Nutraceuticals in Paediatric Patients with Dyslipidaemia
Abstract
:1. Introduction
2. Nutraceuticals and Dyslipidaemias
- Inhibitors of intestinal cholesterol absorption;
- Inhibitors of liver cholesterol synthesis;
- Inducers of cholesterol excretion.
3. Nutraceuticals Inhibitors of Intestinal Cholesterol Absorption
3.1. Fibres
- (1)
- Insoluble fibre (bran): fibre not soluble in water and poorly fermented in the gut, with a possible mechanical laxative effect.
- (2)
- Soluble fibre (inulin, dextrin, oligosaccharides), non-viscous, rapidly fermented; it does not cause any increase in viscosity and is completely fermented by gut microbiota; it can exert a prebiotic effect, without any laxative effect.
- (3)
- Viscous soluble fibre, rapidly fermented (β-glucan, guar gum, pectin); it creates a viscous gel in water, thus increasing chime viscosity and consequently slowing nutrients absorption. It is quickly fermented in the gut, losing its laxative effect.
- (4)
- Soluble viscous non-fermentable fibre (psyllium, multicellulose): it reduces nutrients absorption thanks to its viscosity and can exert a laxative effect.
3.2. Phytosterols and Stanols
3.3. Chitosan
3.4. Probiotics
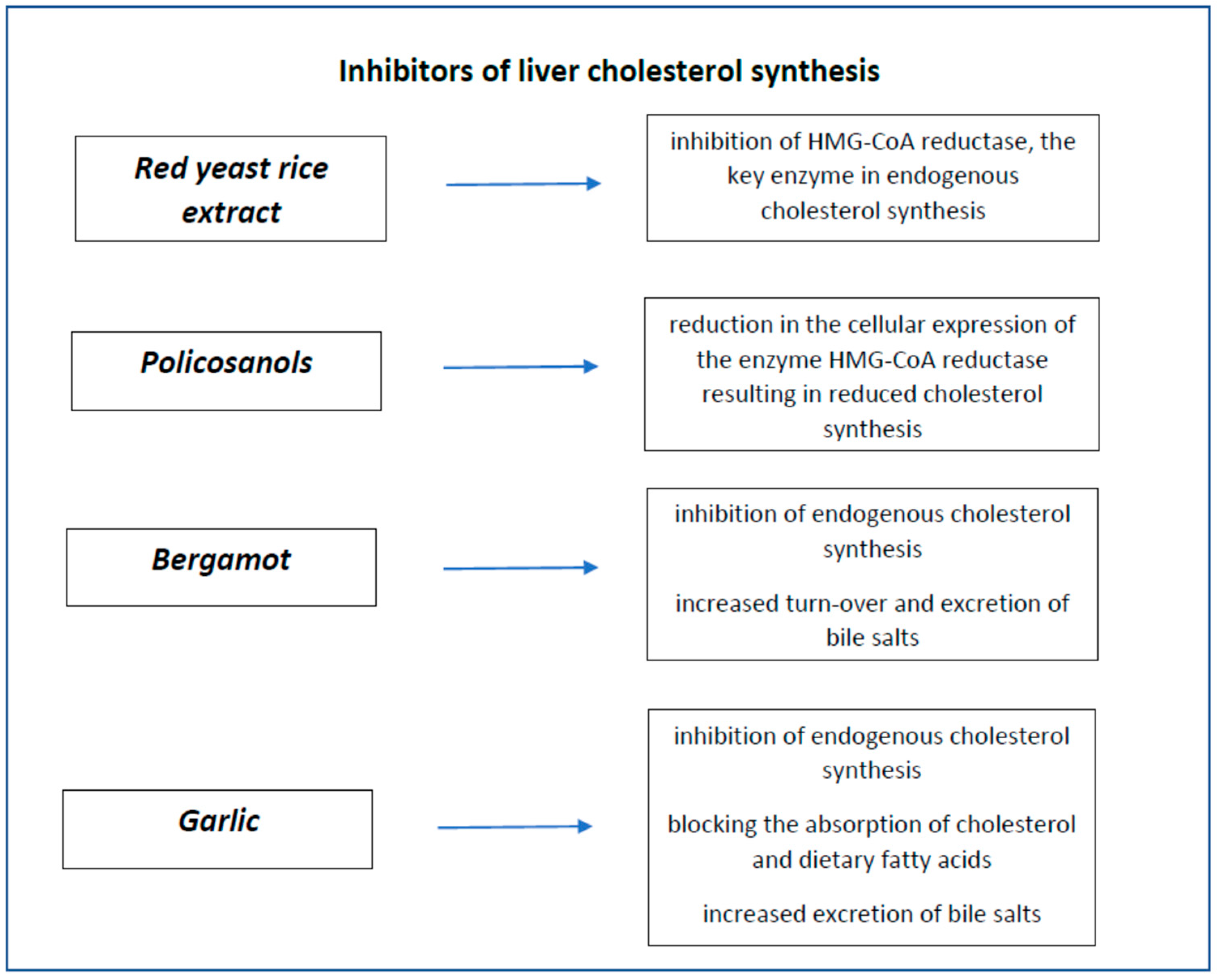
4. Nutraceuticals Inhibitors of Liver Cholesterol Synthesis
4.1. Red Yeast Rice
4.2. Policosanols
4.3. Bergamot
4.4. Garlic
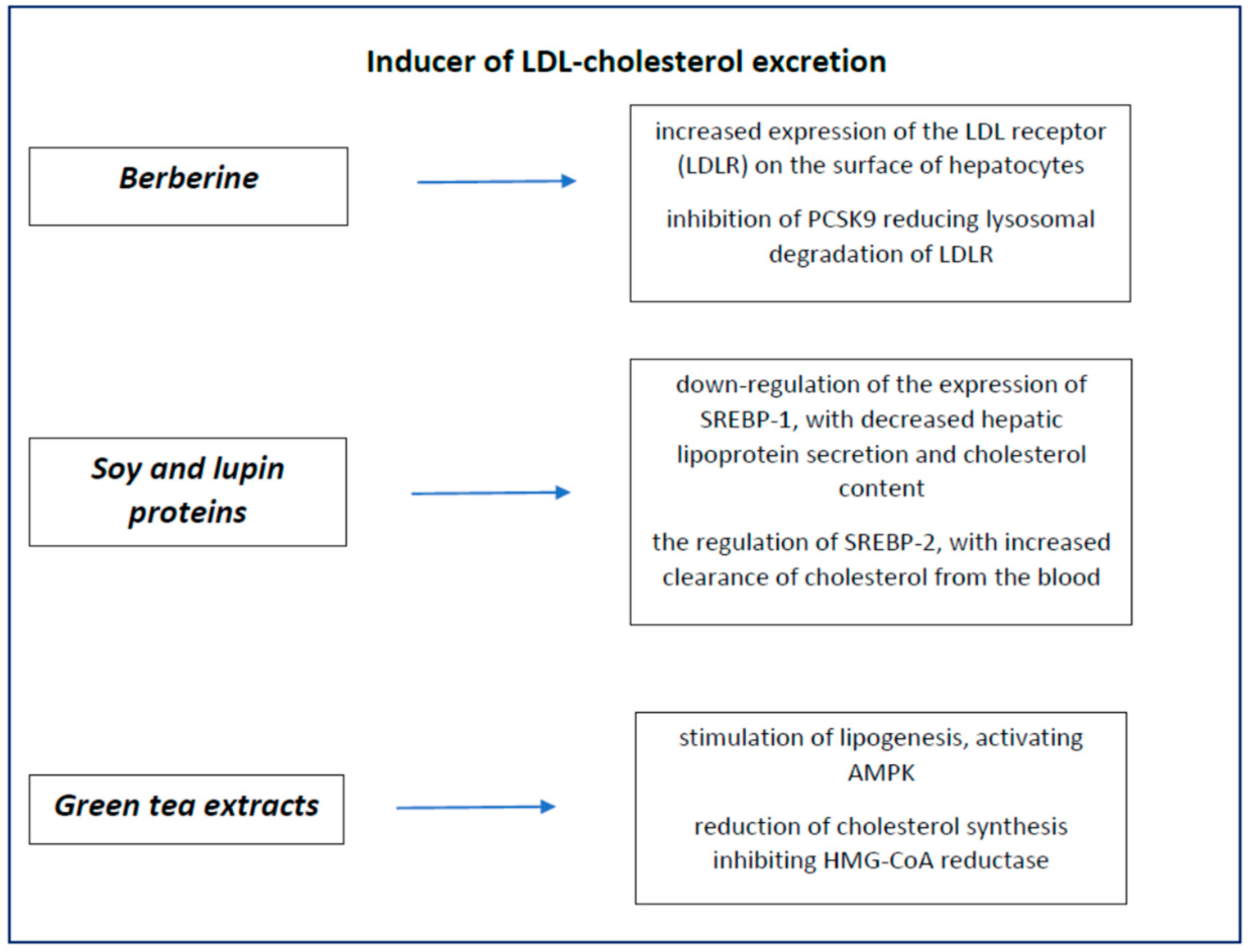
5. Nutraceuticals Inducer of LDL Cholesterol Excretion
5.1. Berberine
5.2. Soy and Lupins
5.3. Green Tea Extracts
6. Nutraceuticals with Mixed Action
6.1. Omega-3 Polyunsaturated Long Chain Fatty Acids
6.2. Curcumin
7. Nutraceuticals in Combined Therapy
8. Nutraceuticals and Pharmacologic Therapy
9. Final Considerations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAP | American Academy of Pediatrics |
| AHA | American Heart Association |
| CHD | Coronary heart disease |
| DHA | Docosahexaenoic acid |
| EAS | European Atherosclerosis Society |
| EFSA | European Food Safety Authority |
| EPA | Eicosapentaenoic acid |
| EU | European Union |
| FCHL | Familial combined hypercholesterolaemia |
| FH | Familial hypercholesterolaemia |
| HDL | High-density lipoprotein |
| HMGCoA | HydroxyMethylGlutaryl CoA |
| LDL | Low-density lipoprotein |
| NCEP | National Cholesterol Education Programme |
| NPC1L1 | Niemann–Pick C1-like protein |
| PUFA | Polyunsaturated fatty acids |
| RYR | Red yeast rice |
| SCFA | Short-chain fatty acids |
| TG | Triglycerides |
References
- Townsend, N.; Wilson, L.; Bhatnagar, P.; Wickramasinghe, K.; Rayner, M.; Nichols, M. Cardiovascular disease in Europe: Epidemiological update 2016. Eur. Heart J. 2016, 37, 3232–3245. [Google Scholar] [CrossRef]
- Spinelli, A.; Nardone, P.; Buoncristiano, M.; Lauria, L.; Pierannunzio, D. Okkio Alla Salute: I Dati Nazionali 2016; Centro Nazionale per la Prevenzione Delle Malattie e la Promozione Della Salute (Cnapps-Iss): Rome, Italy, 2016. [Google Scholar]
- Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: Summary report. Pediatrics 2011, 128 (Suppl. 5), S213–S256. [Google Scholar] [CrossRef] [Green Version]
- Wiegman, A.; Gidding, S.S.; Watts, G.F.; Chapman, M.J.; Ginsberg, H.N.; Cuchel, M.; Ose, L.; Averna, M.; Boileau, C.; Borén, J.; et al. Familial hypercholesterolaemia in children and adolescents: Gaining decades of life by optimizing detection and treatment. Eur. Heart J. 2015, 36, 2425–2437. [Google Scholar] [CrossRef] [Green Version]
- Giovannini, M.; De Carlis, S. Raccomandazioni per la prevenzione in età pediatrica dell’aterosclerosi. Riv. Ital. Pediat. 2000, 26, 13–28. [Google Scholar]
- Catapano, A.L.; Graham, I.; De Backer, G.; Wiklund, O.; Chapman, M.J.; Drexel, H.; Hoes, A.W.; Jennings, C.S.; Landmesser, U.; Pedersen, T.R.; et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias: The Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis 2016, 253, 281–344. [Google Scholar] [PubMed] [Green Version]
- The DISC Collaborative Research Group. The efficacy and safety of lowering dietary intake of total fat, saturated fat, and cholesterol in children with elevated LDL-C: The Dietary Intervention Study in Children (DISC). JAMA 1995, 273, 1429–1435. [Google Scholar] [CrossRef]
- Sahebkar, A.; Serban, M.-C.; Gluba-Brzózka, A.; Mikhailidis, D.P.; Cicero, A.F.; Rysz, J.; Banach, M. Lipid-modifying effects of nutraceuticals: An evidence-based approach. Nutrition 2016, 32, 1179–1192. [Google Scholar] [CrossRef] [PubMed]
- Houston, M. The role of nutraceutical supplements in the treatment of dyslipidemia. J. Clin. Hypertens. 2012, 14, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Banach, M.; Rizzo, M.; Toth, P.P.; Farnier, M.; Davidson, M.H.; Al-Rasadi, K.; Aronow, W.S.; Athyros, V.; Djuric, D.M.; Ezhov, M.V.; et al. Statin intolerance—An attempt at a unified definition. Position paper from an International Lipid Expert Panel. Arch. Med. Sci. 2015, 11, 1–23. [Google Scholar] [CrossRef]
- Banach, M.; Serban, M.C. Discussion around statin discontinuation in older adults and patients with wasting diseases. J. Cachexia Sarcopenia Muscle 2016, 7, 396–399. [Google Scholar] [CrossRef] [Green Version]
- Banach, M.; Aronow, W.S.; Serban, M.C.; Rysz, J.; Voroneanu, L.; Covic, A. Lipids, blood pressure and kidney update 2015. Lipids Health Dis. 2015, 14, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massini, G.; Buganza, R.; De Sanctis, L.; Guardamagna, O. La nutraceutica nel bambino dislipidemico. GIA 2019, 10, 32–48. [Google Scholar]
