Preventive Effect of a Postbiotic and Prebiotic Mixture in a Rat Model of Early Life Rotavirus Induced-Diarrhea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
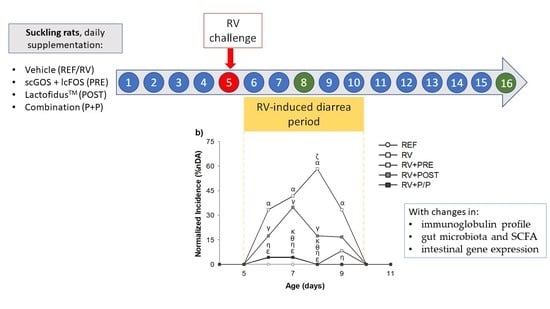
2.2. Experimental Design
2.3. Clinical Evaluation and Fecal Specimen Collection Design
2.4. Stool and Stomach Content pH
2.5. Fecal SA-11 Shedding
2.6. In Vitro Blocking Assay
2.7. Intestinal Permeability Assay
2.8. Anti-RV Antibodies
2.9. Quantification of Immunoglobulins
2.10. Gene Expression Analysis
2.11. Colorectal Microbiota Composition
2.12. Quantification of Short-Chain Fatty Acids in the Cecal Content
2.13. Statistical Analysis
3. Results
3.1. Growth and Morphometry
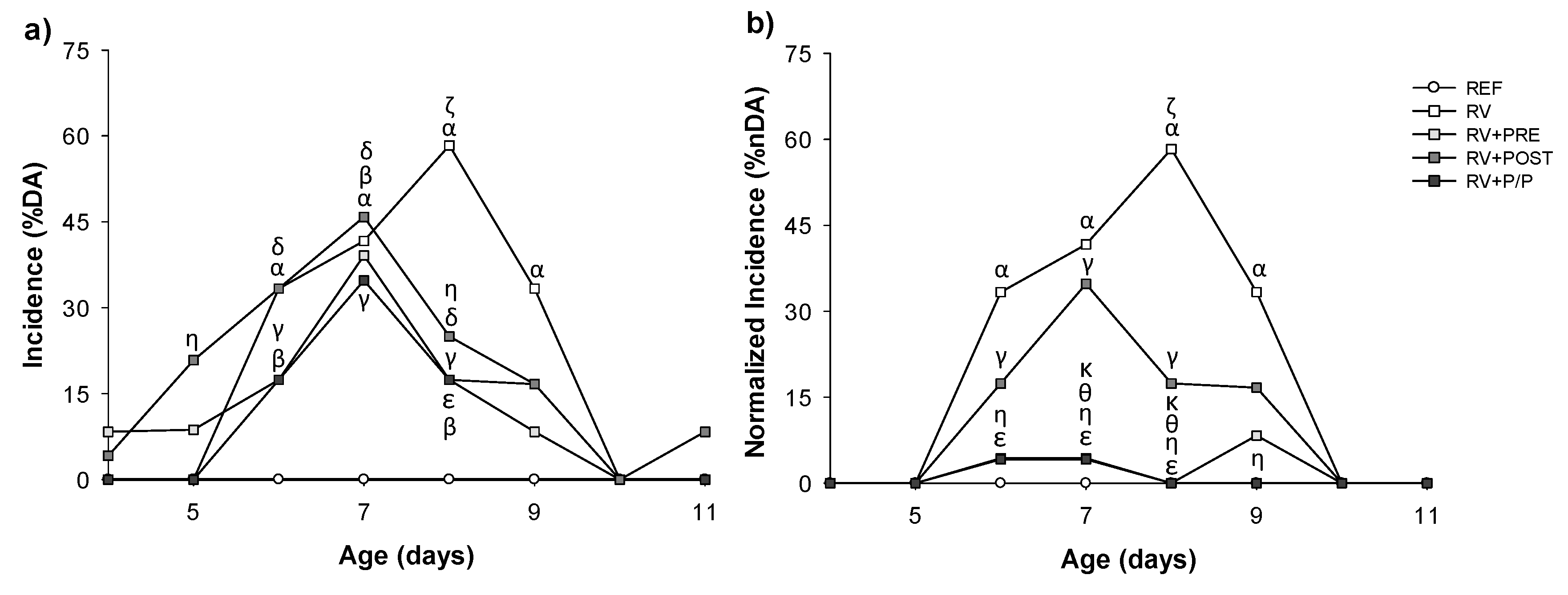
3.2. Incidence and Duration of the Diarrhea Process
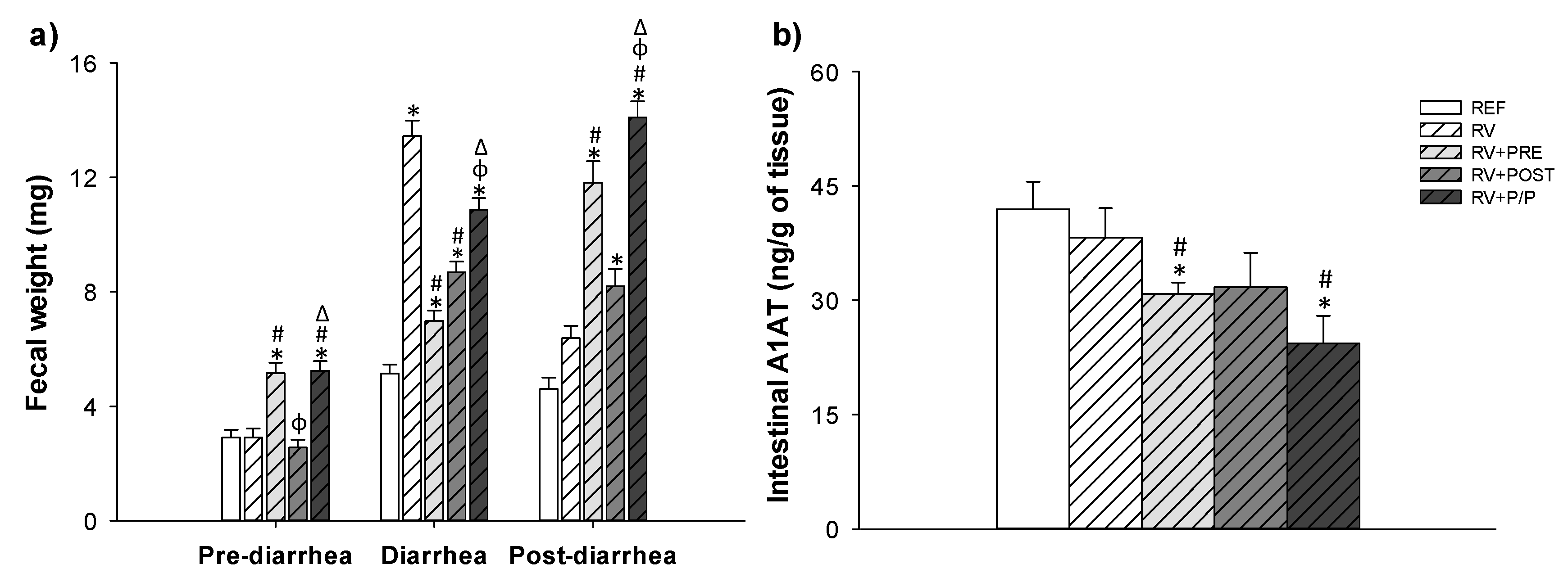
3.3. Severity of Diarrhea
3.4. Intestinal Permeability
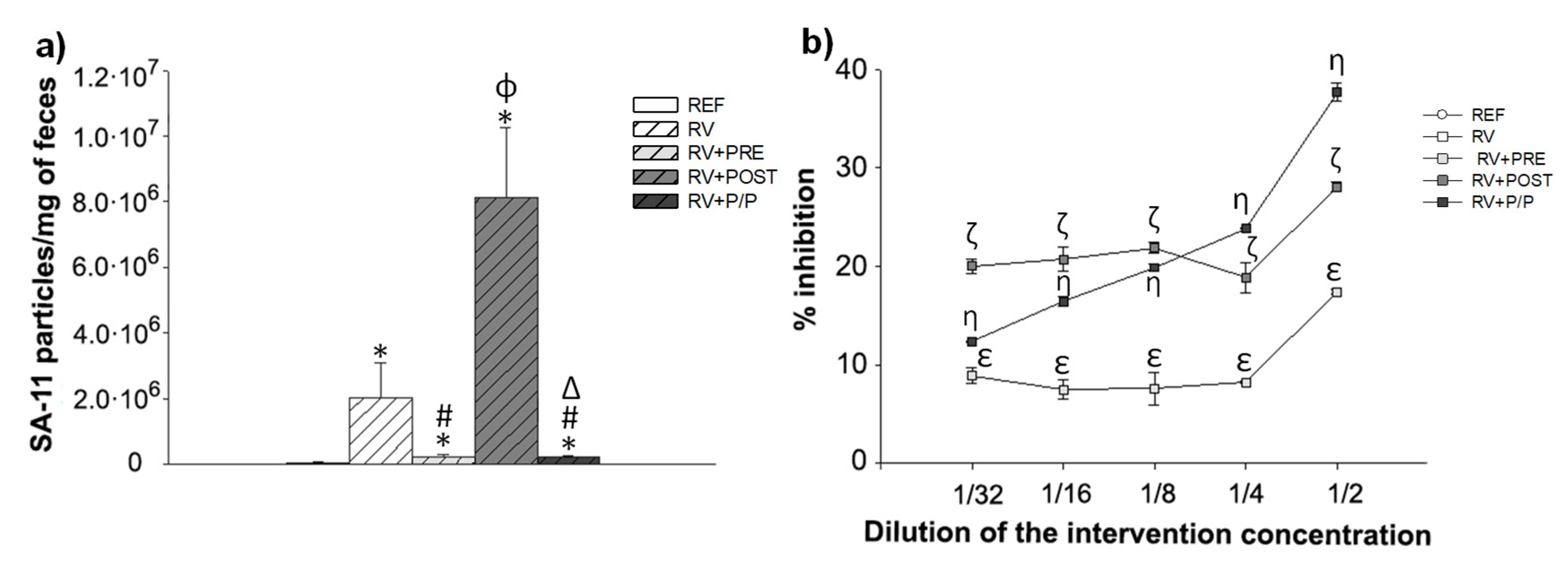
3.5. Fecal SA-11 Shedding and Blocking Assay
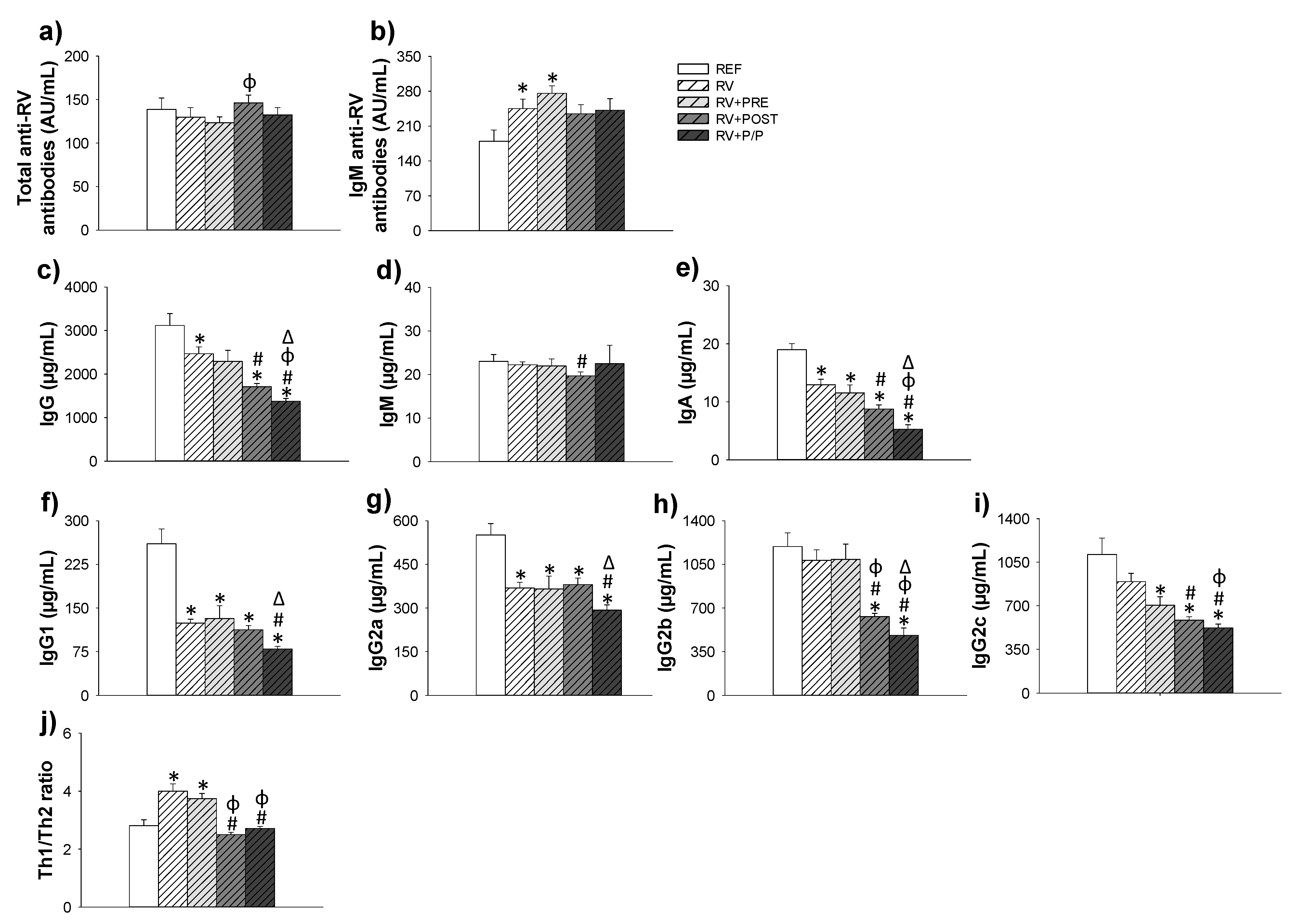
3.6. Antibody Production
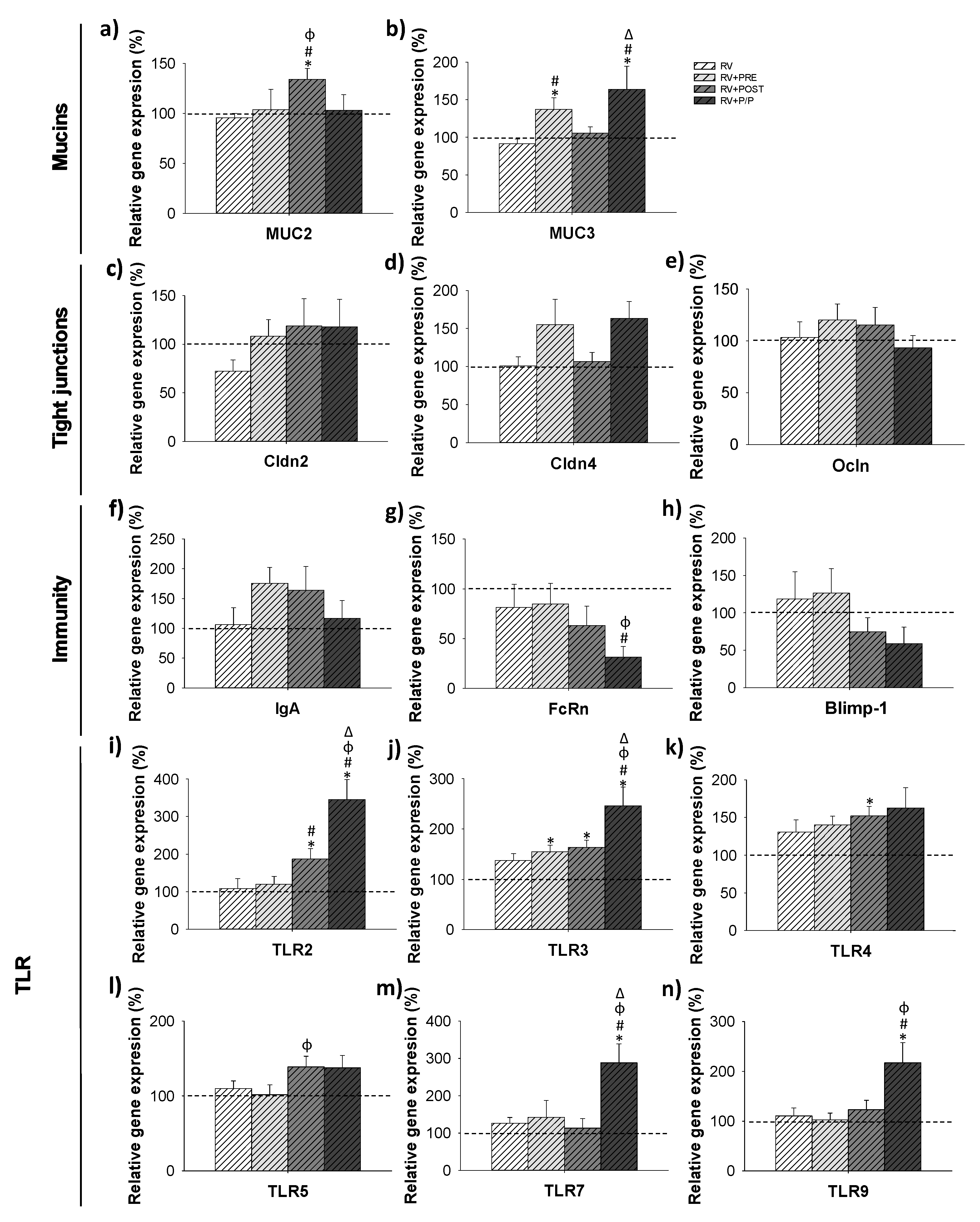
3.7. Gene Expression Analysis
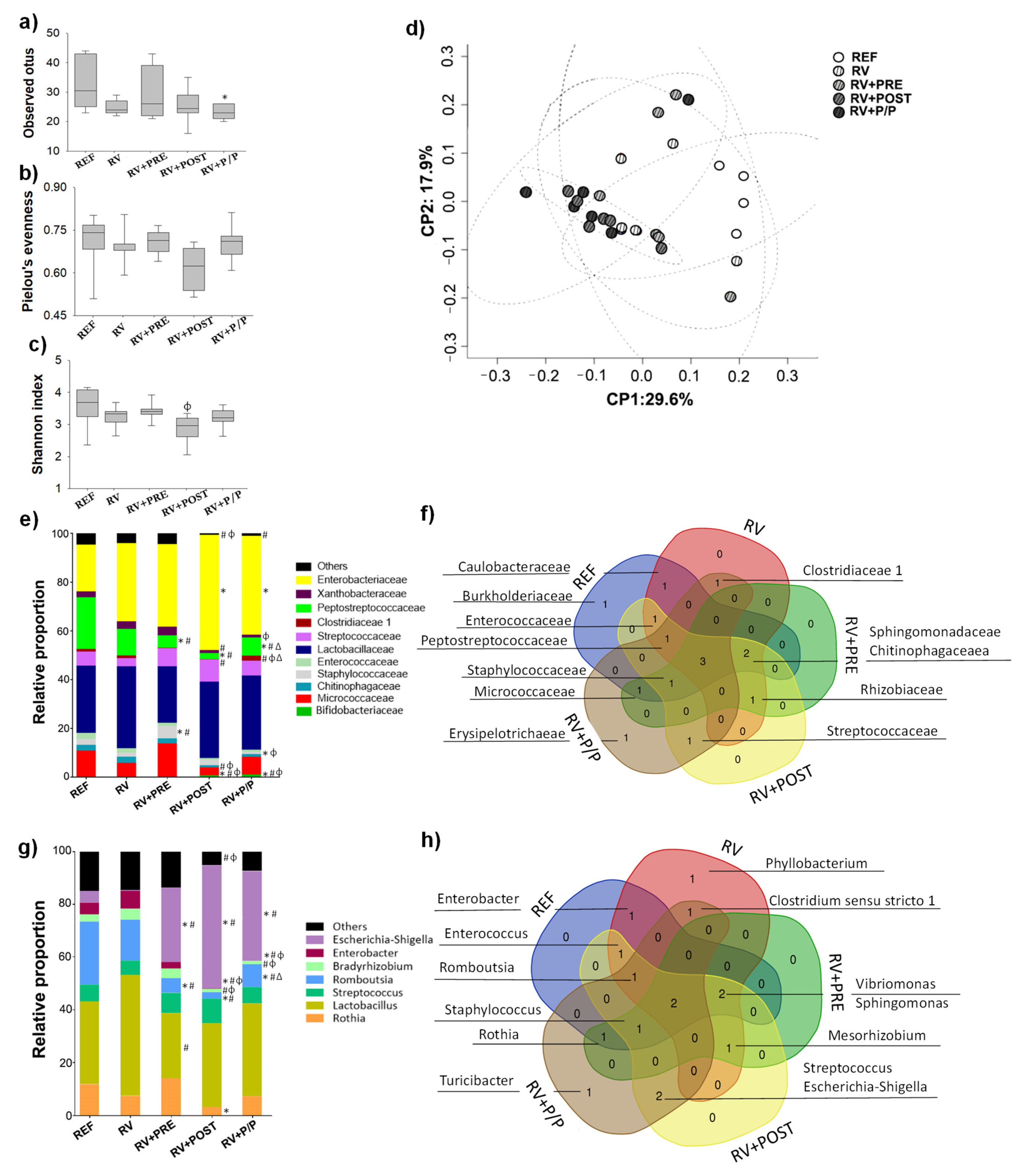
3.8. Colorectal Microbiota Composition
3.9. Quantification of Short-Chain Fatty Acids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crawford, S.E.; Ramani, S.; Tate, J.E.; Parashar, U.D.; Svensson, L.; Hagbom, M.; Franco, M.A.; Greenberg, H.B.; O’Ryan, M.; Kang, G.; et al. Rotavirus infection. Nat. Rev. Dis. Prim. 2017, 3, 17083. [Google Scholar] [CrossRef] [Green Version]
- Seid, U.; Ahmednur, M.; Dawo, F. Rotavirus in Calves and Its Zoonotic Importance. Vet. Med. Int. 2021, 2021, 6639701. [Google Scholar] [CrossRef]
- Megumi, F.; Dall, A.M.; Arruda, R.; Silva, T.; Arlington, S.; Fernandes, T.; Gonçalves, A.; Fernandes, A.; Alcindo, A. Porcine rotavirus B as primary causative agent of diarrhea outbreaks in newborn piglets. Affiliations expand. Sci. Rep. 2020, 10, 22002. [Google Scholar] [CrossRef]
- Sadiq, A.; Bostan, N.; Yinda, K.C.; Naseem, S.; Sattar, S. Rotavirus: Genetics, pathogenesis and vaccine advances. Rev. Med. Virol. 2018, 28, e2003. [Google Scholar] [CrossRef]
- Parashar, U.D.; Nelson, E.A.S.; Kang, G. Diagnosis, management, and prevention of rotavirus gastroenteritis in children. BMJ 2013, 347, f7204. [Google Scholar] [CrossRef] [Green Version]
