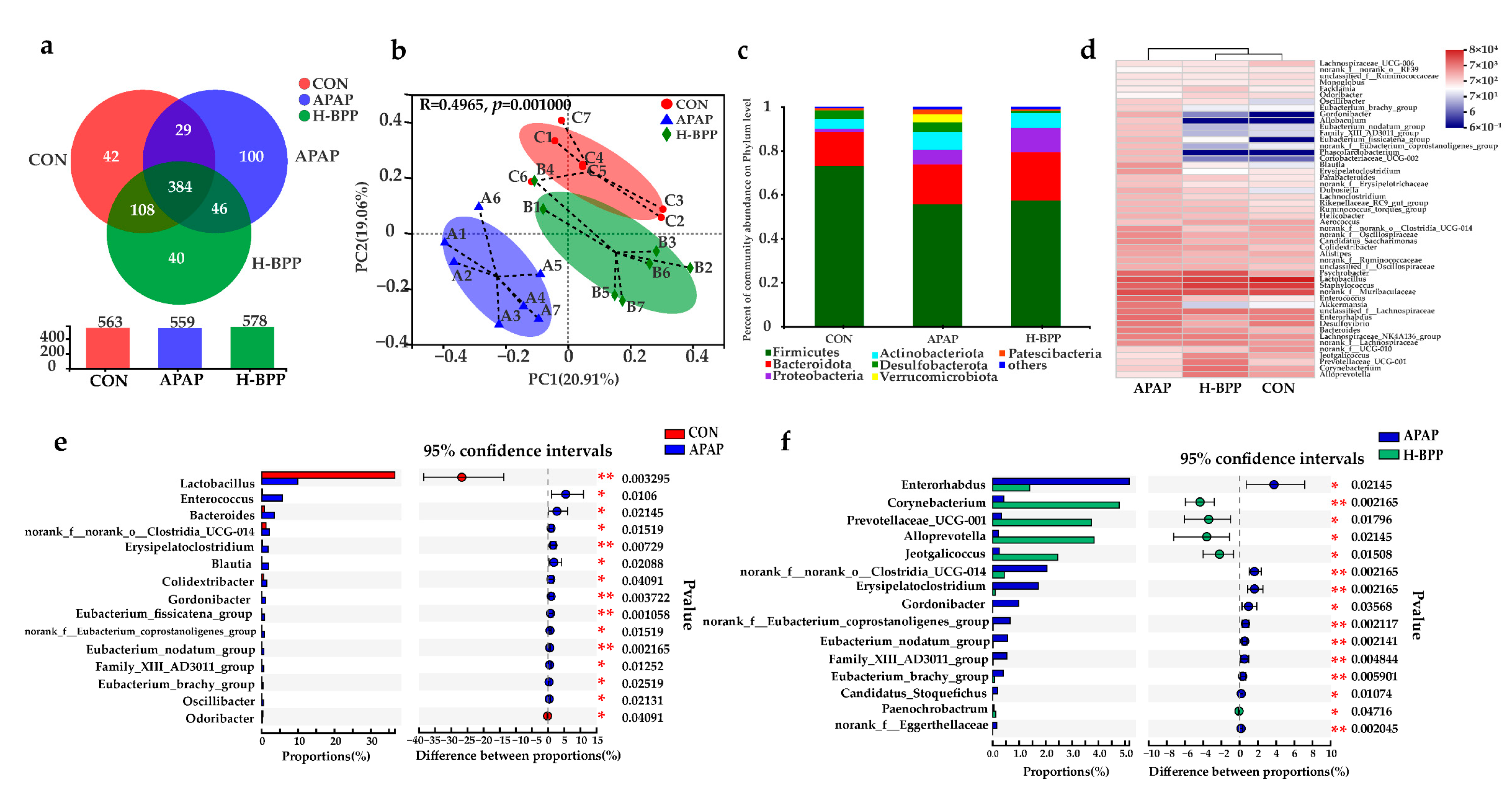
3.8. BPP Improved the Structure of Intestinal Microflora in Injured Mice
3.9. FMT Alleviated the Symptoms of Liver Injury in APAP-Induced Mice
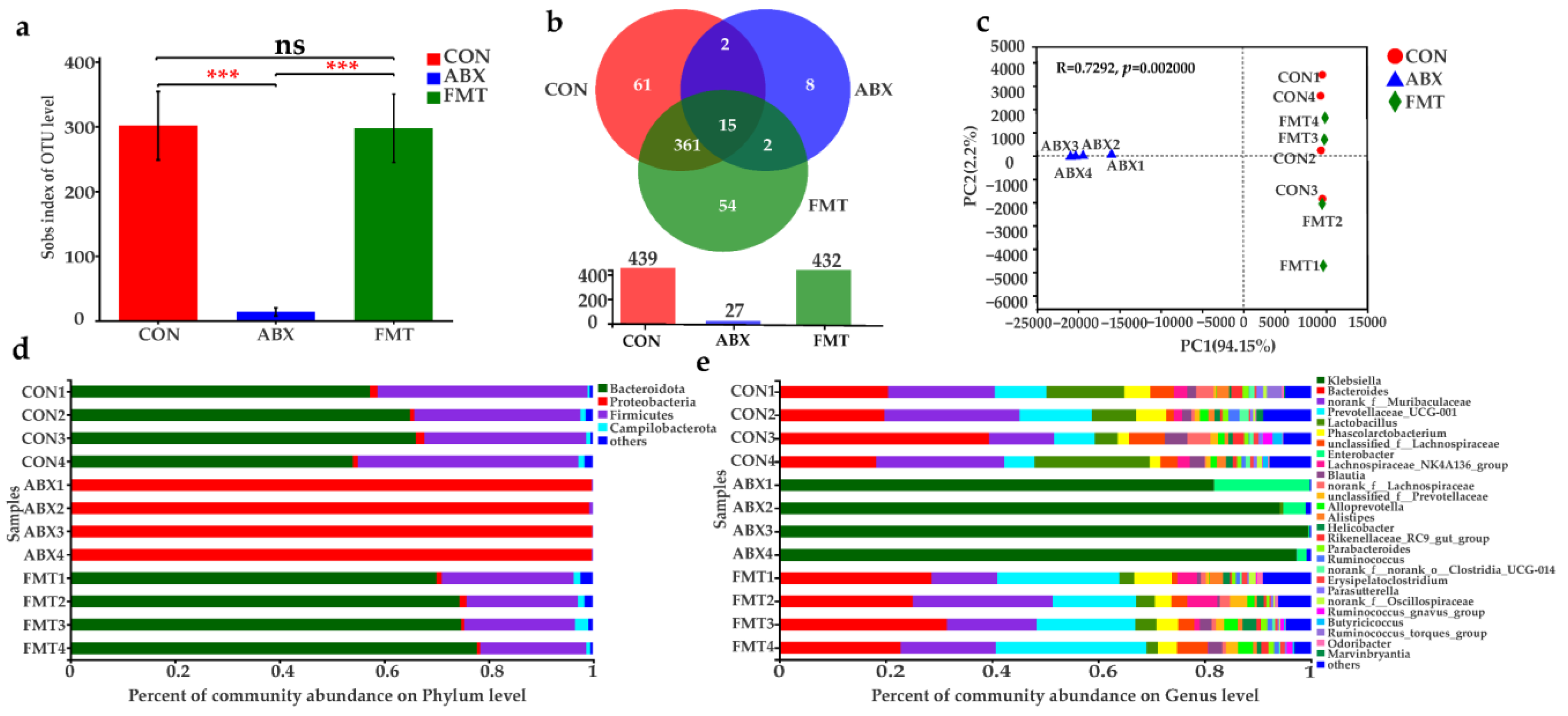
3.10. Changes of Intestinal Microbiota in Mice after ABX and FMT
3.11. Evaluation of the Liver Protective Effect of BPP on Pseudo Sterile Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, W.M. Acetaminophen (APAP) hepatotoxicity-isn’t it time for APAP to go away? J. Hepatol. 2017, 67, 1324–1331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myers, R.P.; Shaheen, A.A.M.; Li, B.; Dean, S.; Quan, H. Impact of liver disease, alcohol abuse, and unintentional ingestions on the outcomes of acetaminophen overdose. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2008, 6, 918–925, 837. [Google Scholar] [CrossRef] [PubMed]
- Ntamo, Y.; Ziqubu, K.; Chellan, N.; Nkambule, B.B.; Nyambuya, T.M.; Mazibuko-Mbeje, S.E.; Gabuza, K.B.; Marcheggiani, F.; Tiano, L.; Dludla, P.V. Drug-induced liver injury: Clinical evidence of n-acetyl cysteine protective effects. Oxid. Med. Cell. Longev. 2021, 2021, 3320325. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, E.M.; Hiatt, J.R.; Zarrinpar, A. Acetaminophen hepatotoxicity: An updated review. Arch. Toxicol. 2015, 89, 193–199. [Google Scholar] [CrossRef]
- Schneider, K.M.; Elfers, C.; Ghallab, A.; Schneider, C.V.; Galvez, E.J.C.; Mohs, A.; Gui, W.; Candels, L.S.; Wirtz, T.H.; Zuehlke, S.; et al. Intestinal dysbiosis amplifies acetaminophen-induced acute liver injury. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 909–933. [Google Scholar] [CrossRef]
- Gong, S.; Lan, T.; Zeng, L.; Luo, H.; Yang, X.; Li, N.; Chen, X.; Liu, Z.; Li, R.; Win, S.; et al. Gut microbiota mediates diurnal variation of acetaminophen induced acute liver injury in mice. J. Hepatol. 2018, 69, 51–59. [Google Scholar] [CrossRef]
- Zheng, N.; Gu, Y.; Hong, Y.; Sheng, L.; Chen, L.; Zhang, F.; Hou, J.; Zhang, W.; Zhang, Z.; Jia, W.; et al. Vancomycin pretreatment attenuates acetaminophen-induced liver injury through 2-hydroxybutyric acid. J. Pharm. Anal. 2020, 10, 560–570. [Google Scholar] [CrossRef]
- Kim, J.; Choi, M.S.; Jeong, J.; Lim, S.; Kim, I.S.; Yoo, H.H.; Kim, D. Effect of probiotics on pharmacokinetics of orally administered acetaminophen in mice. Drug Metab. Dispos. Biol. Fate Chem. 2018, 46, 122–130. [Google Scholar] [CrossRef]