- Cicero, A.F.G.; Colletti, A.; Bajraktari, G.; Descamps, O.; Djuric, D.M.; Ezhov, M.; Fras, Z.; Katsiki, N.; Langlois, M.; Latkovskis, G.; et al. Lipid lowering nutraceuticals in clinical practice: Position paper from an International Lipid Expert Panel. Arch. Med. Sci. 2017, 13, 965–1005. [Google Scholar] [CrossRef] [PubMed]
- McRorie, J.W., Jr. Evidence-Based Approach to Fiber Supplements and Clinically Meaningful Health Benefits, Part 1, What to Look for and How to Recommend an Effective Fiber Therapy. Nutr. Today 2015, 50, 82–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vuksan, V.; Jenkins, A.L.; Rogovik, A.L.; Fairgrieve, C.D.; Jovanovski, E.; Leiter, L.A. Viscosity rather than quantity of dietary fibre predicts cholesterol-lowering effect in healthy individuals. Br. J. Nutr. 2011, 106, 1349–1352. [Google Scholar] [CrossRef] [Green Version]
- Assmann, G.; Buono, P.; Daniele, A.; Della Valle, E.; Farinaro, E.; Ferns, G.; Krogh, V.; Kromhout, D.; Masana, L.; Merino, J.; et al. Functional foods and cardiometabolic diseases* International Task Force for Prevention of Cardiometabolic Diseases. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1272–1300. [Google Scholar] [CrossRef]
- Giacco, R.; Clemente, G.; Cipriano, D.; Luongo, D.; Viscovo, D.; Patti, L.; Di Marino, L.; Giacco, A.; Naviglio, D.; Bianchi, M.; et al. Effects of the regular consumption of wholemeal wheat foods on cardiovascular risk factors in healthy people. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 186–194. [Google Scholar] [CrossRef]
- European Food Safety Authority. Scientific opinion on dietary reference values for carbohydrates and dietary fiber. EFSA J. 2010, 8, 1462. [Google Scholar]
- Yang, Y.; Zhao, L.-G.; Wu, Q.-J.; Ma, X.; Xiang, Y.-B. Association between dietary fiber and lower risk of all-cause mortality: A meta-analysis of cohort studies. Am. J. Epidemiol. 2015, 181, 83–91. [Google Scholar] [CrossRef]
- Pereira, M.A.; O’Reilly, E.; Augustsson, K.; Fraser, G.E.; Goldbourt, U.; Heitmann, B.L.; Hallmans, G.; Knekt, P.; Liu, S.; Pietinen, P.; et al. Dietary fiber and risk of coronary heart disease: A pooled analysis of cohort studies. Arch. Intern. Med. 2004, 164, 370–376. [Google Scholar] [CrossRef] [Green Version]
- Bazzano, L.A.; Thompson, A.M.; Tees, M.T.; Nguyen, C.H.; Winham, D.M. Nonsoy legume consumption lowers cholesterol levels: A meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 94–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estruch, R.; Martinez-Gonzalez, M.; Corella, D.; Basora-Gallisa, J.; Ruiz-Gutierrez, V.; Covas, M.; Fiol, M.; Gomez-Gracia, E.; Lopez-Sabater, M.C.; Escoda, R.; et al. PREDIMED Study Investigators. Effects of dietary fibre intake on risk factors for cardiovascular disease in subjects at high risk. J. Epidemiol. Community Health 2009, 63, 582–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grooms, K.N.; Ommerborn, M.J.; Pham, D.Q.; Djousse, L.; Clark, C.R. Dietary fiber intake and cardiometabolic risks among US adults, NHANES 1999–2010. Am. J. Med. 2013, 126, 1059–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sette, S.; Le Donne, C.; Piccinelli, R.; Arcella, D.; Turrini, A.; Leclercq, C. INRAN-SCAI 2005-6 Study Group. The third Italian National Food Consumption Survey, INRAN-SCAI 2005-06-part 1, Nutrient intakes in Italy. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 922–932. [Google Scholar] [CrossRef]
- Brown, L.; Rosner, B.; Willett, W.W.; Sacks, F.M. Cholesterol-lowering effects of dietary fiber: A meta-analysis. Am. J. Clin. Nutr. 1999, 69, 30–42. [Google Scholar] [CrossRef]
- Whitehead, A.; Beck, E.J.; Tosh, S.; Wolever, T.M. Cholesterol-lowering effects of oat β-glucan: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2014, 100, 1413–1421. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.-H.; Wang, H.; Chen, X.-Y.; Wang, B.-S.; Rong, Z.-X.; Su, B.-H.; Chen, H.-Z. Time- and dose-dependent effect of psyllium on serum lipids in mild-to-moderate hypercholesterolemia: A meta-analysis of controlled clinical trials. Eur. J. Clin. Nutr. 2009, 63, 821–827. [Google Scholar] [CrossRef] [Green Version]
- Sood, N.; Baker, W.L.; Coleman, C.I. Effect of glucomannan on plasma lipid and glucose concentrations, body weight, and blood pressure: Systematic review and meta-analysis. Am. J. Clin. Nutr. 2008, 88, 1167–1175. [Google Scholar] [CrossRef] [Green Version]
- Reppas, C.; Swidan, S.Z.; Tobey, S.W.; Turowski, M.; Dressman, J.B. Hydroxypropylmethylcellulose significantly lowers blood cholesterol in mildly hypercholesterolemic human subjects. Eur. J. Clin. Nutr. 2009, 63, 71–77. [Google Scholar] [CrossRef]
- Singh, B. Psyllium as therapeutic and drug delivery agent. Int. J. Pharm. 2007, 334, 1–14. [Google Scholar] [CrossRef]
- Sadiq Butt, M.; Tahir-Nadeem, M.; Khan, M.K.I.; Shabir, R.; Butt, M.S. Oat: Unique among the cereals. Eur. J. Nutr. 2008, 47, 68–97. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.V.T.; Sievenpiper, J.L.; Zurbau, A.; Blanco Mejia, S.; Jovanovski, E.; Au-Yeung, F.; Jenkins, A.L.; Vuksan, V. A systematic review and meta-analysis of randomized controlled trials of the effect of barley b-glucan on LDL-C, non-HDL-C and apoB for cardiovascular disease risk reduction (i-iv). Eur. J. Clin. Nutr. 2016, 70, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Kranz, S.; Brauchla, M.; Slavin, J.L.; Miller, K.B. What do we know about dietary fiber intake in children and health? The effects of fiber intake on constipation, obesity, and diabetes in children. Adv. Nutr. 2012, 3, 47–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniels, S.R.; Greer, F.R. The Committee on Nutrition. Lipid screening and cardiovascular health in childhood. Pediatrics 2008, 122, 198–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidson, M.H.; Dugan, L.D.; Burns, J.H.; Sugimoto, D.; Story, K.; Drennan, K. A psyllium-enriched cereal for the treatment of hypercholesterolemia in children: A controlled, double-blind, crossover study. Am. J. Clin. Nutr. 1996, 63, 96–102. [Google Scholar] [CrossRef] [Green Version]