- Martin, C.; Ling, P.-R.; Blackburn, G. Review of Infant Feeding: Key Features of Breast Milk and Infant Formula. Nutrients 2016, 8, 279. [Google Scholar] [CrossRef] [Green Version]
- Fernández, L.; Langa, S.; Martín, V.; Maldonado, A.; Jiménez, E.; Martín, R.; Rodríguez, J.M. The human milk microbiota: Origin and potential roles in health and disease. Pharmacol. Res. 2013, 69, 1–10. [Google Scholar] [CrossRef]
- Szajewska, H.; Skórka, A.; Pieścik-Lech, M. Fermented infant formulas without live bacteria: A systematic review. Eur. J. Pediatr. 2015, 174, 1413–1420. [Google Scholar] [CrossRef]
- Salminen, S.; Stahl, B.; Vinderola, G.; Szajewska, H. Infant Formula Supplemented with Biotics: Current Knowledge and Future Perspectives. Nutrients 2020, 12, 1952. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Rigo-Adrover, M.; Pérez-Berezo, T.; Ramos-Romero, S.; van Limpt, K.; Knipping, K.; Garssen, J.; Knol, J.; Franch, À.; Castell, M.; Pérez-Cano, F.J. A fermented milk concentrate and a combination of short-chain galacto-oligosaccharides/long-chain fructo-oligosaccharides/pectin-derived acidic oligosaccharides protect suckling rats from rotavirus gastroenteritis. Br. J. Nutr. 2017, 117, 209–217. [Google Scholar] [CrossRef] [Green Version]
- Arslanoglu, S.; Moro, G.E.; Schmitt, J.; Tandoi, L.; Rizzardi, S.; Boehm, G. Early Dietary Intervention with a Mixture of Prebiotic Oligosaccharides Reduces the Incidence of Allergic Manifestations and Infections during the First Two Years of Life. J. Nutr. 2008, 138, 1091–1095. [Google Scholar] [CrossRef]
- Azagra-Boronat, I.; Massot-Cladera, M.; Knipping, K.; Van’t Land, B.; Stahl, B.; Garssen, J.; Rodríguez-Lagunas, M.J.; Franch, À.; Castell, M.; Pérez-Cano, F.J. Supplementation With 2′-FL and scGOS/lcFOS Ameliorates Rotavirus-Induced Diarrhea in Suckling Rats. Front. Cell. Infect. Microbiol. 2018, 8, 372. [Google Scholar] [CrossRef]
- Bruzzese, E.; Volpicelli, M.; Squeglia, V.; Bruzzese, D.; Salvini, F.; Bisceglia, M.; Lionetti, P.; Cinquetti, M.; Iacono, G.; Amarri, S.; et al. A formula containing galacto- and fructo-oligosaccharides prevents intestinal and extra-intestinal infections: An observational study. Clin. Nutr. 2009, 28, 156–161. [Google Scholar] [CrossRef] [Green Version]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Shin, D.; Chang, S.Y.; Bogere, P.; Won, K.; Choi, J.-Y.; Choi, Y.-J.; Lee, H.K.; Hur, J.; Park, B.-Y.; Kim, Y.; et al. Beneficial roles of probiotics on the modulation of gut microbiota and immune response in pigs. PLoS ONE 2019, 14, e0220843. [Google Scholar] [CrossRef] [Green Version]