- Jourová, L.; Vavreckova, M.; Zemanova, N.; Anzenbacher, P.; Langova, K.; Hermanova, P.; Hudcovic, T.; Anzenbacherova, E. Gut microbiome alters the activity of liver cytochromes P450 in mice with sex-dependent differences. Front. Pharmacol. 2020, 11, 1303. [Google Scholar] [CrossRef]
- Chen, T.; Li, R.; Chen, P. Gut microbiota and chemical-induced acute liver injury. Front. Physiol. 2021, 12, 688780. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, Y.; Zhang, R.; Pan, J.; Qi, D.; Wang, J.; Yang, X. The protective effects of walnut green husk polysaccharide on liver injury, vascular endothelial dysfunction and disorder of gut microbiota in high fructose-induced mice. Int. J. Biol. Macromol. 2020, 162, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Xu, R.; Aruhan; Xiu, L.; Sheng, S.; Liang, Y.; Zhang, H.; Liu, Y.; Tong, H.; Du, R.; et al. Exopolysaccharides from Lactobacillus buchneri TCP016 attenuate LPS- and d-GalN-induced liver injury by modulating the gut microbiota. J. Agric. Food Chem. 2019, 67, 11627–11637. [Google Scholar] [CrossRef]
- Hong, Y.; Shen, M.; Huang, L.; Wu, T.; Xie, J. Mesona chinensis benth polysaccharides alleviates liver injury by beneficial regulation of gut microbiota in cyclophosphamide-induced mice. Food Sci. Hum. Wellness 2022, 11, 74–84. [Google Scholar] [CrossRef]
- Ryu, H.W.; Park, M.H.; Kwon, O.; Kim, D.; Hwang, J.; Jo, Y.H.; Ahn, K.; Hwang, B.Y.; Oh, S. Anti-inflammatory flavonoids from root bark of Broussonetia papyrifera in LPS-stimulated Raw264.7 cells. Bioorg. Chem. 2019, 92, 103233. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Kwon, J.; Shin, J.; Kim, J.; Minai-Tehrani, A.; Yu, K.; Lee, S.; Park, S.; Chang, S.; Jiang, H.; et al. Therapeutic effect of Broussonetia papyrifera and Lonicera japonica in ovalbumin-induced murine asthma model. Nat. Prod. Commun. 2013, 8, 1609–1614. [Google Scholar] [CrossRef] [Green Version]
- Dou, C.; Liu, Y.; Zhang, L.; Chen, S.; Hu, C.; Liu, Y.; Zhao, Y. Polyphenols from Broussonetia papyrifera induce apoptosis of Hepg2 cells via inactivation of ERK and AKT signaling pathways. Evid.-Based Complementary Altern. Med. Ecam. 2021, 2021, 8841706. [Google Scholar] [CrossRef]
- Mei, R.; Wang, Y.; Du, G.; Liu, G.; Zhang, L.; Cheng, Y. Antioxidant lignans from the fruits of Broussonetia papyrifera. J. Nat. Prod. 2009, 72, 621–625. [Google Scholar] [CrossRef]