- Glassman, M.; Spark, A.; Berezin, S.; Schwarz, S.; Medow, M.; Newman, L.J. Treatment of type IIa hyperlipidemia in childhood by a simplified American Heart Association diet and fiber supplementation. Am. J. Dis. Child. 1990, 144, 193–197. [Google Scholar] [CrossRef]
- Williams, C.L.; Bollella, M.; Spark, A.; Puder, D. Soluble Fiber Enhances the Hypercholesterolemic Effect of the Step I Diet in Childhood. J. Am. Coll. Nutr. 1995, 3, 251–257. [Google Scholar] [CrossRef]
- Ribas, S.; Cunha, D.B.; Sichieri, R.; Da Silva, L.C.S. Effects of psyllium on LDL-Cholesterol concentrations in Brazilian children and adolescents: A randomised, placebo-controlled, parallel clinical trial. Br. J. Nutr. 2015, 113, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Guardamagna, O.; Abello, F.; Cagliero, P.; Visioli, F. Could dyslipidemic children benefit from glucomannan intake? Nutrition 2013, 29, 1060–1065. [Google Scholar] [CrossRef]
- Martino, F.; Puddu, P.E.; Pannarale, G.; Colantoni, C.; Martino, E.; Niglio, T.; Zanoni, C.; Barillà, F. Low dose chromium-polynicotinate or policosanol is effective in hypercholesterolemic children only in combination with glucomannan. Atherosclerosis 2013, 228, 198–202. [Google Scholar] [CrossRef]
- Ho, H.V.T.; Jovanovski, E.; Zurbau, A.; Mejia, S.B.; Sievenpiper, J.L.; Au-Yeung, F.; Jenkins, A.L.; Duvnjak, L.; Leiter, L.; Vuksan, V. A systematic review and meta-analysis of randomized controlled trials of the effect of konjac glucomannan, a viscous soluble fiber, on LDL cholesterol and the new lipid targets non-HDL cholesterol and apolipoprotein B. Am. J. Clin. Nutr. 2017, 105, 1239–1247. [Google Scholar] [CrossRef] [Green Version]
- Jane, M.; McKay, J.; Pal, S. Effects of daily consumption of psyllium, oat bran and polyGlycopleX on obesity-related disease risk factors: A critical review. Nutrition 2019, 57, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Zavoral, J.H.; Hannan, P.; Fields, D.J.; Hanson, M.N.; Frantz, I.D.; Kuba, K.; Elmer, P.; Jacobs, D.R., Jr. The hypolipidemic effect of locust bean gum food products in familial hypercholesterolemic adults and children. Am. J. Clin. Nutr. 1983, 38, 285–294. [Google Scholar] [CrossRef] [Green Version]
- Devaraj, S.; Jialal, I. The role of dietary supplementation with plant sterols and stanols in the prevention of cardiovascular disease. Nutr. Rev. 2006, 64, 348–354. [Google Scholar] [CrossRef]
- Ras, R.T.; Hiemstra, H.; Lin, Y.; Vermeer, M.A.; Duchateau, G.S.M.J.E.; Trautwein, E.A. Consumption of plant sterol-enriched foods and effects on plasma plant sterol concentrations: A meta-analysis of randomized controlled studies. Atherosclerosis 2013, 230, 336–346. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, J.J.; Stojanovski, E.; MacDonald-Wicks, L.; Garg, M.L. Fat type in phytosterol products influence their cholesterol-lowering potential: A systematic review and meta-analysis of RCTs. Prog. Lipid Res. 2016, 64, 16–29. [Google Scholar] [CrossRef]
- Andersson, S.W.; Skinner, J.; Ellegård, L.; Welch, A.A.; Bingham, S.; Mulligan, A.; Andersson, H.; Khaw, K.T. Intake of dietary plant sterols is inversely related to serum cholesterol concentration in men and women in the EPIC Norfolk population: A cross-sectional study. Eur. J. Clin. Nutr. 2004, 58, 1378–1385. [Google Scholar] [CrossRef]
- Klingberg, S.; Ellegård, L.; Johansson, I.; Hallmans, G.; Weinehall, L.; Andersson, H.; Winkvist, A. Inverse relation between dietary intake of naturally occurring plant sterols and serum cholesterol in northern Sweden. Am. J. Clin. Nutr. 2008, 87, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Ribas, S.; Sichieri, R.; Moreira, A.; Souza, D.; Cabral, C.; Gianinni, D.; Cunha, D. Phytosterol-enriched milk lowers LDL-Cholesterol levels in Brazilian children and adolescents: Double-blind, cross-over trial. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 971–977. [Google Scholar] [CrossRef]
- Amundsen, L.; Ose, L.; Nenseter, M.S.; Ntanios, F.Y. Plant sterol ester-enriched spread lowers plasma total and LDL cholesterol in children with familial hypercholesterolemia. Am. J. Clin. Nutr. 2002, 76, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Garoufi, A.; Vorre, S.; Soldatou, A.; Tsentidis, C.; Kossiva, L.; Drakatos, A.; Marmarinos, A.; Gourgiotis, D. Plant sterols-enriched diet decreases small, dense LDL-Cholesterol levels in children with hypercholesterolemia: A prospective study. Ital. J. Pediatr. 2014, 40, 42. [Google Scholar] [CrossRef] [Green Version]
- Patti, A.M.; Katsiki, N.; Nikolic, D.; Al-Rasadi, K.; Rizzo, M. Nutraceuticals in lipid-lowering treatment: A narrative review on the role of chitosan. Angiology 2015, 66, 416–421. [Google Scholar] [CrossRef]
