Punicalagin Protects against Diabetic Liver Injury by Upregulating Mitophagy and Antioxidant Enzyme Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies
2.2. Animals and Treatment
2.3. HOMA-IR
2.4. Detection of Serum and Liver Biochemical Indicators
2.5. H&E Staining
2.6. Detection of Mitochondrial Membrane Potential
2.7. Cell Culture and Grouping
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
3.1. Effects of Punicalagin on T2DM
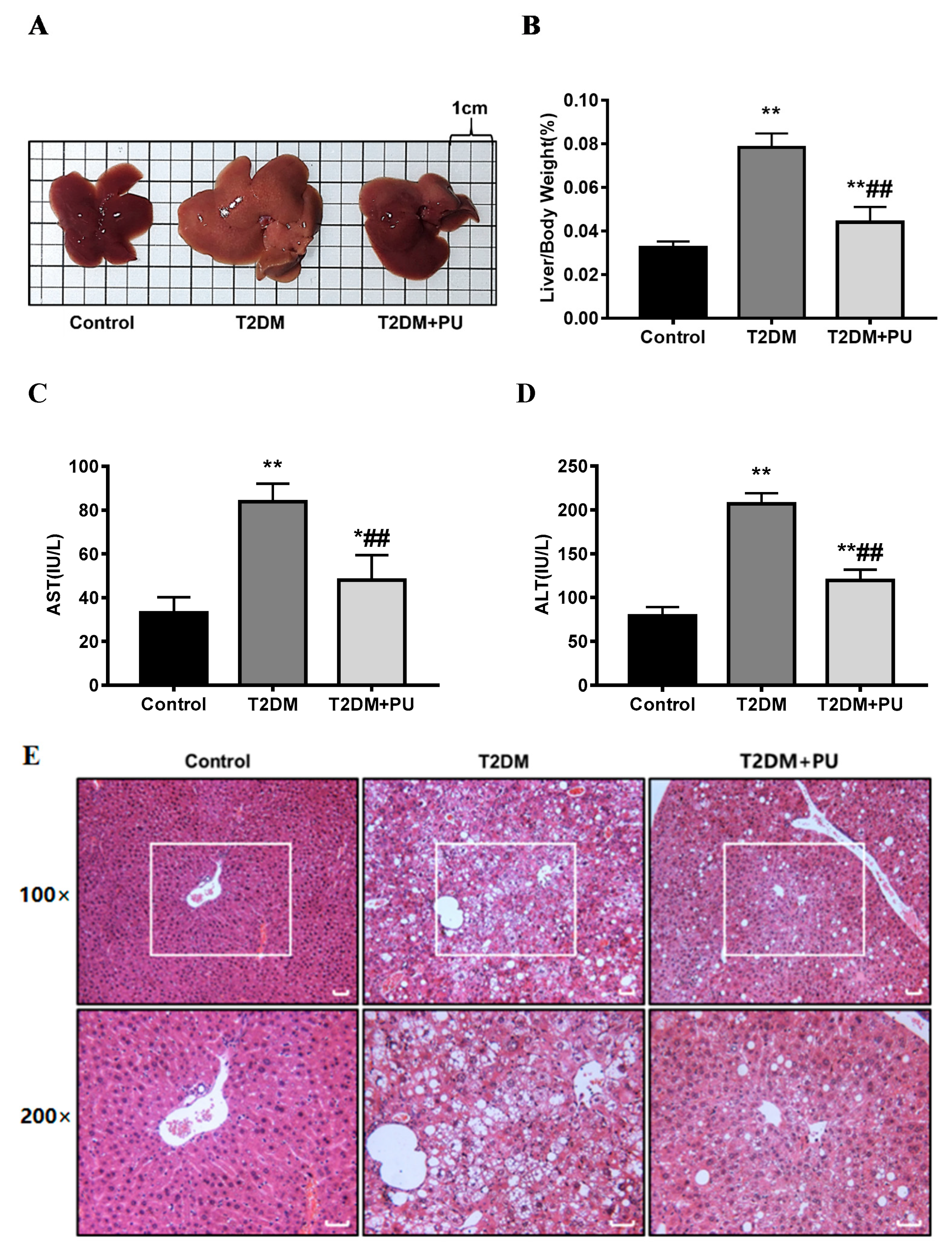
3.2. Punicalagin Protects Diabetic Liver Injury
3.3. Punicalagin Protects against Diabetic Liver Injury by Attenuating Oxidative Stress
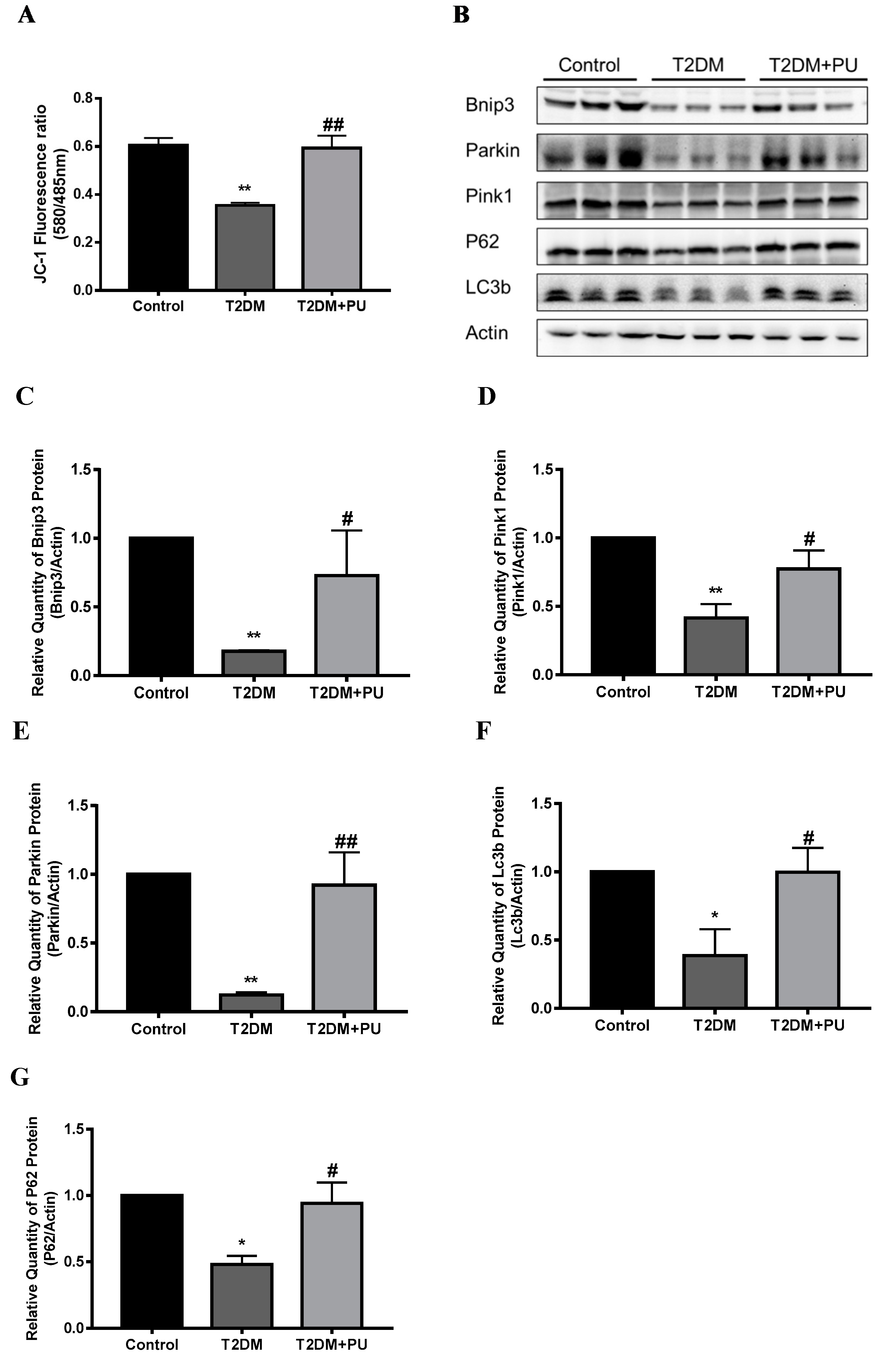
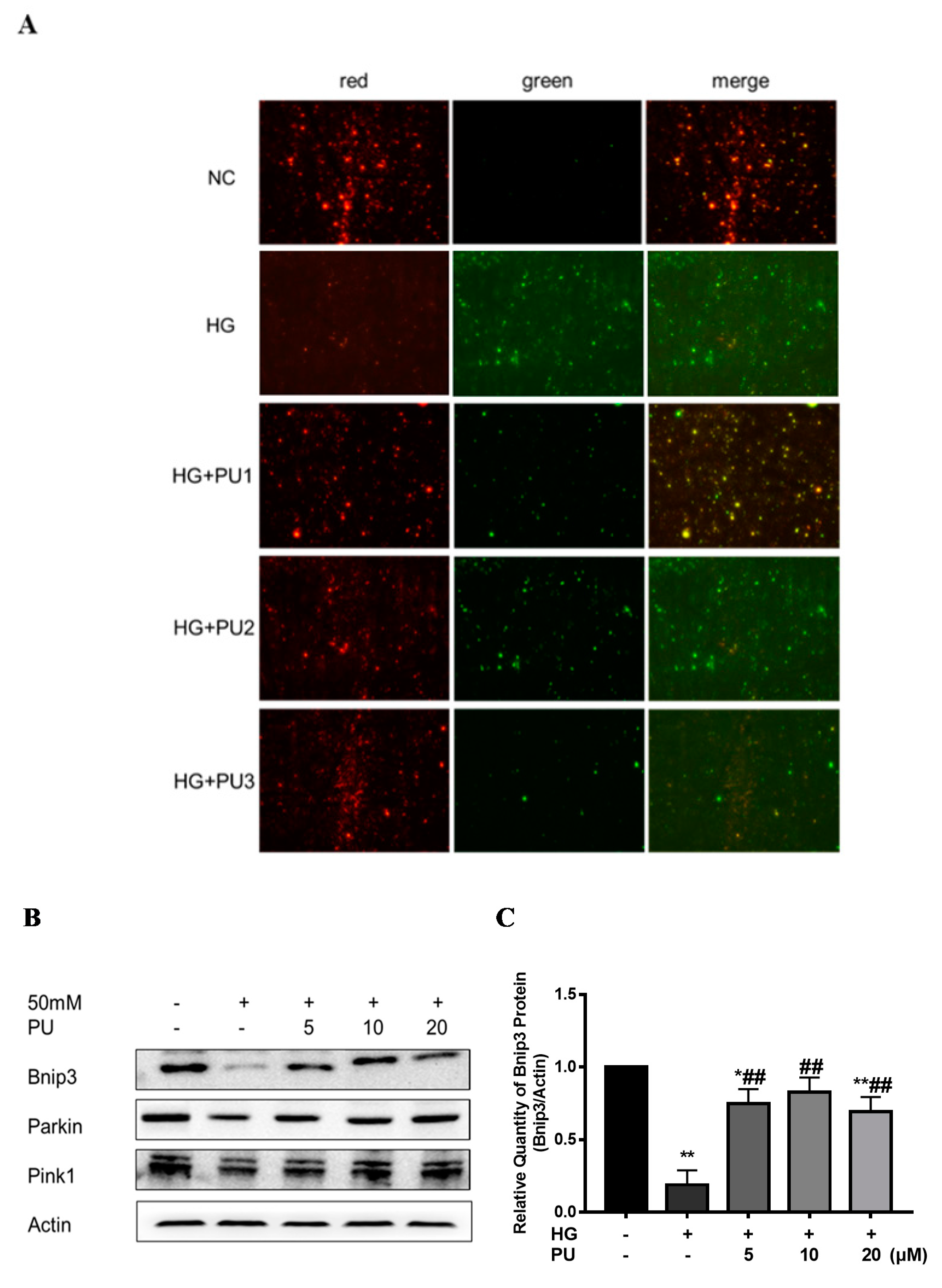
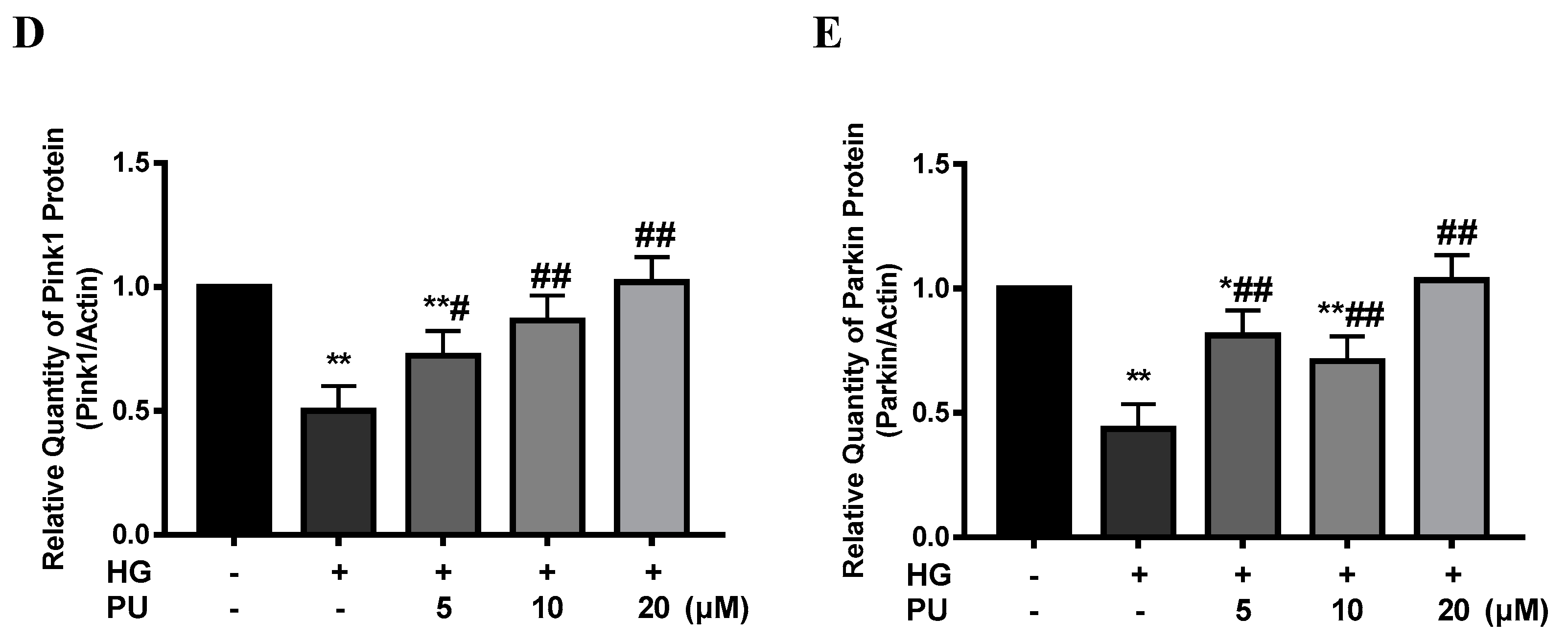
3.4. Punicalagin Regulates Mitophagy to Attenuate Oxidative Stress
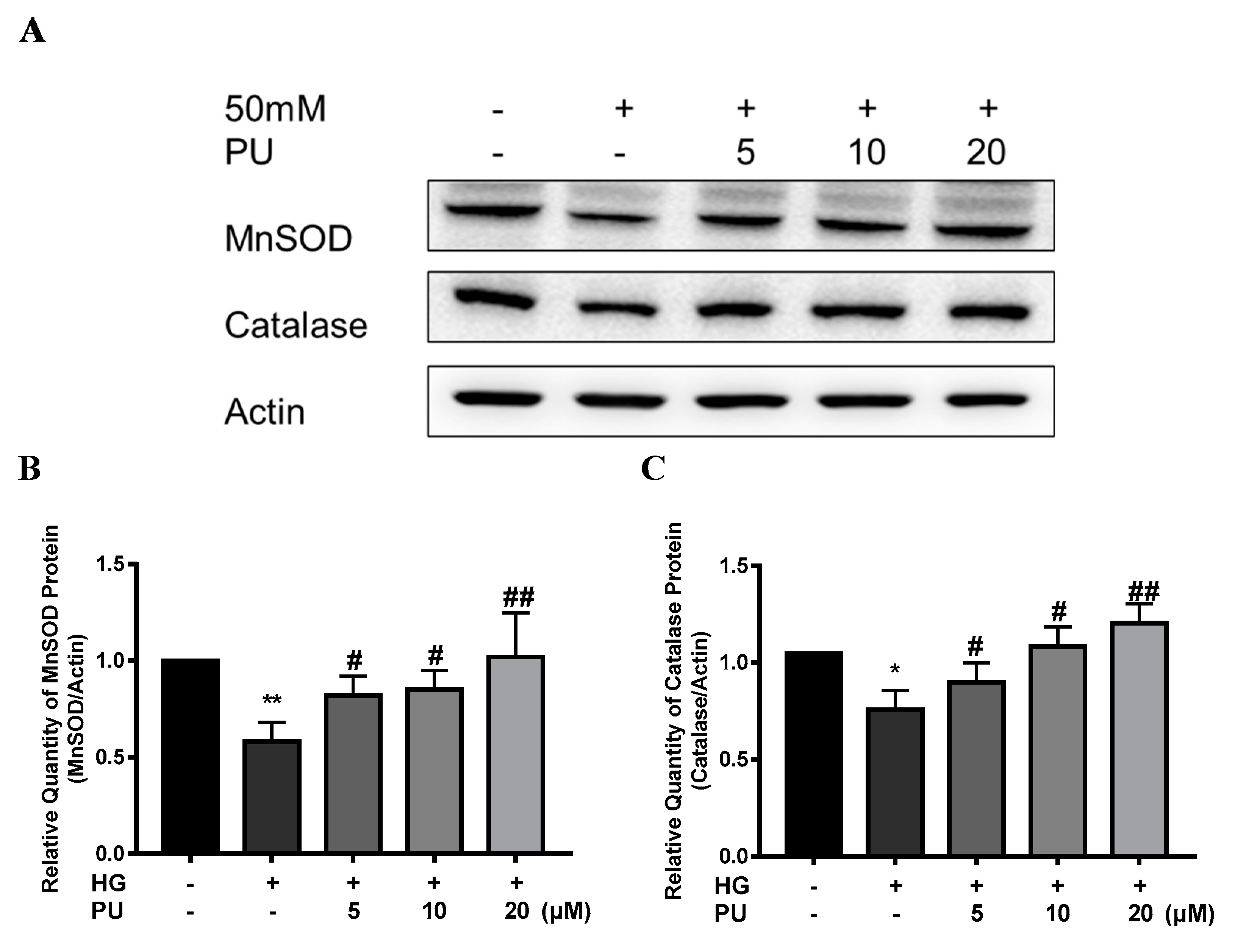
3.5. Punicalagin Regulates Antioxidant Enzyme System to Attenuate Oxidative Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; Available online: https://www.idf.org/aboutdiabetes/type-2-diabetes.html (accessed on 29 May 2022).
- Hazlehurst, J.M.; Woods, C.; Marjot, T.; Cobbold, J.F.; Tomlinson, J.W. Non-alcoholic fatty liver disease and diabetes. Metabolism 2016, 65, 1096–1108. [Google Scholar] [CrossRef] [Green Version]
- Ahmadieh, H.; Azar, S.T. Liver disease and diabetes: Association, pathophysiology, and management. Diabetes Res. Clin. Pract. 2014, 104, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.; Yan, L.; Zheng, Y. Effect of Tanggan decoction on glucolipid metabolism of diabetic and fatty liver rats. Chin. J. Integr. Trad. West. Med. Dig. 2010, 18, 316–318. [Google Scholar]