- Yin, Z.; Zhang, W.; Zhang, J.; Kang, W. Isolation, purification, structural analysis and coagulatory activity of water-soluble polysaccharides from Ligustrum lucidum ait flowers. Chem. Cent. J. 2017, 11, 98. [Google Scholar] [CrossRef] [Green Version]
- Han, Q.; Wu, Z.; Huang, B.; Sun, L.; Ding, C.; Yuan, S.; Zhang, Z.; Chen, Y.; Hu, C.; Zhou, L.; et al. Extraction, antioxidant and antibacterial activities of Broussonetia papyrifera fruits polysaccharides. Int. J. Biol. Macromol. 2016, 92, 116–124. [Google Scholar] [CrossRef]
- Fan, H.; Meng, Q.; Xiao, T.; Zhang, L. Partial characterization and antioxidant activities of polysaccharides sequentially extracted from Dendrobium officinale. J. Food Meas. Charact. 2018, 12, 1054–1064. [Google Scholar] [CrossRef]
- Yang, X.; Chen, F.; Huang, G. Extraction and analysis of polysaccharide from Momordica charantia. Ind. Crops Prod. 2020, 153, 112588. [Google Scholar] [CrossRef]
- Yang, X.; Nisar, T.; Hou, Y.; Gou, X.; Sun, L.; Guo, Y. Pomegranate peel pectin can be used as an effective emulsifier. Food Hydrocoll. 2018, 85, 30–38. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, X.; Wang, K.; Cao, X. Structural characterisation and bioactivity of polysaccharides isolated from fermented Dendrobium officinale. J. Sci. Food Agric. 2022, 102, 280–290. [Google Scholar] [CrossRef]
- Li, Q.; Cui, Y.; Xu, B.; Wang, Y.; Lv, F.; Li, Z.; Li, H.; Chen, X.; Peng, X.; Chen, Y.; et al. Main active components of jiawei gegen qinlian decoction protects against ulcerative colitis under different dietary environments in a gut microbiota-dependent manner. Pharmacol. Res. 2021, 170, 105694. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, B.; Wang, F.; Tang, L.; Lei, Y.; Luo, Y.; Huang, S.; Yang, M.; Wu, L.; Wang, W.; et al. Parthenolide ameliorates colon inflammation through regulating Treg/Th17 balance in a gut microbiota-dependent manner. Theranostics 2020, 10, 5225–5241. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Lin, Y.; Li, Z.; Ma, X.; Han, Q.; Liu, Y.; Zhou, Q.; Liu, J.; Li, R.; Li, J.; et al. Glutathione s-transferase a1 (Gsta1) release, an early indicator of acute hepatic injury in mice. Food Chem. Toxicol. 2014, 71, 225–230. [Google Scholar] [CrossRef]