- Baker, W.L.; Tercius, A.; Anglade, M.; White, C.M.; Coleman, C.I. A meta-analysis evaluating the impact of chitosan on serum lipids in hypercholesterolemic patients. Ann. Nutr. Metab. 2008, 55, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.; Giglio, R.V.; Nikolic, D.; Patti, A.M.; Campanella, C.; Cocchi, M.; Katsiki, N.; Montalto, G. Effects of chitosan on plasma lipids and lipoproteins: A 4-month prospective pilot study. Angiology 2014, 65, 538–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mhurchu, C.N.; Poppitt, S.D.; McGill, A.-T.; Leahy, F.E.; Bennett, D.A.; Lin, R.B.; Ormrod, D.; Ward, L.; Strik, C.; Rodgers, A. The effect of the dietary supplement, chitosan, on body weight: A randomised controlled trial in 250 overweight and obese adults. Int. J. Obes. 2004, 28, 1149–1156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilliland, S.E.; Nelson, C.R.; Maxwell, C. Assimilation of cholesterol by Lactobacillus acidophilus. Appl. Environ. Microbiol. 1985, 49, 377–381. [Google Scholar] [CrossRef] [Green Version]
- Mistry, P. Natural cholesterol-lowering products: Focus on probiotics. Br. J. Community Nurs. 2014, 19 (Suppl. 11), S14–S18. [Google Scholar] [CrossRef]
- Kim, G.B.; Yi, S.H.; Lee, B.H. Purification and characterization of three different types of bile salt hydrolases from Bifidobacterium strains. J. Dairy Sci. 2004, 87, 258–266. [Google Scholar] [CrossRef] [Green Version]
- Liong, M.T.; Dunshea, F.R.; Shah, N.P. Effects of a synbiotic containing Lactobacillus acidophilus ATCC 4962 on plasma lipid profiles and morphology of erythrocytes in hypercholesterolaemic pigs on high- and low-fat diets. Br. J. Nutr. 2007, 98, 736–744. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, M.; Hashiguchi, M.; Shiga, T.; Tamura, H.O.; Mochizuki, M. Meta-analysis: Effects of probiotic supplementation on lipid profiles in normal to mildly hypercholesterolemic individuals. PLoS ONE 2015, 10, e0139795. [Google Scholar]
- Guardamagna, O.; Amaretti, A.; Puddu, P.E.; Raimondi, S.; Abello, F.; Cagliero, P.; Rossi, M. Bifidobacteria supplementation: Effects on plasma lipid profiles in dyslipidemic children. Nutrition 2014, 30, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, Y.; Ye, Q.; Li, J.; Hua, Y.; Ju, D.; Zhang, D.; Cooper, R.; Chang, M. Constituents of read yeast rice a traditional Chinese food and Medicine. J. Agic. Food Chem. 2000, 48, 5220–5533. [Google Scholar] [CrossRef] [PubMed]
- Burke, F.M. Red yeast rice for the treatment of dyslipidemia. Curr. Atheroscler. Rep. 2015, 17, 495. [Google Scholar] [CrossRef] [PubMed]
- Heber, D.; Yip, I.; Ashley, J.M.; Elashoff, D.A.; Elashoff, R.M.; Go, V.L. Cholesterol-lowering effects of a proprietary Chinese Red-yeast-rice dietary supplement. Am. J. Clin. Nutr. 1999, 69, 231–236. [Google Scholar] [CrossRef]
- Geralds, M.C.; Terlou, R.J.; Hu, H.; Koks, C.H.; Gerdes, V.E. Traditional Chinese lipid-loweing agent red yeast rice results in significant LDL reduction but safety is uncertain- a systematic rewiev and meta-analysis. Atherosclerosis 2015, 240, 415–423. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.; Kou, W.; Du, B.; Wu, Y.; Zhao, S.; Brusco, O.A.; Morgan, J.M.; Capuzzi, D.M. Effect of Xuezhikang, an extract from red yeast Chinese rice, on coronary events in a Chinese population with previous myocardial infarction. Am. J. Cardiol. 2008, 101, 1689–1693. [Google Scholar] [CrossRef]
- Gordon, R.Y.; Cooperman, T.; Obermeyer, W.; Becker, D.J. Marked variability of monakolin levels in commercial red yeast rice products: Buyer baware! Arch. Intern. Med. 2010, 170, 1722–1727. [Google Scholar] [CrossRef] [Green Version]
- EFSA, Scientific Opinion on the risks for public and animal health related to the presence of citrinin in food and feed. EFSA Panel of Contaminats in the Food Chain (CONTAM). European Food Safety Authoruty (EFSA), Parma, Italy. EFSA J. 2012, 10, 2605. [Google Scholar]
- Pirro, M.; Vetrani, C.; Bianchi, C.; Mannarino, M.R.; Bernini, F.; Rivellese, A.A. Joint position statement on “Nutraceuticals for the treatment of hypercholesterolemia” of the Italian Society for the Study of Arteriosclerosis (SISA). Nutr. Metab. Cardiovasc. Dis. 2017, 27, 2–17. [Google Scholar] [CrossRef] [Green Version]
- Guardamagna, O.; Abello, F.; Baracco, V.; Stasiowska, B.; Martino, F. The treatment of hypercholesterolemic children: Efficacy and safety of a combination of red yeast rice extract and policosanols. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 424–429. [Google Scholar] [CrossRef]
- Gouni-Berthold, I.; Berthold, H.K. Policosanol: Clinical pharmacology and therapeutic significance of a new lipid-lowering agent. Am. Heart J. 2002, 143, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.T.; Wesley, R.; Shamburek, R.D.; Pucino, F.; Csako, G. Meta-analysis of natural therapies for hyperlipidemia: Plant sterols and stanols versus policosanol. Pharmacotherapy 2005, 25, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Greyling, A.; De Witt, C.; Oosthuizen, W.; Jerling, J.C. Effects of a policosanol supplement on serum lipid concentrations in hypercholesterolemic and heterozigous familial hypercholesterolemic subjects. Br. J. Nutr. 2006, 95, 968–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dulin, M.F.; Hatcher, L.F.; Sasser, H.C.; Barringer, T.A. Policosanol in ineffective in the treatment of hypercholesterolemia: A randomized controlled trial. Am. J. Clin. Nutr. 2006, 84, 1543–1548. [Google Scholar] [CrossRef] [Green Version]
- European Food Safety Authority. Scientific Opinion on the substatiation of health claims related to policosanols from sugar cane wax and maintenance of normal blood LDL-cholesterol concentration (ID 1747, 1748, 1864, 1951, 1954, 4693) and maintenance of normal blood HDL-cholesterol concentration (ID 1474, 1478, 1684, 1951, 1954, 4693) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2255. [Google Scholar]
