Ideal Cardiovascular Health Metrics Are Associated with Reduced Severity of Hepatic Steatosis and Liver Fibrosis Detected by Transient Elastography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. CVH Metrics
2.3. Definition of Advanced Liver Fibrosis, Cirrhosis, and NAFLD
2.4. Covariates
2.5. Statistical Methods
3. Results
3.1. Baseline Characteristic
3.2. Association between CAP, LSM, and CVH Metrics
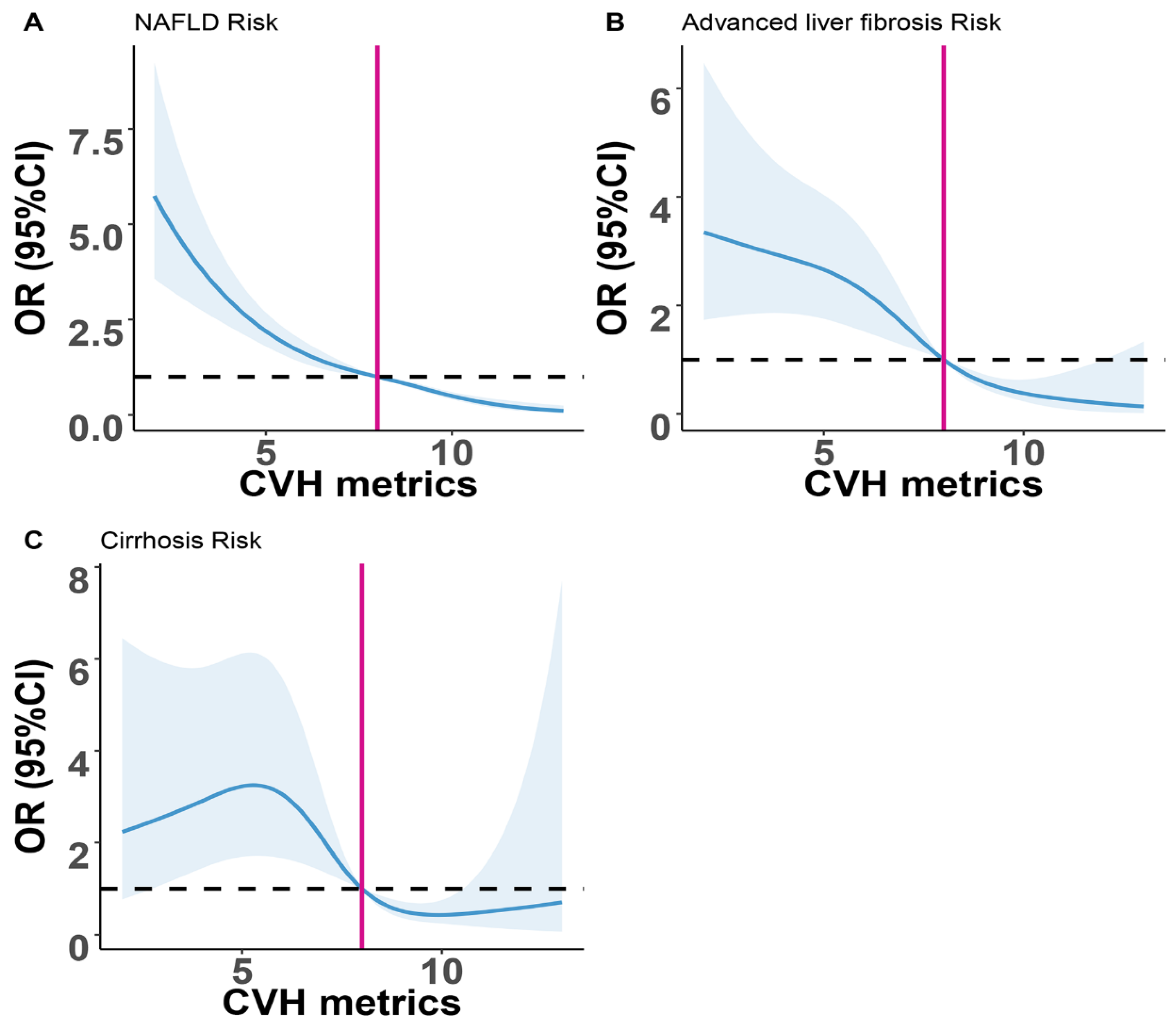
3.3. Association between NAFLD, Advanced Liver Fibrosis, Cirrhosis and CVH Metrics
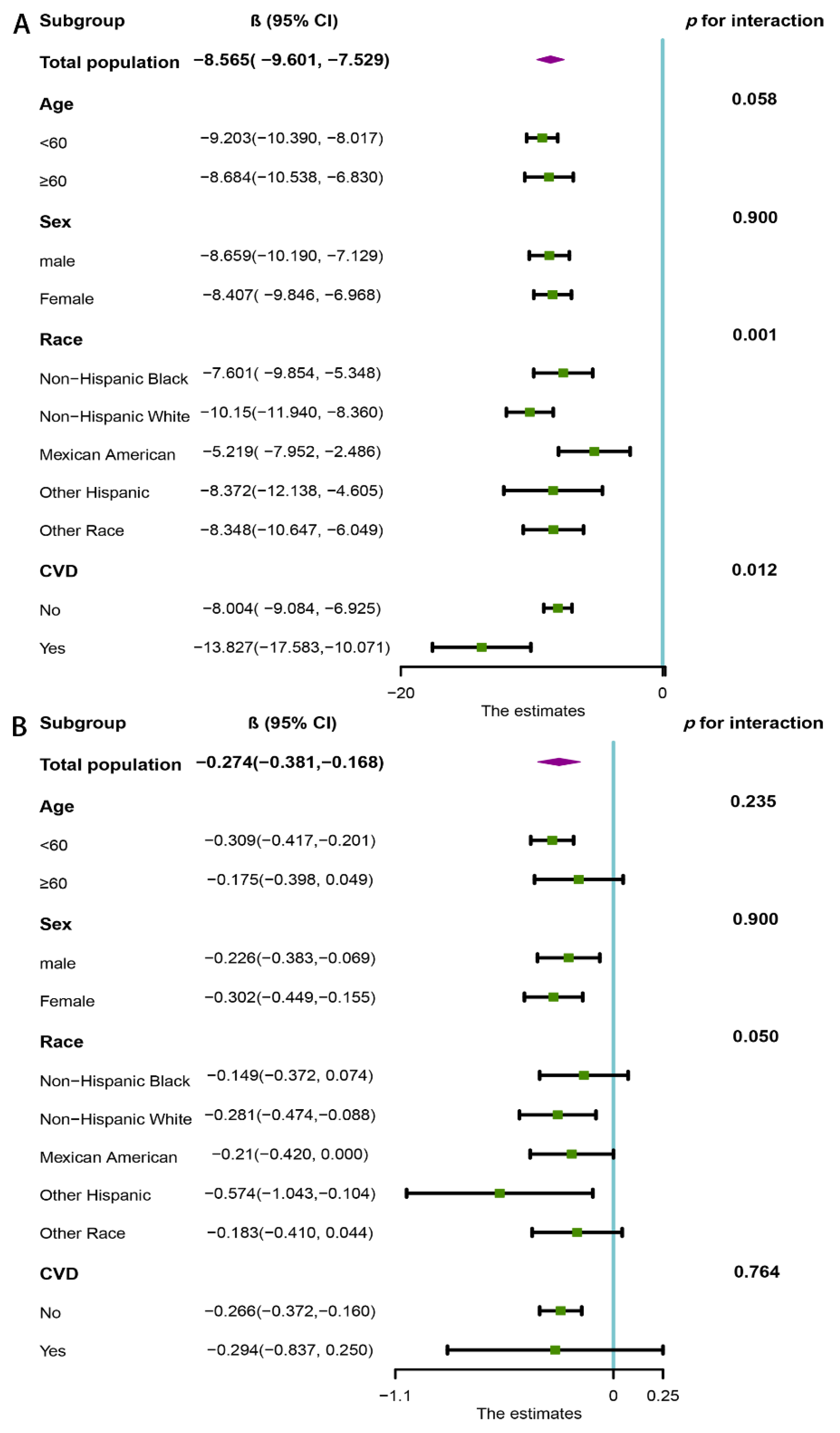
3.4. Subgroup Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lloyd-Jones, D.M.; Hong, Y.; Labarthe, D.; Mozaffarian, D.; Appel, L.J.; Van Horn, L.; Greenlund, K.; Daniels, S.; Nichol, G.; Tomaselli, G.F.; et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: The American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation 2010, 121, 586–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oni, E.; Ogunmoroti, O.; Allen, N.; A-Mallah, M.H.; Blankstein, R.; Martin, S.S.; Zeb, I.; Cushman, M.; Joshi, P.H.; Budoff, M.J.; et al. Life’s Simple 7 and Nonalcoholic Fatty Liver Disease: The Multiethnic Study of Atherosclerosis. Am. J. Med. 2021, 134, 519–525. [Google Scholar] [CrossRef] [PubMed]
- DeCoste, L.R.; Wang, N.; Palmisano, J.N.; Mendez, J.; Hoffmann, U.; Benjamin, E.J.; Long, M.T. Adherence to Ideal Cardiovascular Health Metrics Is Associated With Reduced Odds of Hepatic Steatosis. Hepatol. Commun. 2021, 5, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.H.; Chang, Y.; Ryu, S.; Kim, S.; Kim, Y.H.; Sung, K.C.; Cho, Y.K.; Lee, S.J.; Shin, H.; Wild, S.H.; et al. Cardiovascular Health Metrics in the Development and Regression of Nonalcoholic Fatty Liver Disease: A Cohort Study. J. Clin. Med. 2019, 8, 610. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Li, M.; Zhao, Z.; Xu, M.; Lu, J.; Wang, T.; Chen, Y.; Wang, S.; Dai, M.; Hou, Y.; et al. Ideal Cardiovascular Health Is Inversely Associated with Nonalcoholic Fatty Liver Disease: A Prospective Analysis. Am. J. Med. 2018, 131, e1511–e1515. [Google Scholar] [CrossRef]
- Mazidi, M.; Katsiki, N.; Mikhailidis, D.P.; Banach, M. Ideal cardiovascular health associated with fatty liver: Results from a multi-ethnic survey. Atherosclerosis 2019, 284, 129–135. [Google Scholar] [CrossRef]