- Scherz-Shouval, R.; Elazar, Z. Regulation of autophagy by ROS: Physiology and pathology. Trends Biochem. Sci. 2011, 36, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strappazzon, F.; Nazio, F.; Corrado, M.; Cianfanelli, V.; Romagnoli, A.; Fimia, G.M.; Campello, S.; Nardacci, R.; Piacentini, M.; Campanella, M.; et al. AMBRA1 is able to induce mitophagy via LC3 binding, regardless of PARKIN and p62/SQSTM1. Cell Death Differ. 2014, 22, 419–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Chen, Z.; Wang, Y.; Tan, Z.; Zhu, C.; Li, Y.; Han, Z.; Chen, L.; Gao, R.; Liu, L.; et al. Mitophagy receptor FUNDC1 regulates mitochondrial dynamics and mitophagy. Autophagy 2016, 12, 689–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandoval, H.; Thiagarajan, P.; Dasgupta, S.K.; Schumacher, A.; Prchal, J.T.; Chen, M.; Wang, J. Essential role for Nix in autophagic maturation of erythroid cells. Nature 2008, 454, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Shao, N.; Yu, X.-Y.; Ma, X.-F.; Lin, W.-J.; Hao, M.; Kuang, H.-Y. Exenatide Delays the Progression of Nonalcoholic Fatty Liver Disease in C57BL/6 Mice, Which May Involve Inhibition of the NLRP3 Inflammasome through the Mitophagy Pathway. Gastroenterol. Res. Pract. 2018, 2018, 1864307. [Google Scholar] [CrossRef]
- Zhou, T.; Chang, L.; Luo, Y.; Zhou, Y.; Zhang, J. Mst1 inhibition attenuates non-alcoholic fatty liver disease via reversing Parkin-related mitophagy. Redox Biol. 2019, 21, 101120. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, B.; Longtine, M.; Nelson, D.M. Punicalagin promotes autophagy to protect primary human syncytiotrophoblasts from apoptosis. Reproduction 2016, 151, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Johanningsmeier, S.D.; Harris, G.K. Pomegranate as a Functional Food and Nutraceutical Source. Annu. Rev. Food Sci. Technol. 2011, 2, 181–201. [Google Scholar] [CrossRef] [PubMed]
- Cerdá, B.; Cerón, J.J.; Tomás-Barberán, A.F.A.; Espín, J.C. Repeated Oral Administration of High Doses of the Pomegranate Ellagitannin Punicalagin to Rats for 37 Days Is Not Toxic. J. Agric. Food Chem. 2003, 51, 3493–3501. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Li, J.; Zhao, W.; Liu, Y. Effect of pomegranate peel polyphenols on lipid peroxidation in vitro. J. Food Sci. Biotechnol. 2012, 31, 159–165. [Google Scholar]
- Mestry, S.N.; Dhodi, J.B.; Kumbhar, S.B.; Juvekar, A. Attenuation of diabetic nephropathy in streptozotocin-induced diabetic rats by Punica granatum Linn. leaves extract. J. Tradit. Complement. Med. 2017, 7, 273–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mu, J. Effects of Punicalagin on Glucolipid Metabolism and Hepatic Oxidative Stress in T2DM Mice; Beijing University of Chinese Medicine: Beijing, China, 2018. [Google Scholar]
- Cao, Y.; Chen, J.; Ren, G.; Zhang, Y.; Tan, X.; Yang, L. Punicalagin Prevents Inflammation in LPS- Induced RAW264.7 Macrophages by Inhibiting FoxO3a/Autophagy Signaling Pathway. Nutrients 2019, 11, 2794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.-G.; Huang, M.-H.; Li, J.-H.; Lai, F.-I.; Lee, H.-M.; Hsu, Y.-N. Punicalagin induces apoptotic and autophagic cell death in human U87MG glioma cells. Acta Pharmacol. Sin. 2013, 34, 1411–1419. [Google Scholar] [CrossRef]