- Di Donna, L.; De Luca, G.; Mazzotti, F.; Napoli, A.; Salerno, R.; Taverna, D.; Sindona, G. Statin-like principles of bergamot fruit (Citrus bergamia): Isolation of 3-hydroxymethylglutaryl flavonoid glycosides. J. Nat. Prod. 2009, 72, 1352–1354. [Google Scholar] [CrossRef]
- Giglio, R.V.; Patti, A.M.; Nikolic, D.; Volti, G.L.; Al-Rasadi, K.; Katsiki, N.; Mikhailidis, D.P.; Montalto, G.; Ivanova, E.; Orekhov, A.N.; et al. The effect of bergamot on dyslipidemia. Phytomedicine 2016, 23, 1175–1181. [Google Scholar] [CrossRef]
- Gliozzi, M.; Walker, R.; Muscoli, S.; Vitale, C.; Gratteri, S.; Carresi, C.; Musolino, V.; Russo, V.; Janda, E.; Ragusa, S.; et al. Bergamot polyphenolic fraction enhances rosuvastatin-induced effect on LDL-cholesterol, LOX-1 expression and protein kinase B phosphorylation in patients with hyperlipideamia. Int. J. Cardiol. 2013, 170, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Mollace, V.; Sacco, I.; Janda, E.; Malara, C.; Ventrice, D.; Colica, C.; Visalli, V.; Muscoli, S.; Ragusa, S.; Muscoli, C.; et al. Hypolipemic and hypoglycaemic activity of bergamot polyphenols: From animal models to human studies. Fitoterapia 2011, 82, 309–316. [Google Scholar] [CrossRef]
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.H.; Nwachukwu, I.; Slusarenko, A.J. Allicin: Chemistry and biological properties. Molecules 2014, 19, 12591–12618. [Google Scholar] [CrossRef] [Green Version]
- Ried, K.; Toben, C.; Fackler, P. Effect of garlic on serum lipids: An updated meta-analisys. Nutr. Rev. 2013, 71, 282–299. [Google Scholar] [CrossRef]
- Ried, K. Garlic lowers blood pressure in hypertensive individuals, regulates serum cholesterol and stimulates immunity: An updated meta-analisys and review. J. Nutr. 2016, 146, 3895–3965. [Google Scholar] [CrossRef] [Green Version]
- Jung, E.-S.; Park, S.-H.; Choi, E.-K.; Ryu, B.-H.; Park, B.-H.; Kim, D.-S.; Kim, Y.-G.; Chae, S.-W. Reduction of blood lipid parameters by a 12-wk supplementation of aged black garlic: A randomized controlled trial. Nutrition 2014, 30, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Morihara, N.; Hino, A. Aged glarlic extract suppresses platelet aggregation by changing property of platelets. J. Nat. Med. 2017, 71, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, R.T.; Mulrow, C.D.; Ramirez, G.; Gardner, C.D.; Morbidoni, L.; Lawrence, V.A. Garlic shows promise for improving some cardiovascular risk factors. Arch. Intern. Med. 2001, 161, 813–824. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.-S.; Zheng, Y.-R.; Zhang, Y.-F.; Long, X.-Y. Research progress on berberine with a special focus on its oral bioavailability. Fitoterapia 2016, 109, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lim, H.J.; Park, J.H.; Lee, K.S.; Jang, Y.; Park, H.Y. Berberine induced LDLR upregulation involves JNK pathway. Biochem. Biophys. Res. Commun. 2007, 362, 853–857. [Google Scholar] [CrossRef]
- Kong, W.; Wei, J.; Abidi, P.; Lin, M.; Inaba, S.; Li, C.; Wang, Y.; Wang, Z.; Si, S.; Pan, H.; et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat. Med. 2004, 10, 1344–1351. [Google Scholar] [CrossRef]
- Cameron, J.; Ranheim, T.; Kulseth, M.A.; Leren, T.P.; Berge, K.E. Berberine decrease PCSK9 expression in HepG2 cells. Atherosclerosis 2008, 201, 266–273. [Google Scholar] [CrossRef]
- Lan, J.; Zhao, Y.; Dong, F.; Yan, Z.; Zheng, W.; Fan, J.; Sun, G. Meta-analysis of the effect and safety of berberine in the treatment of type 2 diabetes mellitus, hyperlipemia and hypertension. J. Ethnopharmacol. 2015, 161, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Wang, N.; Zhao, L.; Lu, F. Berberine in the treatment of type 2 diabetes mellitus: A systemic review and meta-analysis. Evid. Based Complementary Altern. Med. 2012, 2012, 591654. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Zhao, Y.; Zhao, L.; Lu, F. The effects of berberine on blood lipids: A systemic review and meta-analysis of randomized controlled trials. Planta Med. 2013, 79, 437–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Ee, J.H. Soy constituents: Modes of action in low-density lipoprotein management. Nutr. Rev. 2009, 67, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.R. Phytoestrogens: The biochemistry, physiology, and implications for human health of soy isoflavones. Am. J. Clin. Nutr. 1998, 68, 1333S–1346S. [Google Scholar] [CrossRef] [PubMed]
- Descovich, G.C.; Ceredi, C.; Gaddi, A.; Cattin, L.; Senin, U.; Caruzzo, C.; Fragiacomo, C.; Sirtori, M.; Ceredi, C.; Benassi, M.; et al. Multicentre study of soybean protein diet for outpatient hypercholesterolaemic patients. Lancet 1980, 2, 709–712. [Google Scholar] [CrossRef]
- Marlett, J.A. Sites and mechanism for the hypocholesterolemic actions of soluble dietary fiber sources. Adv. Exp. Med. Biol. 1997, 427, 109–121. [Google Scholar] [PubMed]
- Cho, S.J.; Juillerat, M.A.; Lee, C.H. Cholesterol lowering mechanism of soybean protein hydrolysate. J. Agric. Food Chem. 2007, 55, 10599–10604. [Google Scholar] [CrossRef] [PubMed]
- Potter, S.M. Overview of proposed mechanisms for the hypocholesterolemic effect of soy. J. Nutr. 1995, 125 (Suppl. 3), 606S–611S. [Google Scholar]
- Lammi, C.; Zanoni, C.; Scigliuolo, G.M.; D’Amato, A.; Arnoldi, A. Lupin peptides lower low-density lipoprotein (LDL) cholesterol through an up-regulation of the LDL receptor/sterol regulatory lement binding protein 2 (SREBP2) pathway at HepG2 cell line. J. Agric. Food Chem. 2014, 62, 7151–7159. [Google Scholar] [CrossRef]