- Wong, V.W.; Vergniol, J.; Wong, G.L.; Foucher, J.; Chan, H.L.; Le Bail, B.; Choi, P.C.; Kowo, M.; Chan, A.W.; Merrouche, W.; et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology 2010, 51, 454–462. [Google Scholar] [CrossRef]
- de Lédinghen, V.; Wong, G.L.; Vergniol, J.; Chan, H.L.; Hiriart, J.B.; Chan, A.W.; Chermak, F.; Choi, P.C.; Foucher, J.; Chan, C.K.; et al. Controlled attenuation parameter for the diagnosis of steatosis in non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2016, 31, 848–855. [Google Scholar] [CrossRef]
- Aykut, U.E.; Akyuz, U.; Yesil, A.; Eren, F.; Gerin, F.; Ergelen, R.; Celikel, C.A.; Yilmaz, Y. A comparison of FibroMeter™ NAFLD Score, NAFLD fibrosis score, and transient elastography as noninvasive diagnostic tools for hepatic fibrosis in patients with biopsy-proven non-alcoholic fatty liver disease. Scand. J. Gastroenterol. 2014, 49, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Zhu, S.; Xiao, X.; Yan, L.; Yang, J.; Wu, G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: A meta-analysis. Hepatology 2017, 66, 1486–1501. [Google Scholar] [CrossRef] [Green Version]
- Vilar-Gomez, E.; Nephew, L.D.; Vuppalanchi, R.; Gawrieh, S.; Mladenovic, A.; Pike, F.; Samala, N.; Chalasani, N. High-quality diet, physical activity, and college education are associated with low risk of NAFLD among the US population. Hepatology 2022, 75, 1491–1506. [Google Scholar] [CrossRef]
- Krebs-Smith, S.M.; Pannucci, T.E.; Subar, A.F.; Kirkpatrick, S.I.; Lerman, J.L.; Tooze, J.A.; Wilson, M.M.; Reedy, J. Update of the Healthy Eating Index: HEI-2015. J. Acad. Nutr. Diet. 2018, 118, 1591–1602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ford, E.S.; Greenlund, K.J.; Hong, Y. Ideal cardiovascular health and mortality from all causes and diseases of the circulatory system among adults in the United States. Circulation 2012, 125, 987–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Shi, S.; Jiang, Y.; Chen, K.; Liao, Y.; Huang, R.; Huang, K. Association of sarcopenia with ideal cardiovascular health metrics among US adults: A cross-sectional study of NHANES data from 2011 to 2018. BMJ Open 2022, 12, e061789. [Google Scholar] [CrossRef] [PubMed]