- Cheng, A.Y.; Fantus, I.G. Oral antihyperglycemic therapy for type 2 diabetes mellitus. CMAJ Can. Med. Assoc. J. 2005, 172, 213–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elasy, T.A.; Griffin, M. Thiazolidinedione use, fluid retention, and congestive heart failure: A consensus statement from the American Heart Association and American Diabetes Association: Response to Nesto. Diabetes Care 2004, 27, 2096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmet, P.; Alberti, K.G.M.M.; Shaw, J. Global and societal implications of the diabetes epidemic. Nature 2001, 414, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, W.; Kishi, H.; Yagasaki, K.; Ohhira, S. Urolithin A attenuates pro-inflammatory mediator production by suppressing PI3-K/Akt/NF-kappaB and JNK/AP-1 signaling pathways in lipopolysaccharide-stimulated RAW264 macrophages: Possible involvement of NADPH oxidase-derived reactive oxygen species. Eur. J. Pharmacol. 2018, 833, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yuan, C.; Wang, G.; Luo, J.; Ma, H.; Xu, L.; Mu, Y.; Li, Y.; Seeram, N.P.; Huang, X.; et al. Urolithins Attenuate LPS-Induced Neuroinflammation in BV2Microglia via MAPK, Akt, and NF-κB Signaling Pathways. J. Agric. Food Chem. 2018, 66, 571–580. [Google Scholar] [CrossRef] [PubMed]
- BenSaad, L.A.; Kim, K.H.; Quah, C.C.; Kim, W.R.; Shahimi, M. Anti-inflammatory potential of ellagic acid, gallic acid and punicalagin A&B isolated from Punica granatum. BMC Complement. Altern. Med. 2017, 17, 47. [Google Scholar]