- Shi, C.; Lin, Y.; Liu, F.; Chang, Y.; Li, R.; Li, C.; Li, Y.; He, J.; Ma, X.; Li, Z. Hepatoprotective effects of ethanol extracts from Folium syringae against acetaminophen-induced hepatotoxicity in vitro and in vivo. J. Chin. Med. Assoc. JCMA 2017, 80, 623–629. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Z.; Hou, J.; Zhou, Y.; He, Y.; Jiang, S.; Wang, Y.; Ren, S.; Li, W. The liver protection effects of maltol, a flavoring agent, on carbon tetrachloride-induced acute liver injury in mice via inhibiting apoptosis and inflammatory response. Molecules 2018, 23, 2120. [Google Scholar] [CrossRef] [Green Version]
- Du, K.; Ramachandran, A.; Jaeschke, H. Oxidative stress during acetaminophen hepatotoxicity: Sources, pathophysiological role and therapeutic potential. Redox Biol. 2016, 10, 148–156. [Google Scholar] [CrossRef] [Green Version]
- Fu, S.; Wu, D.; Jiang, W.; Li, J.; Long, J.; Jia, C.; Zhou, T. Molecular biomarkers in drug-induced liver injury: Challenges and future perspectives. Front. Pharmacol. 2019, 10, 1667. [Google Scholar] [CrossRef] [Green Version]
- Vermeulen, N.P.; Bessems, J.G.; Van de Straat, R. Molecular aspects of paracetamol-induced hepatotoxicity and its mechanism-based prevention. Drug Metab. Rev. 1992, 24, 367–407. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Jia, F.; He, Y.; Zhang, B. Protective effects of 5-methoxypsoralen against acetaminophen-induced hepatotoxicity in mice. World J. Gastroentero. 2012, 18, 2197–2202. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.; Han, X.D.; Kan, Y.W. An important function of Nrf2 in combating oxidative stress: Detoxification of acetaminophen. Proc. Natl. Acad. Sci. USA 2001, 98, 4611–4616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, S.R.M.; El-Agamy, D.S.; Abdallah, H.M.; Ahmed, N.; Elkablawy, M.A.; Mohamed, G.A. Protective activity of tovophyllin a, a xanthone isolated from Garcinia mangostana pericarps, against acetaminophen-induced liver damage: Role of Nrf2 activation. Food Funct. 2018, 9, 3291–3300. [Google Scholar] [CrossRef]
- Li, Y.; Xu, J.; Li, D.; Ma, H.; Mu, Y.; Huang, X.; Li, L. Guavinoside b from Psidium guajava alleviates acetaminophen-induced liver injury via regulating the Nrf2 and JNK signaling pathways. Food Funct. 2020, 11, 8297–8308. [Google Scholar] [CrossRef]
- Chen, C.; Liu, X.; Qi, S.C.P.; Dias, A.; Yan, J.; Zhang, X. Hepatoprotective effect of Phellinus linteus mycelia polysaccharide (Pl-n1) against acetaminophen-induced liver injury in mouse. Int. J. Biol. Macromol. 2020, 154, 1276–1284. [Google Scholar] [CrossRef]