- Bucholz, E.M.; Butala, N.M.; Allen, N.B.; Moran, A.E.; de Ferranti, S.D. Age, Sex, Race/Ethnicity, and Income Patterns in Ideal Cardiovascular Health Among Adolescents and Adults in the U.S. Am. J. Prev. Med. 2022, 62, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Eddowes, P.J.; Sasso, M.; Allison, M.; Tsochatzis, E.; Anstee, Q.M.; Sheridan, D.; Guha, I.N.; Cobbold, J.F.; Deeks, J.J.; Paradis, V.; et al. Accuracy of FibroScan Controlled Attenuation Parameter and Liver Stiffness Measurement in Assessing Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1717–1730. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, M.S.; Vuppalanchi, R.; Van Natta, M.L.; Hallinan, E.; Kowdley, K.V.; Abdelmalek, M.; Neuschwander-Tetri, B.A.; Loomba, R.; Dasarathy, S.; Brandman, D.; et al. Vibration-Controlled Transient Elastography to Assess Fibrosis and Steatosis in Patients With Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2019, 17, 156–163.e2. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Liu, H.; Yao, Y.; Wang, Y.; Ma, L.; Liu, X.; Guo, S.; Feng, X.; Chen, Y.; Chen, X.; Liu, Z.; et al. Ideal cardiovascular health metrics and the risk of non-alcoholic fatty liver disease: A cross-sectional study in northern China. Liver Int. 2019, 39, 950–955. [Google Scholar] [CrossRef] [PubMed]
- Heredia, N.I.; Zhang, X.; Balakrishnan, M.; Daniel, C.R.; Hwang, J.P.; McNeill, L.H.; Thrift, A.P. Physical activity and diet quality in relation to non-alcoholic fatty liver disease: A cross-sectional study in a representative sample of U.S. adults using NHANES 2017–2018. Prev. Med. 2022, 154, 106903. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Hennein, R.; Liu, C.; Long, M.T.; Hoffmann, U.; Jacques, P.F.; Lichtenstein, A.H.; Hu, F.B.; Levy, D. Improved Diet Quality Associates With Reduction in Liver Fat, Particularly in Individuals With High Genetic Risk Scores for Nonalcoholic Fatty Liver Disease. Gastroenterology 2018, 155, 107–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loeffelholz, C.V.; Roth, J.; Coldewey, S.M.; Birkenfeld, A.L. The Role of Physical Activity in Nonalcoholic and Metabolic Dysfunction Associated Fatty Liver Disease. Biomedicines 2021, 9, 1853. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Clauss, M.; Gérard, P.; Mosca, A.; Leclerc, M. Interplay Between Exercise and Gut Microbiome in the Context of Human Health and Performance. Front. Nutr. 2021, 8, 637010. [Google Scholar] [CrossRef] [PubMed]
- Zein, C.O. Clearing the smoke in chronic liver diseases. Hepatology 2010, 51, 1487–1490. [Google Scholar] [CrossRef] [Green Version]