- Ryu, D.; Mouchiroud, L.; Andreux, P.A.; Katsyuba, E.; Moullan, N.; Nicolet-Dit-Félix, A.A.; Williams, E.G.; Jha, P.; Lo Sasso, G.; Huzard, D.; et al. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat. Med. 2016, 22, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, Y.; Chen, J.; Qin, H.; Yang, L. A New Possible Mechanism by Which Punicalagin Protects against Liver Injury Induced by Type 2 Diabetes Mellitus: Upregulation of Autophagy via the Akt/FoxO3a Signaling Pathway. J. Agric. Food Chem. 2019, 67, 13948–13959. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Sun, W.; Wang, X.; Long, J.; Liu, X.; Feng, Z.; Liu, J. Punicalagin attenuates palmitate-induced lipotoxicity in HepG2 cells by activating the Keap1-Nrf2 antioxidant defense system. Mol. Nutr. Food Res. 2016, 60, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Wang, L. Ameliorative Effect and Mechanisms of Punicalagin on Non-Alcoholic Fatty Liver Disease; Northwest A&F University: Xianyang, China, 2016. [Google Scholar]
- Li, L.; Yang, X. The Essential Element Manganese, Oxidative Stress, and Metabolic Diseases: Links and Interactions. Oxid. Med. Cell. Longev. 2018, 2018, 7580707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corpas, F.; González-Gordo, S.; Palma, J. Nitric Oxide (NO) Scaffolds the Peroxisomal Protein–Protein Interaction Network in Higher Plants. Int. J. Mol. Sci. 2021, 22, 2444. [Google Scholar] [CrossRef] [PubMed]