- Wegh, C.A.M.; Geerlings, S.Y.; Knol, J.; Roeselers, G.; Belzer, C. Postbiotics and Their Potential Applications in Early Life Nutrition and Beyond. Int. J. Mol. Sci. 2019, 20, 4673. [Google Scholar] [CrossRef] [Green Version]
- Borriello, S.P.; Hammes, W.P.; Holzapfel, W.; Marteau, P.; Schrezenmeir, J.; Vaara, M.; Valtonen, V. Safety of Probiotics That Contain Lactobacilli or Bifidobacteria. Clin. Infect. Dis. 2003, 36, 775–780. [Google Scholar] [CrossRef]
- Rowan, N.J.; Deans, K.; Anderson, J.G.; Gemmell, C.G.; Hunter, I.S.; Chaithong, T. Putative Virulence Factor Expression by Clinical and Food Isolates of Bacillus spp. after Growth in Reconstituted Infant Milk Formulae. Appl. Environ. Microbiol. 2001, 67, 3873–3881. [Google Scholar] [CrossRef] [Green Version]
- Gueimonde, M.; Sánchez, B.; de los Reyes-Gavilán, C.G.; Margolles, A. Antibiotic resistance in probiotic bacteria. Front. Microbiol. 2013, 4, 202. [Google Scholar] [CrossRef] [Green Version]
- de Almada, C.N.; Almada, C.N.; Martinez, R.C.R.; Sant’Ana, A.S. Paraprobiotics: Evidences on their ability to modify biological responses, inactivation methods and perspectives on their application in foods. Trends Food Sci. Technol. 2016, 58, 96–114. [Google Scholar] [CrossRef]
- Malagón-Rojas, J.N.; Mantziari, A.; Salminen, S.; Szajewska, H. Postbiotics for Preventing and Treating Common Infectious Diseases in Children: A Systematic Review. Nutrients 2020, 12, 389. [Google Scholar] [CrossRef] [Green Version]
- Morales-Ferré, C.; Azagra-Boronat, I.; Massot-Cladera, M.; Tims, S.; Knipping, K.; Garssen, J.; Knol, J.; Franch, À.; Castell, M.; Rodríguez-Lagunas, M.J.; et al. Effects of a Postbiotic and Prebiotic Mixture on Suckling Rats’ Microbiota and Immunity. Nutrients 2021, 13, 2975. [Google Scholar] [CrossRef]
- Gao, J.; Li, Y.; Wan, Y.; Hu, T.; Liu, L.; Yang, S.; Gong, Z.; Zeng, Q.; Wei, Y.; Yang, W.; et al. A Novel Postbiotic From Lactobacillus rhamnosus GG With a Beneficial Effect on Intestinal Barrier Function. Front. Microbiol. 2019, 10, 477. [Google Scholar] [CrossRef] [Green Version]