- Zein, C.O.; Unalp, A.; Colvin, R.; Liu, Y.C.; McCullough, A.J. Smoking and severity of hepatic fibrosis in nonalcoholic fatty liver disease. J. Hepatol. 2011, 54, 753–759. [Google Scholar] [CrossRef] [Green Version]
- Ou, H.; Fu, Y.; Liao, W.; Zheng, C.; Wu, X. Association between Smoking and Liver Fibrosis among Patients with Nonalcoholic Fatty Liver Disease. Can. J. Gastroenterol. Hepatol. 2019, 2019, 6028952. [Google Scholar] [CrossRef]
- Koehler, E.M.; Schouten, J.N.; Hansen, B.E.; van Rooij, F.J.; Hofman, A.; Stricker, B.H.; Janssen, H.L. Prevalence and risk factors of non-alcoholic fatty liver disease in the elderly: Results from the Rotterdam study. J. Hepatol. 2012, 57, 1305–1311. [Google Scholar] [CrossRef]
- Caballería, L.; Pera, G.; Auladell, M.A.; Torán, P.; Muñoz, L.; Miranda, D.; Alumà, A.; Casas, J.D.; Sánchez, C.; Gil, D.; et al. Prevalence and factors associated with the presence of nonalcoholic fatty liver disease in an adult population in Spain. Eur. J. Gastroenterol. Hepatol. 2010, 22, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Ciardullo, S.; Monti, T.; Perseghin, G. High Prevalence of Advanced Liver Fibrosis Assessed by Transient Elastography Among U.S. Adults With Type 2 Diabetes. Diabetes Care 2021, 44, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.L.; Tai, C.M.; Huang, J.F.; Liu, C.J.; Chen, H.F.; Cheng, P.N.; Chen, C.Y.; Peng, C.Y.; Wang, C.C.; Weng, S.H.; et al. The impact of body mass index on clinicopathological features of nonalcoholic fatty liver disease in Taiwan. J. Gastroenterol. Hepatol. 2022, 37, 1901–1910. [Google Scholar] [CrossRef]
- Alexopoulos, A.S.; Crowley, M.J.; Wang, Y.; Moylan, C.A.; Guy, C.D.; Henao, R.; Piercy, D.L.; Seymour, K.A.; Sudan, R.; Portenier, D.D.; et al. Glycemic Control Predicts Severity of Hepatocyte Ballooning and Hepatic Fibrosis in Nonalcoholic Fatty Liver Disease. Hepatology 2021, 74, 1220–1233. [Google Scholar] [CrossRef] [PubMed]
- Aneni, E.C.; Oni, E.T.; Martin, S.S.; Blaha, M.J.; Agatston, A.S.; Feldman, T.; Veledar, E.; Conçeicao, R.D.; Carvalho, J.A.; Santos, R.D.; et al. Blood pressure is associated with the presence and severity of nonalcoholic fatty liver disease across the spectrum of cardiometabolic risk. J. Hypertens. 2015, 33, 1207–1214. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, Y.; Lin, C.; Chen, Z. Hypertension and non-alcoholic fatty liver disease proven by transient elastography. Hepatol. Res. 2016, 46, 1304–1310. [Google Scholar] [CrossRef]