- Hu, J.; Yan, D.; Gao, J.; Xu, C.; Yuan, Y.; Zhu, R.; Xiang, D.; Weng, S.; Han, W.; Zang, G.; et al. Rhil-1ra reduces hepatocellular apoptosis in mice with acetaminophen-induced acute liver failure. Lab. Investig. 2010, 90, 1737–1746. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Hu, J.; Yan, M.; Xing, J.; Liu, W.; Li, W. Caspase-mediated anti-apoptotic effect of ginsenoside rg5, a main rare ginsenoside, on acetaminophen-induced hepatotoxicity in mice. J. Agric. Food Chem. 2017, 65, 9226–9236. [Google Scholar] [CrossRef]
- Cao, P.; Sun, J.; Sullivan, M.A.; Huang, X.; Wang, H.; Zhang, Y.; Wang, N.; Wang, K. Angelica sinensis polysaccharide protects against acetaminophen-induced acute liver injury and cell death by suppressing oxidative stress and hepatic apoptosis in vivo and in vitro. Int. J. Biol. Macromol. 2018, 111, 1133–1139. [Google Scholar] [CrossRef]
- Wu, K.; Fan, J.; Huang, X.; Wu, X.; Guo, C. Hepatoprotective effects exerted by Poria cocos polysaccharides against acetaminophen-induced liver injury in mice. Int. J. Biol. Macromol. 2018, 114, 137–142. [Google Scholar] [CrossRef]
- Saeedi, B.J.; Liu, K.H.; Owens, J.A.; Hunter-Chang, S.; Camacho, M.C.; Eboka, R.U.; Chandrasekharan, B.; Baker, N.F.; Darby, T.M.; Robinson, B.S.; et al. Gut-resident Lactobacilli activate hepatic Nrf2 and protect against oxidative liver injury. Cell Metab. 2020, 31, 956–968. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Lv, L.; Liu, B.; Wang, S.; Zhang, S.; Wu, Z.; Yang, L.; Bian, X.; Wang, Q.; Wang, K.; et al. Akkermansia muciniphila ameliorates acetaminophen-induced liver injury by regulating gut microbial composition and metabolism. Microbiol. Spectr. 2022, 10, e159621. [Google Scholar] [CrossRef] [PubMed]
- Jian, H.; Miao, S.; Liu, Y.; Wang, X.; Xu, Q.; Zhou, W.; Li, H.; Dong, X.; Zou, X. Dietary valine ameliorated gut health and accelerated the development of nonalcoholic fatty liver disease of laying hens. Oxid. Med. Cell. Longev. 2021, 2021, 4704771. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Ryu, S.H.; Kim, S.; Lee, H.W.; Lim, M.; Seong, S.J.; Kim, S.; Yoon, Y.; Kim, K. Pattern recognition analysis for hepatotoxicity induced by acetaminophen using plasma and urinary 1 h Nmr-based metabolomics in humans. Anal. Chem. 2013, 85, 11326–11334. [Google Scholar] [CrossRef]