- Popović, N.; Djokić, J.; Brdarić, E.; Dinić, M.; Terzić-Vidojević, A.; Golić, N.; Veljović, K. The Influence of Heat-Killed Enterococcus faecium BGPAS1-3 on the Tight Junction Protein Expression and Immune Function in Differentiated Caco-2 Cells Infected With Listeria monocytogenes ATCC 19111. Front. Microbiol. 2019, 10, 412. [Google Scholar] [CrossRef]
- Dunand, E.; Burns, P.; Binetti, A.; Bergamini, C.; Peralta, G.H.; Forzani, L.; Reinheimer, J.; Vinderola, G. Postbiotics produced at laboratory and industrial level as potential functional food ingredients with the capacity to protect mice against Salmonella infection. J. Appl. Microbiol. 2019, 127, 219–229. [Google Scholar] [CrossRef]
- Rigo-Adrover, M.; Saldaña-Ruíz, S.; van Limpt, K.; Knipping, K.; Garssen, J.; Knol, J.; Franch, A.; Castell, M.; Pérez-Cano, F.J. A combination of scGOS/lcFOS with Bifidobacterium breve M-16V protects suckling rats from rotavirus gastroenteritis. Eur. J. Nutr. 2017, 56, 1657–1670. [Google Scholar] [CrossRef]
- Isolauri, E.; Juntunen, M.; Rautanen, T.; Sillanaukee, P.; Koivula, T. A human Lactobacillus strain (Lactobacillus casei sp strain GG) promotes recovery from acute diarrhea in children. Pediatrics 1991, 88, 90–97. [Google Scholar]
- Oli, M.W.; Petschow, B.W.; Buddington, R.K. Evaluation of fructooligosaccharide supplementation of oral electrolyte solutions for treatment of diarrhea. Recovery of the intestinal bacteria. Dig. Dis. Sci. 1998, 43, 138–147. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Azagra-Boronat, I.; Massot-Cladera, M.; Mayneris-Perxachs, J.; Knipping, K.; Van’t Land, B.; Tims, S.; Stahl, B.; Garssen, J.; Franch, À.; Castell, M.; et al. Immunomodulatory and Prebiotic Effects of 2′-Fucosyllactose in Suckling Rats. Front. Immunol. 2019, 10, 1773. [Google Scholar] [CrossRef] [Green Version]
- Rigo-Adrover, M.M.; van Limpt, K.; Knipping, K.; Garssen, J.; Knol, J.; Costabile, A.; Franch, À.; Castell, M.; Pérez-Cano, F.J. Preventive Effect of a Synbiotic Combination of Galacto- and Fructooligosaccharides Mixture With Bifidobacterium breve M-16V in a Model of Multiple Rotavirus Infections. Front. Immunol. 2018, 9, 1318. [Google Scholar] [CrossRef] [Green Version]