- Ma, J.; Hwang, S.J.; Pedley, A.; Massaro, J.M.; Hoffmann, U.; Chung, R.T.; Benjamin, E.J.; Levy, D.; Fox, C.S.; Long, M.T. Bi-directional analysis between fatty liver and cardiovascular disease risk factors. J. Hepatol. 2017, 66, 390–397. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Allen, A.M.; Wang, Z.; Prokop, L.J.; Murad, M.H.; Loomba, R. Fibrosis progression in nonalcoholic fatty liver vs. nonalcoholic steatohepatitis: A systematic review and meta-analysis of paired-biopsy studies. Clin. Gastroenterol. Hepatol. 2015, 13, 643–654.e1–e9; quiz e39–e40. [Google Scholar] [CrossRef] [Green Version]
- Sozen, E.; Ozer, N.K. Impact of high cholesterol and endoplasmic reticulum stress on metabolic diseases: An updated mini-review. Redox. Biol. 2017, 12, 456–461. [Google Scholar] [CrossRef]
- Ballestri, S.; Nascimbeni, F.; Romagnoli, D.; Lonardo, A. The independent predictors of non-alcoholic steatohepatitis and its individual histological features.: Insulin resistance, serum uric acid, metabolic syndrome, alanine aminotransferase and serum total cholesterol are a clue to pathogenesis and candidate targets for treatment. Hepatol. Res. 2016, 46, 1074–1087. [Google Scholar]
- Carulli, L.; Ballestri, S.; Lonardo, A.; Lami, F.; Violi, E.; Losi, L.; Bonilauri, L.; Verrone, A.M.; Odoardi, M.R.; Scaglioni, F.; et al. Is nonalcoholic steatohepatitis associated with a high-though-normal thyroid stimulating hormone level and lower cholesterol levels? Intern. Emerg. Med. 2013, 8, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Touros, A.; Kim, W.R. Nonalcoholic Fatty Liver Disease and Metabolic Syndrome. Clin. Liver Dis. 2018, 22, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Ballestri, S.; Zona, S.; Targher, G.; Romagnoli, D.; Baldelli, E.; Nascimbeni, F.; Roverato, A.; Guaraldi, G.; Lonardo, A. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2016, 31, 936–944. [Google Scholar] [CrossRef]
- Cazac, G.D.; Lăcătușu, C.M.; Mihai, C.; Grigorescu, E.D.; Onofriescu, A.; Mihai, B.M. New Insights into Non-Alcoholic Fatty Liver Disease and Coronary Artery Disease: The Liver-Heart Axis. Life 2022, 12, 1189. [Google Scholar] [CrossRef] [PubMed]