- Song, S.B.; Jang, S.-Y.; Kang, H.T.; Wei, B.; Jeoun, U.-W.; Yoon, G.S.; Hwang, E.S. Modulation of Mitochondrial Membrane Potential and ROS Generation by Nicotinamide in a Manner Independent of SIRT1 and Mitophagy. Mol. Cells 2017, 40, 503–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, W.-X. Role of autophagy in liver physiology and pathophysiology. World J. Biol. Chem. 2010, 1, 3–12. [Google Scholar] [CrossRef]
- Franko, A.; von Kleist-Retzow, J.-C.; Neschen, S.; Wu, M.; Schommers, P.; Böse, M.; Kunze, A.; Hartmann, U.; Sanchez-Lasheras, C.; Stoehr, O.; et al. Liver adapts mitochondrial function to insulin resistant and diabetic states in mice. J. Hepatol. 2014, 60, 816–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowley, T.T.; Bitner, B.F.; Ray, J.D.; Lathen, D.R.; Smithson, A.T.; Dallon, B.W.; Plowman, C.J.; Bikman, B.T.; Hansen, J.M.; Dorenkott, M.R.; et al. Monomeric cocoa catechins enhance beta-cell function by increasing mitochondrial respiration. J. Nutr. Biochem. 2017, 49, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, R.; Kakkar, P. Naringenin accords hepatoprotection from streptozotocin induced diabetes in vivo by modulating mitochondrial dysfunction and apoptotic signaling cascade. Toxicol. Rep. 2014, 1, 569–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Lin, H.; Xu, Y.; Zhou, F.; Wang, J.; Liu, J.; Zhu, X.; Guo, X.; Tang, Y.; Yao, P. Frataxin-Mediated PINK1-Parkin-Dependent Mitophagy in Hepatic Steatosis: The Protective Effects of Quercetin. Mol. Nutr. Food Res. 2018, 62, e1800164. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Du, W.; Li, Y.; Shi, C.; Hu, N.; Ma, S.; Wang, W.; Ren, J. Effects of melatonin on fatty liver disease: The role of NR4A1/DNA-PKcs/p53 pathway, mitochondrial fission, and mitophagy. J. Pineal Res. 2018, 64, e12450. [Google Scholar] [CrossRef]