- Sirtori, C.R.; Galli, C.; Anderson, J.W.; Arnoldi, A. Nutritional and nutraceutical approaches to dyslipidemia and atherosclerosis prevention: Focus on dietary proteins. Atherosclerosis 2009, 203, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Tokede, O.A.; Onabanjo, T.A.; Yansane, A.; Gaziano, J.M.; Djoussé, L. Soya products and serum lipids: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2015, 114, 831–843. [Google Scholar] [CrossRef] [PubMed]
- Bähr, M.; Fechner, A.; Kiehntopf, M.; Jahreis, G. Consuming a mixed diet enriched with lupin protein beneficially affects plasma lipids in hypercholesterolemic subjects: A randomized controlled trial. Clin. Nutr. 2015, 34, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Weghuber, D.; Widhalm, K. Effect of 3-month treatment of children and adolescents with familial and polygenic hypercholesterolaemia with a soya-substituted diet. Br. J. Nutr. 2008, 99, 281–286. [Google Scholar] [CrossRef]
- Helk, O.; Widhalm, K. Effects of a low-fat dietary regimen enriched with soy in children affected with heterozygous familial hypercholesterolemia. Clin. Nutr. 2020, 36, 150–156. [Google Scholar] [CrossRef]
- Sosnowska, B.; Penson, P.; Banach, M. The role of nutraceuticals in the prevention of cardiovascular disease. Cardiovasc. Diagn. Ther. 2017, 7 (Suppl. 1), S21–S31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Way, T.-D.; Lin, H.-Y.; Kuo, D.-H.; Tsai, S.-J.; Shieh, J.-C.; Wu, J.-C.; Lee, M.-R.; Lin, J.-K. Pu-erh tea attenuates hyperlipogenesis and induces hepatoma cells growth arrest through activating AMP-activated protein kinase (AMPK) in human HepG2 cells. J. Agric. Food Chem. 2009, 57, 5257–5264. [Google Scholar] [CrossRef]
- Shishikura, Y.; Khokhar, S.; Murray, B.S. Effects of tea polyphenols on emulsification of olive oil in a small intestine model system. J. Agric. Food Chem. 2006, 54, 1906–1913. [Google Scholar] [CrossRef] [PubMed]
- Lavie, C.J.; Milani, R.V.; Mehera, M.R.; Ventura, H.O. Omega-3 polyunsatured fatty acids and cardiovacsular diseases. J. Am. Coll. Cardiol. 2009, 54, 585–594. [Google Scholar] [CrossRef] [Green Version]
- James, M.J.; Gibson, M.A.; Cleland, L.G. Dietary polyunsatured fatty acids and inflammatory mediator production. Am. J. Clin. Nutr. 2000, 71, 343S–348S. [Google Scholar] [CrossRef]
- Miles, E.A.; Wallace, F.A.; Calder, P.C. Dietary fish oil reduces intercellular adhesion molecule I and scavanger receptor expression on murine macrofages. Atherosclerosis 2000, 152, 43–50. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products. EFSA J. 2010, 8, 1796–1828.
- Miller, M.; Stone, N.J.; Ballantyne, C.; Bittner, V.; Criqui, M.H.; Ginsberg, H.N.; Goldberg, A.C.; Howard, W.J.; Jacobson, M.S.; Kris-Etherton, P.M.; et al. Triglycerides and cardiovascular disease: A scientific statement from the American Heart Association. Circulation 2011, 123, 2292–2333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howe, P.; Mori, T.; Buckley, J. Long chain omega-3 fatty acids and cardiovascular disease-FNSAZ consideration of a commisioned revew. Br. J. Nutr. 2012, 107 (Suppl. 2), S201–S213. [Google Scholar]
- Harris, W.S.; Bulchandani, D. Why do omega-3 fatty acids lower serum triglycerides? Curr. Opin. Lipidol. 2006, 17, 377–393. [Google Scholar] [CrossRef] [PubMed]
- Eslick, G.D.; Howe, C.R.; Smith, C.; Priest, R.; Bensoussan, A. Benefits of fish oil supplementation in hyperlipidemia: A systematic review and mata-analysis. Int. J. Cardiol. 2009, 136, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Leslie, M.A.; Cohen, D.J.; Liddle, D.M.; Robinson, L.E.; Ma, D.W. A review of the effect of omega-3 polyunsatured fatty acids on blood triacylglycerol levels in normolipidemic and borderline hyperlipidemic individuals. Lipids Health Dis. 2015, 14, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burr, M.L.; Fehily, A.M.; Gilbert, J.F.; Rogers, S.; Holliday, R.M.; Sweetnam, P.M.; Elwood, P.C.; Deadman, N.M. Effects of changes in fat, fish and fibre intakes on death and myocardial reinfarction: Diet and reinfarction trial (DART). Lancet 1989, 2, 757–761. [Google Scholar] [CrossRef]
- Dietary supplementation with n-3 polyunsatured fatty acids and vitamin E after myocardial infarcion: Results of the GISSI-Prevenzione trial Gruppo Italiano per lo studio della Sopravvivenza nell’infarto miocardico. Lancet 1999, 354, 447–455. [CrossRef]
- Yokoyama, M.; Origasa, H.; Matsuzaki, M. Effects of eicosapentaenoic acid on major coronary events in hypercholsterolaemic patients (JELIS): A randomised open-label, blinded endpoint analysis. Lancet 2007, 369, 1090–1098. [Google Scholar] [CrossRef]
- Hooper, L.; Thompson, R.L.; Harrison, R.A.; Summerbell, C.D.; Ness, A.R.; Moore, H.J.; Worthington, H.V.; Durrington, P.N.; Higgins, J.P.; Capps, N.E.; et al. Risks and benefits of omega 3 fats for mortality, cardiovascular disease, and cancer: Systematic review. BMJ 2006, 332, 752–760. [Google Scholar] [CrossRef] [Green Version]
- Kwak, S.; Myung, S.; Lee, Y.; Seo, H.G. Korean Meta-analysis Study Group. Efficacy of omega-3 fatty acid supplements in the secondary prevention of cardiovascular disease: A meta-analysis of randomized, double blind, placebo controlled trials. Arch. Intern. Med. 2012, 172, 686–694. [Google Scholar] [PubMed] [Green Version]
- Rizos, E.C.; Ntzani, E.E.; Bika, E.; Kostapanos, M.S.; Elisaf, M.S. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: A systematic review and meta-analysis. JAMA 2012, 308, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Institutes of Health. A Study of AMR101 to Evaluate Its Ability to Reduce Cardiovascular Events in High Risk Patients with Hypertriglyceridemia and on Statin (REDUCE-IT); U.S. National Institutes of Health: Washington, DC, USA, 2016.