- Wu, F.; Lei, H.; Chen, G.; Chen, C.; Song, Y.; Cao, Z.; Zhang, C.; Zhang, C.; Zhou, J.; Lu, Y.; et al. In vitro and in vivo studies reveal that hesperetin-7-o-glucoside, a naturally occurring monoglucoside, exhibits strong anti-inflammatory capacity. J. Agric. Food Chem. 2021, 69, 12753–12762. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, D.; Fan, Z.; Li, J.; Gao, L.; Wang, Y.; Wang, L. Microcystin-Lr induces ferroptosis in intestine of common carp (Cyprinus carpio). Ecotox. Environ. Safe. 2021, 223, 112610. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Q.; Zha, X.; Luo, J. Dendrobium fimbriatum hook polysaccharide ameliorates dextran-sodium-sulfate-induced colitis in mice via improving intestinal barrier function, modulating intestinal microbiota, and reducing oxidative stress and inflammatory responses. Food Funct. 2022, 13, 143–160. [Google Scholar] [CrossRef]
- Zou, J.; Li, W.; Wang, G.; Fang, S.; Cai, J.; Wang, T.; Zhang, H.; Liu, P.; Wu, J.; Ma, Y. Hepatoprotective effects of huangqi decoction (Astragali radix and Glycyrrhizae radix et rhizoma) on cholestatic liver injury in mice: Involvement of alleviating intestinal microbiota dysbiosis. J. Ethnopharmacol. 2021, 267, 113544. [Google Scholar] [CrossRef]
- Lee, Y.; Chiu, C.; Chen, Y.; Huang, W.; Wang, Y.; Chiu, C.; Lin, T.; Hung, S.; Liu, J.; Chuang, H. Co-treatment with cefotaxime and high-fructose diet inducing nonalcoholic fatty liver disease and gut microbial dysbiosis in mice. Processes 2021, 9, 434. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, D.; Song, S.; Zhang, M.; Zamaratskaia, G.; Xu, X.; Zhou, G.; Li, C. High-meat-protein high-fat diet induced dysbiosis of gut microbiota and tryptophan metabolism in wistar rats. J. Agric. Food Chem. 2020, 68, 6333–6346. [Google Scholar] [CrossRef]
- Shao, Y.; Jiang, Y.; Li, H.; Zhang, F.; Hu, Z.; Zheng, S. Characteristics of mouse intestinal microbiota during acute liver injury and repair following 50% partial hepatectomy. Exp. Ther. Med. 2021, 22, 953. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Huang, Z.; Jia, R.; Lv, X.; Zhao, C.; Liu, B. Spirulina platensis polysaccharides attenuate lipid and carbohydrate metabolism disorder in high-sucrose and high-fat diet-fed rats in association with intestinal microbiota. Food Res. Int. 2021, 147, 110530. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhong, L.; Lyu, N.; Liu, F.; Li, B.; Hao, Y.; Xue, Y.; Li, J.; Feng, Y.; Ma, Y.; et al. Inulin can alleviate metabolism disorders in ob/ob mice by partially restoring leptin-related pathways mediated by gut microbiota. Genom. Proteom. Bioinform. 2019, 17, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Shen, Y.; Chen, M.; Zhang, Z.; Xiao, S.; Liu, C.; Wan, Y.; Yang, L.; Jiang, S.; Shang, E.; et al. Lizhong decoction ameliorates ulcerative colitis in mice via modulating gut microbiota and its metabolites. Appl. Microbiol. Biot. 2020, 104, 5999–6012. [Google Scholar] [CrossRef]
- Xie, Z.; Bai, Y.; Chen, G.; Rui, Y.; Chen, D.; Sun, Y.; Zeng, X.; Liu, Z. Modulation of gut homeostasis by exopolysaccharides from Aspergillus cristatus (Mk346334), a strain of fungus isolated from fuzhuan brick tea, contributes to immunomodulatory activity in cyclophosphamide-treated mice. Food Funct. 2020, 11, 10397–10412. [Google Scholar] [CrossRef]





| Gene Name | Primer Sequence (5′-3′) | Accession Number |
|---|---|---|
| β-Actin | F: GTGCTATGTTGCTCTAGACTTCG R: ATGCCACAGGATTCCATACC | NM_007393.5 |
| Nrf2 | F: TAGATGACCATGAGTCGCTTGC R: GCCAAACTTGCTCCATGTCC | NM_010902.4 |
| HO-1 | F: AAGCCGAGAATGCTGAGTTCA R: GCCGTGTAGATATGGTACAAGGA | NM_010442.2 |
| NQO-1 | F: AGGATGGGAGGTACTCGAATC R: AGGCGTCCTTCCTTATATGCTA | NM_008706.5 |
| GCLc | F: GGGGTGACGAGGTGGAGTA R: GTTGGGGTTTGTCCTCTCCC | NM_010295.2 |
| GCLm | F: CGGGAACCTGCTCAACTG R: CCAAAACATCTGGAAACTCCC | NM_008129.4 |
| bcl-2 | F: GCTACCGTCGTGACTTCGC R: CCCCACCGAACTCAAAGAAGG | NM_009741.5 |
| bax | F: AGACAGGGGCCTTTTTGCTAC R: AATTCGCCGGAGACACTCG | XM_011250780.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, B.; Hao, K.; Chen, X.; Wu, E.; Nie, D.; Zhang, G.; Si, H. Broussonetia papyrifera Polysaccharide Alleviated Acetaminophen-Induced Liver Injury by Regulating the Intestinal Flora. Nutrients 2022, 14, 2636. https://doi.org/10.3390/nu14132636
Xu B, Hao K, Chen X, Wu E, Nie D, Zhang G, Si H. Broussonetia papyrifera Polysaccharide Alleviated Acetaminophen-Induced Liver Injury by Regulating the Intestinal Flora. Nutrients. 2022; 14(13):2636. https://doi.org/10.3390/nu14132636
Chicago/Turabian StyleXu, Baichang, Kaiyuan Hao, Xiaogang Chen, Enyun Wu, Dongyang Nie, Geyin Zhang, and Hongbin Si. 2022. "Broussonetia papyrifera Polysaccharide Alleviated Acetaminophen-Induced Liver Injury by Regulating the Intestinal Flora" Nutrients 14, no. 13: 2636. https://doi.org/10.3390/nu14132636