- Ayechu-Muruzabal, V.; Xiao, L.; Wehkamp, T.; van Ark, I.; Hoogendoorn, E.J.; Leusink-Muis, T.; Folkerts, G.; Garssen, J.; Willemsen, L.E.M.; van’t Land, B. A Fermented Milk Matrix Containing Postbiotics Supports Th1- and Th17-Type Immunity In Vitro and Modulates the Influenza-Specific Vaccination Response In Vivo in Association with Altered Serum Galectin Ratios. Vaccines 2021, 9, 254. [Google Scholar] [CrossRef]
- Abrahamse, E.; Huybers, S.; Alles, M.S.; Renes, I.B.; Knol, J.; Bouritius, H.; Ludwig, T. Fermented infant formula increases ileal protein digestibility and reduces ileal proteolytic activity compared with standard and hydrolyzed infant formulas in piglets. J. Nutr. 2015, 145, 1423–1428. [Google Scholar] [CrossRef] [Green Version]
- Vandenplas, Y.; de Halleux, V.; Arciszewska, M.; Lach, P.; Pokhylko, V.; Klymenko, V.; Schoen, S.; Abrahamse-Berkeveld, M.; Mulder, K.; Porcel Rubio, R. A Partly Fermented Infant Formula with Postbiotics Including 3′-GL, Specific Oligosaccharides, 2′-FL, and Milk Fat Supports Adequate Growth, Is Safe and Well-Tolerated in Healthy Term Infants: A Double-Blind, Randomised, Controlled, Multi-Country Trial. Nutrients 2020, 12, 3560. [Google Scholar] [CrossRef]
- Rodriguez-herrera, A.; Mulder, K.; Bouritius, H.; Rubio, R.; Muñoz, A.; Agosti, M.; Lista, G.; Corvaglia, L.; Ludwig, T.; Abrahamse-berkeveld, M.; et al. Gastrointestinal Tolerance, Growth and Safety of a Partly Fermented Formula with Specific Prebiotics in Healthy Infants: A Double-Blind, Randomized, Controlled Trial. Nutrients 2019, 11, 1530. [Google Scholar] [CrossRef] [Green Version]
- Rigo-Adrover, M.; Knipping, K.; Garssen, J.; van Limpt, K.; Knol, J.; Franch, À.; Castell, M.; Rodríguez-lagunas, M.; Pérez-Cano, F. Prevention of Rotavirus Diarrhea in Suckling Rats by a Specific Fermented Milk Concentrate with Prebiotic Mixture. Nutrients 2019, 11, 189. [Google Scholar] [CrossRef] [Green Version]
- Azagra-Boronat, I.; Massot-Cladera, M.; Knipping, K.; Van’t Land, B.; Tims, S.; Stahl, B.; Knol, J.; Garssen, J.; Franch, À.; Castell, M.; et al. Oligosaccharides Modulate Rotavirus-Associated Dysbiosis and TLR Gene Expression in Neonatal Rats. Cells 2019, 8, 876. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Cano, F.J.; Castell, M.; Castellote, C.; Franch, À. Characterization of clinical and immune response in a rotavirus diarrhea model in suckling Lewis rats. Pediatr. Res. 2007, 62, 658–663. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [Green Version]