- U.S. National Institutes of Health. Outcomes Study to Assess STatin Residual Risk Reduction with EpaNova in HiGh CV Risk PatienTs with Hypertrygliceridemia (STRENGHT); U.S. National Institutes of Health: Washington, DC, USA, 2016.
- Agostoni, C.; Breaegger, C.; Decsi, T.; Kolacek, S.; Mihatsch, W.; Moreno, L.A.; Puntis, J.; Shamir, R.; Szajewska, H.; Turck, D.; et al. Supplementation of n-3 LPUFA in the diet of children older than 2 years: A commentary by the ESPGHAN commitee on nutrition. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 2–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engler, M.M.; Engler, M.B.; Malloy, M.J.; Paul, S.M.; Kulkarni, K.R.; Mietus-Snyder, M.L. Effect of docosahexaenoic acid on lipoprotein subclasses in hyperlipidemic children (the EARLY Study). Am. J. Cardiol. 2005, 95, 869–871. [Google Scholar] [CrossRef] [PubMed]
- Dangardt, F.; Osika, W.; Chen, Y.; Nilsson, U.; Gan, L.M.; Gronowitz, E.; Strandvik, B.; Friberg, P. Omega-3 fatty acid supplementation improves vacular function and reduces inflammation in obese adolescents. Atherosclerosis 2010, 212, 580–585. [Google Scholar] [CrossRef]
- Nobili, V.; Bedogni, G.; Alisi, A.; Pietrobattista, A.; Risé, P.; Galli, C.; Agostoni, C. Docosahexaenoic acid supplementation decreases liver fat content in children with non-alcoholic fatty liver disease: Double blind randomized controlled clinical trial. Arch. Dis. Child. 2011, 96, 350–353. [Google Scholar] [CrossRef]
- Gidding, S.S.; Prospero, C.; Hossain, J.; Zappalla, F.; Balagopal, P.B.; Falkner, B.; Kwiterovich, P. A Double-Blind Randomized Trial of Fish Oil to Lower Triglycerides and Improve Cardiometabolic Risk in Adolescents. J. Pediatr. 2014, 165, 497–503. [Google Scholar] [CrossRef] [Green Version]
- Del Bo, C.; Deon, V.; Abello, F.; Massini, G.; Porrini, M.; Riso, P.; Guardamagna, O. Eight-week hempseed oil intervention improves the fatty acid composition of erythrocyte phospholipids and the omega-3 index, but does not affect the lipid profile in children and adolescents with primary hyperlipidemia. Food Res. Int. 2019, 119, 469–476. [Google Scholar] [CrossRef]
- Rahmani, S.; Asgary, S.; Askary, G.; Keshvari, M.; Hatamipour, M.; Feizi, A.; Sahebkar, A. Treatment of non-alcoholic fatty liver disease with curcumin: A randomized placebo-controlled trial. Phytother. Res. 2016, 30, 1540–1548. [Google Scholar] [CrossRef]
- Kumar, P.; Malhotra, P.; Ma, K.; Singla, A.; Hedroug, O.; Saksena, S.; Dudeja, P.K.; Gill, R.K.; Alrefai, W.A. SREBP2 mediates the modulation of intestinal NPC1L1 expression by curcumin. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G148–G155. [Google Scholar] [CrossRef]
- Liu, X.L.; Liu, M.H.; Hu, H.J.; Feng, H.R.; Fan, X.J.; Zou, W.W.; Pan, Y.Q.; Hu, X.M.; Wang, Z. Curcumin enhanced cholesterol efflux by upregulating ABCA1 expression through AMPK-SIRT1-LXRalpha signaling in THP-1 macrophage-derived foam cells. DNA Cell Biol. 2015, 34, 561–572. [Google Scholar]
- Tai, M.H.; Chen, P.K.; Chen, P.Y.; Wu, M.J.; Ho, C.T.; Yen, J.H. Curcumin enhances cell-surface LDLR level and promotes LDL uptake through downregulation of PCSK9 gene expression in HepG2 cells. Mol. Nutr. Food Res. 2014, 58, 2133–2145. [Google Scholar] [CrossRef]
- Momtazi, A.A.; Derosa, G.; Maffioli, P.; Banach, M.; Sahebkar, A. Role of microRNAs in the therapeutic effects of curcumin in non-cancer diseases. Mol. Diagn. Ther. 2016, 20, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A. A systematic review and meta-analysis of randomized controlled trials investigating the effects of curcumin on blood lipid levels. Clin. Nutr. 2014, 33, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.S.; Su, Y.F.; Yang, H.W.; Lee, Y.H.; Chou, J.I.; Ueng, K.C. Lipid-lowering effects of curcumin in patients with metabolic syndrome: A randomized, double-blind, placebo-controlled trial. Phytother. Res. 2014, 28, 1770–1777. [Google Scholar] [CrossRef] [PubMed]
- DeRosa, G.; Limas, C.P.; Macías, P.C.; Estrella, A.; Maffioli, P. Dietary and nutraceutical approach to type 2 diabetes. Arch. Med. Sci. 2014, 10, 336–344. [Google Scholar] [CrossRef] [Green Version]
- Rahimi, H.R.; Nedaeinia, R.; Sepehri Shamloo, A.; Nikdoust, S.; Kazemi Oskuee, R. Novel delivery system for natural products: Nano-curcumin formulations. Avicenna J. Phytomed. 2016, 6, 383–398. [Google Scholar]
- Cicero, A.F.; Ferroni, A.; Ertek, S. Tolerability and safety of commonly used dietary supplements and nutraceuticals with lipid-lowering effects. Expert Opin. Drug Saf. 2012, 11, 753–766. [Google Scholar] [CrossRef]
- Šimić, I.; Reiner, Ž. Adverse effects of statins—Myths and reality. Curr. Pharm. Des. 2015, 21, 1220. [Google Scholar] [CrossRef]
- Banach, M.; Štulc, T.; Dent, R.; Toth, P.P. Statin non-adherence and residual cardiovascular risk: There is need for substantial improvement. Int. J. Cardiol. 2016, 225, 184–196. [Google Scholar] [CrossRef]
- Alevizos, A.; Mihas, C.; Mariolis, A. Advertising campaigns of sterol-enriched food. An often neglected cause of reduced compliance to lipid lowering drug therapy. Cardiovasc. Drugs Ther. 2007, 21, 133–134. [Google Scholar] [CrossRef] [PubMed]
- Katsarou, A.; Tyrovolas, S.; Psaltopoulou, T.; Zeimbekis, A.; Tsakountakis, N.; Bountziouka, V.; Gotsis, E.; Metallinos, G.; Polychronopoulos, E.; Lionis, C.; et al. Socio-economic status, place of residence and dietary habits among the elderly: The Mediterranean islands study. Public Health Nutr. 2010, 13, 1614–1621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capra, M.E.; Pederiva, C.; Viggiano, C.; De Santis, R.; Banderali, G.; Biasucci, G. Nutritional Approach to Prevention and Treatment of Cardiovascular Disease in Childhood. Nutrients 2021, 13, 2359. [Google Scholar] [CrossRef] [PubMed]
- Pederiva, C.; Capra, M.E.; Viggiano, C.; Rovelli, V.; Banderali, G.; Biasucci, G. Early Prevention of Atherosclerosis: Detection and Management of Hypercholesterolaemia in Children and Adolescents. Life 2021, 11, 34. [Google Scholar] [CrossRef] [PubMed]



|