- Sun, J.; Yu, F.; Wang, T.; Bian, J.; Liu, Z.; Zou, H. The role of DRP1-PINK1-Parkin-mediated mitophagy in early cadmium-induced liver damage. Toxicology 2021, 466, 153082. [Google Scholar] [CrossRef]
- He, F.; Huang, Y.; Song, Z.; Zhou, H.J.; Zhang, H.; Perry, R.J.; Shulman, G.I.; Min, W. Mitophagy-mediated adipose inflammation contributes to type 2 diabetes with hepatic insulin resistance. J. Exp. Med. 2020, 218, e20201416. [Google Scholar] [CrossRef]
- Zhang, Y.; Goldman, S.; Baerga, R.; Zhao, Y.; Komatsu, M.; Jin, S. Adipose-specific deletion of autophagy-related gene 7 (atg7) in mice reveals a role in adipogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 19860–19865. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Pires, K.M.; Ferhat, M.; Chaurasia, B.; Buffolo, M.A.; Smalling, R.; Sargsyan, A.; Atkinson, D.L.; Summers, S.A.; Graham, T.E.; et al. Autophagy Ablation in Adipocytes Induces Insulin Resistance and Reveals Roles for Lipid Peroxide and Nrf2 Signaling in Adipose-Liver Crosstalk. Cell Rep. 2018, 25, 1708–1717.e5. [Google Scholar] [CrossRef] [Green Version]




| Weeks | Control | T2DM | T2DM + PU |
|---|---|---|---|
| 1 | 4.52 ± 0.88 | 12.94 ± 1.74 ** | 11.94 ± 1.53 ** |
| 2 | 4.38 ± 0.89 | 12.64 ± 0.76 ** | 11.25 ± 1.25 **, ## |
| 3 | 4.76 ± 0.95 | 13.94 ± 0.86 ** | 11.78 ± 1.27 **, ## |
| 4 | 4.97 ± 0.83 | 13.99 ± 1.41 ** | 11.45 ± 1.27 **, ## |
| 5 | 4.51 ± 0.70 | 13.89 ± 1.71 ** | 11.06 ± 1.44 **, ## |