- Morales-Ferré, C.; Azagra-Boronat, I.; Massot-Cladera, M.; Franch, À.; Castell, M.; Rodríguez-Lagunas, M.J.; Pérez-Cano, F.J. Sexual Dimorphism Has Low Impact on the Response against Rotavirus Infection in Suckling Rats. Vaccines 2020, 8, 345. [Google Scholar] [CrossRef]
- Anderson, M.J.; Walsh, D.C.I. PERMANOVA, ANOSIM, and the Mantel test in the face of heterogeneous dispersions: What null hypothesis are you testing? Ecol. Monogr. 2013, 83, 557–574. [Google Scholar] [CrossRef]
- Mandal, S.; Van Treuren, W.; White, R.A.; Eggesbø, M.; Knight, R.; Peddada, S.D. Analysis of composition of microbiomes: A novel method for studying microbial composition. Microb. Ecol. Health Dis. 2015, 26, 27663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huet, F.; Abrahamse-Berkeveld, M.; Tims, S.; Simeoni, U.; Beley, G.; Savagner, C.; Vandenplas, Y.; Hourihane, J.O.B. Partly Fermented Infant Formulae With Specific Oligosaccharides Support Adequate Infant Growth and Are Well-Tolerated. J. Pediatr. Gastroenterol. Nutr. 2016, 63, e43–e53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Indrio, F.; Ladisa, G.; Mautone, A.; Montagna, O. Effect of a fermented formula on thymus size and stool pH in healthy term infants. Pediatr. Res. 2007, 62, 98–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowman, E.P.; Kuklin, N.A.; Youngman, K.R.; Lazarus, N.H.; Kunkel, E.J.; Pan, J.; Greenberg, H.B.; Butcher, E.C. The intestinal chemokine thymus-expressed chemokine (CCL25) attracts IgA antibody-secreting cells. J. Exp. Med. 2002, 195, 269–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thibault, H.; Aubert-Jacquin, C.; Goulet, O. Effects of Long-term Consumption of a Fermented Infant Formula (with Bifidobacterium breve c50 and Streptococcus thermophilus 065) on Acute Diarrhea in Healthy Infants. J. Pediatr. Gastroenterol. Nutr. 2004, 39, 147–152. [Google Scholar] [CrossRef]
- Rao, S.; Srinivasjois, R.; Patole, S. Prebiotic Supplementation in Full-term Neonates. Arch. Pediatr. Adolesc. Med. 2009, 163, 755–764. [Google Scholar] [CrossRef] [Green Version]
- Beasley, D.E.; Koltz, A.M.; Lambert, J.E.; Fierer, N.; Dunn, R.R. The Evolution of Stomach Acidity and Its Relevance to the Human Microbiome. PLoS ONE 2015, 10, e0134116. [Google Scholar] [CrossRef]