- Muzurović, E.; Peng, C.C.; Belanger, M.J.; Sanoudou, D.; Mikhailidis, D.P.; Mantzoros, C.S. Nonalcoholic Fatty Liver Disease and Cardiovascular Disease: A Review of Shared Cardiometabolic Risk Factors. Hypertension 2022, 79, 1319–1326. [Google Scholar] [CrossRef]
- Drożdż, K.; Nabrdalik, K.; Hajzler, W.; Kwiendacz, H.; Gumprecht, J.; Lip, G.Y.H. Metabolic-Associated Fatty Liver Disease (MAFLD), Diabetes, and Cardiovascular Disease: Associations with Fructose Metabolism and Gut Microbiota. Nutrients 2021, 14, 103. [Google Scholar] [CrossRef]
- Targher, G.; Corey, K.E.; Byrne, C.D. NAFLD, and cardiovascular and cardiac diseases: Factors influencing risk, prediction and treatment. Diabetes Metab. 2021, 47, 101215. [Google Scholar] [CrossRef]
- Adams, L.A.; Anstee, Q.M.; Tilg, H.; Targher, G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 2017, 66, 1138–1153. [Google Scholar] [CrossRef] [Green Version]
- Stahl, E.P.; Dhindsa, D.S.; Lee, S.K.; Sandesara, P.B.; Chalasani, N.P.; Sperling, L.S. Nonalcoholic Fatty Liver Disease and the Heart: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 948–963. [Google Scholar] [CrossRef]

| Variables | Total (n = 2679) | Poor (Score 0–7) (n = 1310) | Intermediate (Score 8–10) (n = 1033) | Ideal (Score 11–14) (n = 3,36) | p-Value |
|---|---|---|---|---|---|
| Age (years) | 50.02 (17.11) | 56.62 (14.93) | 46.31 (16.67) | 35.70 (13.90) | <0.001 |
| Sex, male, n, (%) | 1348 (50.32%) | 715 (54.58%) | 517 (50.05%) | 116 (34.52%) | <0.001 |
| MS, n, (%) | <0.001 | ||||
| Married/living with partner | 1634 (60.99%) | 806 (61.53%) | 643 (62.25%) | 185 (55.06%) | |
| Widowed/divorced/separated | 533.00 (19.90%) | 334 (25.50%) | 170 (16.46%) | 29 (8.63%) | |
| Never married | 512.00 (19.11%) | 170 (12.98%) | 220 (21.30%) | 122 (36.31%) | |
| Education level (%) | <0.001 | ||||
| High school degree/equivalency or less | 1026 (38.30%) | 554 (42.29%) | 375 (36.30%) | 97 (28.87%) | |
| Some college or associates degree | 887 (33.11%) | 456 (34.81%) | 323 (31.27%) | 108 (32.14%) | |
| College graduate or above | 766 (28.59%) | 300 (22.90%) | 335 (32.43%) | 131 (38.99%) | |
| Race, (%) | <0.001 | ||||
| Mexican American | 333 (12.43%) | 152 (11.60%) | 134 (12.97%) | 47 (13.99%) | |
| Non-Hispanic white | 970 (36.21%) | 493 (37.63%) | 371 (35.91%) | 106 (31.55%) | |
| Non-Hispanic black | 603 (22.51%) | 331 (25.27%) | 208 (20.14%) | 64 (19.05%) | |
| Other Hispanic | 241 (9.00%) | 115 (8.78%) | 89 (8.62%) | 37 (11.01%) | |