| 6 | 5.07 ± 1.00 | 14.48 ± 0.79 ** | 10.5 ± 0.92 **, ## |
| 7 | 4.86 ± 0.74 | 14.84 ± 1.18 ** | 9.41 ± 1.27 **, ## |
| 8 | 4.80 ± 0.42 | 14.27 ± 1.40 ** | 8.64 ± 1.01 **, ## |
| Control | T2DM | T2DM + Punicalagin (PU) | |
|---|---|---|---|
| Serum | |||
| TC (mmol/L) | 7.18 ± 1.07 | 12.25 ± 1.82 ** | 8.42 ± 1.44 ## |
| TG (mmol/L) | 0.79 ± 0.11 | 1.31 ± 0.25 ** | 1.05 ± 0.15 *, # |
| LDL-C (mmol/L) | 0.22 ± 0.02 | 0.67 ± 0.21 ** | 0.26 ± 0.13 ## |
| FFA (μmol/L) | 849.4 ± 50.0 | 964.7 ± 89.7 * | 845.7 ± 39.6 # |
| Liver tissue | |||
| TC (mmol/gprot) | 0.09 ± 0.01 | 0.21 ± 0.03 ** | 0.13 ± 0.04 *, ## |
| TG (mmol/gprot) | 0.2 ± 0.02 | 1.33 ± 0.63 ** | 0.61 ± 0.32 # |
| LDL-C (mmol/gprot) | 0.02 ± 0.01 | 0.09 ± 0.04 * | 0.02 ± 0.01 # |
| FFA (μmol/L) | 1140.6 ± 165.5 | 1351.8 ± 91.6 * | 1147.2 ± 89.3 # |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Tan, X.; Cao, Y.; An, X.; Chen, J.; Yang, L. Punicalagin Protects against Diabetic Liver Injury by Upregulating Mitophagy and Antioxidant Enzyme Activities. Nutrients 2022, 14, 2782. https://doi.org/10.3390/nu14142782
Zhang Y, Tan X, Cao Y, An X, Chen J, Yang L. Punicalagin Protects against Diabetic Liver Injury by Upregulating Mitophagy and Antioxidant Enzyme Activities. Nutrients. 2022; 14(14):2782. https://doi.org/10.3390/nu14142782
Chicago/Turabian StyleZhang, Yahui, Xiuying Tan, Yuan Cao, Xin An, Jihua Chen, and Lina Yang. 2022. "Punicalagin Protects against Diabetic Liver Injury by Upregulating Mitophagy and Antioxidant Enzyme Activities" Nutrients 14, no. 14: 2782. https://doi.org/10.3390/nu14142782