- Yang, X.; Twitchell, E.; Li, G.; Wen, K.; Weiss, M.; Kocher, J.; Lei, S.; Ramesh, A.; Ryan, E.P.; Yuan, L. High protective efficacy of rice bran against human rotavirus diarrhea via enhancing probiotic growth, gut barrier function, and innate immunity. Sci. Rep. 2015, 5, 15004. [Google Scholar] [CrossRef]
- Krumbeck, J.A.; Rasmussen, H.E.; Hutkins, R.W.; Clarke, J.; Shawron, K.; Keshavarzian, A.; Walter, J. Probiotic Bifidobacterium strains and galactooligosaccharides improve intestinal barrier function in obese adults but show no synergism when used together as synbiotics. Microbiome 2018, 6, 121. [Google Scholar] [CrossRef] [PubMed]
- Pelaseyed, T.; Bergström, J.H.; Gustafsson, J.K.; Ermund, A.; Birchenough, G.M.H.; Schütte, A.; van der Post, S.; Svensson, F.; Rodríguez-Piñeiro, A.M.; Nyström, E.E.L.; et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol. Rev. 2014, 260, 8–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leforestier, G.; Blais, A.; Blachier, F.; Marsset-Baglieri, A.; Davila-Gay, A.M.; Perrin, E.; Tomé, D. Effects of galacto-oligosaccharide ingestion on the mucosa-associated mucins and sucrase activity in the small intestine of mice. Eur. J. Nutr. 2009, 48, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, Y.; Katayama, T. Consumption of non-digestible oligosaccharides elevates colonic alkaline phosphatase activity by up-regulating the expression of IAP-I, with increased mucins and microbial fermentation in rats fed a high-fat diet. Br. J. Nutr. 2019, 121, 146–154. [Google Scholar] [CrossRef]
- Figueroa-Lozano, S.; Ren, C.; Yin, H.; Pham, H.; Van Leeuwen, S.; Dijkhuizen, L.; De Vos, P. The impact of oligosaccharide content, glycosidic linkages and lactose content of galacto-oligosaccharides (GOS) on the expression of mucus-related genes in goblet cells. Food Funct. 2020, 11, 3506–3515. [Google Scholar] [CrossRef]
- Paradis, T.; Bègue, H.; Basmaciyan, L.; Dalle, F.; Bon, F. Tight Junctions as a Key for Pathogens Invasion in Intestinal Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 2506. [Google Scholar] [CrossRef]
- Suzuki, T. Regulation of the intestinal barrier by nutrients: The role of tight junctions. Anim. Sci. J. 2020, 91, e13357. [Google Scholar] [CrossRef] [Green Version]