| Other races | 532 (19.86%) | 219 (16.72%) | 231 (22.36%) | 82 (24.40%) | |
| CVD, (%) | 246 (9.18%) | 180 (13.74%) | 63 (6.10%) | 3 (0.89%) | <0.001 |
| Platelet count, 10^9/L | 241.98 (62.50) | 245.44 (67.35) | 239.99 (58.62) | 234.65 (52.95) | 0.057 |
| Hs-CRP, mg/L | 3.84 (6.96) | 4.84 (8.16) | 3.21 (5.96) | 1.88 (2.85) | <0.001 |
| AST, U/L | 21.49 (11.09) | 21.90 (10.43) | 21.23 (12.04) | 20.65 (10.52) | 0.012 |
| ALT, U/L | 21.97 (14.86) | 24.00 (16.21) | 20.89 (13.71) | 17.37 (10.94) | <0.001 |
| HDL, mmol/L | 1.38 (0.39) | 1.31 (0.38) | 1.41 (0.39) | 1.56 (0.37) | <0.001 |
| eGFR, mL/min/1.73 m2 | 93.38 (22.61) | 86.95 (22.26) | 96.97 (21.55) | 107.41 (17.89) | <0.001 |
| Total bilirubin, mg/dL | 7.97 (4.62) | 7.61 (4.34) | 8.17 (4.60) | 8.76 (5.50) | <0.001 |
| Albumin, g/L | 40.79 (3.18) | 40.23 (3.25) | 41.15 (3.06) | 41.85 (2.87) | <0.001 |
| CAP, dB/m | 263.60 (62.31) | 286.68 (60.71) | 250.32 (55.69) | 214.44 (45.01) | <0.001 |
| LSM, kPa | 5.90 (5.45) | 6.76 (6.82) | 5.23 (3.78) | 4.63 (2.39) | <0.001 |
| NAFLD, (%) | 974 (36.36%) | 674 (51.45%) | 275 (26.62%) | 25 (7.44%) | <0.001 |
| Advanced liver fibrosis, (%) | 176 (6.57%) | 141 (10.76%) | 33 (3.19%) | 2 (0.60%) | <0.001 |
| Cirrhosis, (%) | 79 (2.95%) | 65 (4.96%) | 12 (1.16%) | 2 (0.60%) | <0.001 |
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| β, (95% CI) | p | β, (95% CI) | p | β, (95% CI) | p | |
| CAP | ||||||
| Continuous | −11.703 (−12.590, −10.816) | <0.001 | −12.059 (−13.038, −11.081) | <0.001 | −8.565 (−9.601, −7.529) | <0.001 |
| Categorical | ||||||
| Poor (0–7) | 72.241 (65.402, 79.080) | <0.001 | 69.683 (62.333, 77.033) | <0.001 | 45.846 (38.444, 53.248) | <0.001 |
| Intermediate (8–10) | 35.885 (28.861, 42.908) | <0.001 | 33.508 (26.448, 40.569) | <0.001 | 21.105 (14.368, 27.843) | <0.001 |
| Ideal (11–14) | Reference | Reference | Reference | |||
| LSM | ||||||
| Continuous | −0.385 (−0.471, −0.300) | <0.001 | −0.376 (−0.473, −0.279) | <0.001 | −0.274 (−0.381, −0.168) | <0.001 |
| Categorical | ||||||
| Poor (0–7) | 2.176 (1.673, 2.680) | <0.001 | 2.151 (1.638, 2.664) | <0.001 | 1.593 (1.018, 2.168) | <0.001 |
| Intermediate (8–10) | 0.487 (−0.043, 1.017) | 0.072 | 0.314 (−0.225, 0.854) | 0.253 | 0.220 (−0.331, 0.771) | 0.433 |
| Ideal (11–14) | Reference | Reference | Reference | |||
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| NAFLD | ||||||