| Reduce Total Cholesterol | Reduce LDL Cholesterol | Reduce Triglycerides | Increase HDL Cholesterol | |
|---|---|---|---|---|
| Fibres | + | + | ||
| Phytosterols and stanols | + | + | ||
| Probiotics | + | + | ||
| Red yeast rice | + | + | ||
| Soy and lupins | + | + | + | + |
| Omega-3 fatty acids | + |
| Nutraceuticals | Type of Study | Aim | Casuistry | Dose and Duration of the Intervention | Effects | Reference |
|---|---|---|---|---|---|---|
| Psyllium | DB-CO- RCT | Lipid-lowering effect of cereals added to psyllium | 32 children Age: 6–18 years Inclusion criteria: LDL-C ≥ 90th percentile | 8-week diet: 58 g of cereals added to psyllium (6.4 g) or to placebo | ↓ TC: −5% ↓ LDL-C: −6.8% | Davidson MH et al., Am J Clin Nutr 1996 [36] |
| RCT | Reduction of CT and LDL-C after integration with psyllium | 36 children Age: 3–17 years Inclusion criteria: FH | Age ≤ 7 years: 5 g/die Age ≥ 7 years: 10 g/die Duration: 8.0 ± 1.1 months | ↓ TC: −18% ↓ LDL-C: −23% | Glassman M et al., AJDC 1990 [37] | |
| SB-RCT | Effectiveness of psyllium in CT and LDL-C reduction | 50 children Age: 2–11 years Inclusion criteria: LDL-C ≥ 110 mg/dL | CHILD I: all groups. Intervention: cereals containing 3.2 g of psyllium. Duration: 12 weeks | ↓ TC: −9.6% ↓ LDL-C: −15.7% ↑ HDL-C: +9.96% | Williams CL et al., J Am Coll Nutr 1995 [38] | |
| DB-RCT | Effectiveness of psyllium on LDL-C in Brazilians children and teenagers with dyslipidaemia | 51 children Age: 6–19 years Inclusion criteria: TC ≥ 175 mg/dL | CHILD II: 6 weeks. Intervention group: 7 g/die of psyllium Control group: 7.0 g/die of cellulose | ↓ TC: −7.7% ↓ LDL-C: −10.7% | Ribas SA et al., Br J Nutr 2015 [39] | |
| Glucomannan | DB-CO- RCT | Efficacy and tolerability of supplementation with glucomannan | 36 FH children Age: 6–15 years Inclusion criteria: baseline values of CT > 90°percentile by gender and age | CHILD I diet Duration: 4 weeks 2 cps/day of Glucomannan or placebo Duration: 8 weeks Wash-out: 4 weeks | ↓ TC: 5.1% ↓ LDL-C: 7.3% | Guardamagna O et al., Nutrition 2013 [40] |
| DB-RCT | Lipid-lowering effects of glucomannan in combination with CP or PC | 132 children Age: 3–16 years Inclusion criteria: TC ≥ 170 mg/dL, 1 parent with CT 240 mg/dL, or familiarity for CVD | Randomised assignment to 5 neutraceuticals and 1 placebo (only resistant starch) 8-week treatment groups Duration: 8 weeks | GM + CP: ↓ LDL-C: −16% GM + PC: ↓ LDL-C: −10% | Martino F et al., Atherosclerosis 2013 [41] | |
| Phytosterols and stanols | DB-CO-RCT | Lipid-lowering effects of sterols | 30 children Age: 6–9 years Inclusion criteria: TC ≥ 170 mg/dL, LDL-C ≥ 110 mg/dL | Intervention group: milk +1.2 g/day of sterols vegetable. Control group: skim milk Duration: 8 weeks | Intervention group: ↓ TC: −4.5% ↓ LDL-C: −11.1% Control group: TC: +1.4 ↓ LDL-C: −0.9% | Ribas SA et al., NMCD 2017 [50] |
| DB-CO-RCT | Lipid-lowering effects of stanols | 38 children Age: 7–12 years Inclusion criteria: “Definite” diagnosis or “possible” diagnosis of FH | CHILD I + 1.6 g of stanols or placebo Duration: 8 weeks | ↓ LDL-C 10.2% ↓ TC and ApoB 7.4% | Amundsen AL et al., Am J Clin Nutr 2002 [51] | |
| CT | Effects of sterols on LDL-C levels | 64 children 25 LDL-C ≥ 130 mg/dL 34 LDL-C ≤ 130 mg/dL Age: 4.5–15.9 years | CHILD II Intervention group: yoghurt (2 g/die sterols) Duration: 6–12 months | Intervention group: ↓ LDL-C: −13% | Garoufi A et al., IJP 2014 [52] | |
| Red yeast rice | DB-CO-RCT | Efficacy and safety of a combination of red yeast rice extract and policosanols | 80 children Age: 8–16 years | CHILD I red yeast rice 200 mg/die + policosanols 10 mg/ placebo Duration: 8 weeks Wash-out: 4 weeks | ↓ TC: 18.5% ↓ LDL-C: 25.1% ↓ ApoB: 25.3% | Guardamagna O et al., NMCD 2011 [71] |
| Soy | RCT | The effect of integration with protein of soy on lipoproteins | 23 children Age: 4–18 years Inclusion criteria: FH | Step 1: diet Duration: 3 months Step 2: diet + soya protein 0.25 g/kg in replacement animal protein. Duration: 3 months | Step 1: ↓ TC: −12.3% ↓ LDL-C: −11.8% ↓ ApoB: −10.6% Step 2: ↓ TC: −7.7% ↓ LDL-C: −6.4% ↓ ApoB: −12.6% | Weghuber D et al., Br J Nutr 2008 [104] |
| RCT | The effect of soy on LDL-C levels | 17 children Age: 5–13 years Inclusion criteria: FH | Soy group: soy-enriched fat modified diet Control group: fat modified diet Duration: 13 weeks | LDL-C decrease: statistically significantly greater in the soy group | Helk O et al., Clin Nutr 2020 [105] | |
| Omega-3 polyunsaturated long-chain fatty acids | DB-CO-RCT | The efficacy of fish oil in lowering TG and impacting lipoprotein particles | 42 children Age: 10–17 years Inclusion criteria: TG ≥ 150 mg/dL and <750 mg/dL, LDL-C <160 mg/dL | Intervention group: 4 g daily of fish oil Control group: placebo Duration: 8 weeks | TG decrease: greater in the intervention group | Gidding SS et al., J Pediatr 2014 [130] |
| RCT | The effectiveness of hempseed oil in the modulation of hyperlipidaemia and evaluation of fatty acid composition of red blood cells | 36 children Age: 6–16 years Inclusion criteria: hyperlipidaemia primitive and compliance to the alimentary indications | Control group: CHILD I Intervention group: hempseed oil 3 g/die Duration: 8 weeks | Intervention group: ↓ RC SFA: −5.02% ↓ RC MUFA: −2.12% ↑ PUFA n − 3: + 1.57% ↑ PUFAs n − 6: +5.39% ↑ Omega 3 Index: 1.18% ↓ LDL-C: 14.2%, Control group: ↓ LDL-C: −4.94% | del Bo’ C et al., Food Res Int. 2019 [131] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banderali, G.; Capra, M.E.; Viggiano, C.; Biasucci, G.; Pederiva, C. Nutraceuticals in Paediatric Patients with Dyslipidaemia. Nutrients 2022, 14, 569. https://doi.org/10.3390/nu14030569
Banderali G, Capra ME, Viggiano C, Biasucci G, Pederiva C. Nutraceuticals in Paediatric Patients with Dyslipidaemia. Nutrients. 2022; 14(3):569. https://doi.org/10.3390/nu14030569
Chicago/Turabian StyleBanderali, Giuseppe, Maria Elena Capra, Claudia Viggiano, Giacomo Biasucci, and Cristina Pederiva. 2022. "Nutraceuticals in Paediatric Patients with Dyslipidaemia" Nutrients 14, no. 3: 569. https://doi.org/10.3390/nu14030569