| Continuous | 0.696 (0.668, 0.724) | <0.001 | 0.667 (0.637, 0.698) | <0.001 | 0.738 (0.702, 0.776) | <0.001 |
| Categorical | ||||||
| Poor (0–7) | 13.183 (8.826, 20.582) | <0.001 | 14.414 (9.440, 22.925) | <0.001 | 7.140 (4.550, 11.633) | <0.001 |
| Intermediate (8–10) | 4.513 (2.993, 7.099) | <0.001 | 4.545 (2.990, 7.198) | <0.001 | 3.098 (1.997, 4.994) | <0.001 |
| Ideal (11–14) | Reference | Reference | Reference | |||
| Advanced liver fibrosis | ||||||
| Continuous | 0.700 (0.650, 0.751) | <0.001 | 0.707 (0.654, 0.764) | <0.001 | 0.761 (0.697, 0.829) | <0.001 |
| Categorical | ||||||
| Poor (0–7) | 20.143 (6.381,122.273) | <0.001 | 15.846 (4.893, 97.230) | <0.001 | 10.687 (3.146, 67.225) | <0.001 |
| Intermediate (8–10) | 5.511 (1.664, 34.099) | 0.02 | 4.769 (1.428, 29.614) | 0.033 | 3.927 (1.141, 24.781) | <0.001 |
| Ideal (11–14) | Reference | Reference | Reference | |||
| Cirrhosis | ||||||
| Continuous | 0.741 (0.669, 0.818) | <0.001 | 0.732 (0.656, 0.816) | <0.001 | 0.794 (0.699, 0.899) | <0.001 |
| Categorical | ||||||
| Poor (0–7) | 8.719 (2.715, 53.288) | 0.003 | 8.478 (2.505, 53.012) | 0.004 | 6.012 (1.609, 39.902) | 0.022 |
| Intermediate (8–10) | 1.963 (0.532, 12.655) | 0.379 | 1.882 (0.503, 12.217) | 0.412 | 1.553 (0.390, 10.466) | 0.582 |
| Ideal (11–14) | Reference | Reference | Reference | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, H.; Xu, C.; Li, W.; Huang, Y.; Hua, R.; Xiong, Y.; Yang, Y.; Feng, X.; Wang, Z.; Yuan, Z.; et al. Ideal Cardiovascular Health Metrics Are Associated with Reduced Severity of Hepatic Steatosis and Liver Fibrosis Detected by Transient Elastography. Nutrients 2022, 14, 5344. https://doi.org/10.3390/nu14245344
Fan H, Xu C, Li W, Huang Y, Hua R, Xiong Y, Yang Y, Feng X, Wang Z, Yuan Z, et al. Ideal Cardiovascular Health Metrics Are Associated with Reduced Severity of Hepatic Steatosis and Liver Fibrosis Detected by Transient Elastography. Nutrients. 2022; 14(24):5344. https://doi.org/10.3390/nu14245344
Chicago/Turabian StyleFan, Heze, Chenbo Xu, Wenyuan Li, Yuzhi Huang, Rui Hua, Ying Xiong, Yuxuan Yang, Xueying Feng, Zihao Wang, Zuyi Yuan, and et al. 2022. "Ideal Cardiovascular Health Metrics Are Associated with Reduced Severity of Hepatic Steatosis and Liver Fibrosis Detected by Transient Elastography" Nutrients 14, no. 24: 5344. https://doi.org/10.3390/nu14245344