- Alizadeh, A.; Akbari, P.; Difilippo, E.; Schols, H.A.; Ulfman, L.H.; Schoterman, M.H.C.; Garssen, J.; Fink-Gremmels, J.; Braber, S. The piglet as a model for studying dietary components in infant diets: Effects of galacto-oligosaccharides on intestinal functions. Br. J. Nutr. 2016, 115, 605–618. [Google Scholar] [CrossRef] [Green Version]
- Arévalo Sureda, E.; Weström, B.; Pierzynowski, S.G.; Prykhodko, O. Maturation of the Intestinal Epithelial Barrier in Neonatal Rats Coincides with Decreased FcRn Expression, Replacement of Vacuolated Enterocytes and Changed Blimp-1 Expression. PLoS ONE 2016, 11, e0164775. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Kagan, J.C. Toll-like Receptors and the Control of Immunity. Cell 2020, 180, 1044–1066. [Google Scholar] [CrossRef]
- Han, C.; Dai, Y.; Liu, B.; Wang, L.; Wang, J.; Zhang, J. Diversity analysis of intestinal microflora between healthy and diarrheal neonatal piglets from the same litter in different regions. Anaerobe 2019, 55, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Béghin, L.; Tims, S.; Roelofs, M.; Rougé, C.; Oozeer, R.; Rakza, T.; Chirico, G.; Roeselers, G.; Knol, J.; Rozé, J.C.; et al. Fermented infant formula (with Bifidobacterium breve C50 and Streptococcus thermophilus O65) with prebiotic oligosaccharides is safe and modulates the gut microbiota towards a microbiota closer to that of breastfed infants. Clin. Nutr. 2021, 40, 778–787. [Google Scholar] [CrossRef] [PubMed]


| REF | RV | RV + PRE | RV + POST | RV + P/P | |
|---|---|---|---|---|---|
| Growth measurements | |||||
| Naso-anal length (Body, cm) | 6.82 ± 0.11 | 6.95 ± 0.09 | 6.97 ± 0.05 | 7.08 ± 0.11 | 6.91 ± 0.09 |
| Anus-tail length (Tail, cm) | 3.04 ± 0.03 | 3.06 ± 0.05 | 3.03 ± 0.04 | 3.12 ± 0.06 | 2.92 ± 0.07 |
| Naso-tail length (cm) | 9.83 ± 0.13 | 10.01 ± 0.11 | 10.00 ± 0.08 | 10.20 ± 0.15 | 9.83 ± 0.13 Δ |
| Body/Tail length ratio | 2.23 ± 0.02 | 2.28 ± 0.04 | 2.31 ± 0.03 | 2.28 ± 0.04 | 2.38 ± 0.06 * |
| Body mass index (g/cm2) | 0.24 ± 0.01 | 0.25 ± 0.01 | 0.24 ± 0.01 | 0.27 ± 0.00 *ϕ | 0.25 ± 0.01 |
| Lee index (g0.33/cm, ×1000) | 329.65 ± 3.05 | 331.79 ± 3.75 | 325.54 ± 2.29 # | 334.63 ± 2.56 ϕ | 329.91 ± 4.25 |
| Weight of organs | |||||
| Spleen/BW ratio (%) | 0.56 ± 0.03 | 0.53 ± 0.05 | 0.62 ± 0.04 | 0.73 ± 0.05 *# | 0.69 ± 0.03 *# |
| Thymus/BW ratio (%) | 0.32 ± 0.02 | 0.29 ± 0.02 | 0.26 ± 0.01 * | 0.32 ± 0.01 ϕ | 0.26 ± 0.01 *Δ |
| Liver/BW ratio (%) | 2.99 ± 0.05 | 2.90 ± 0.08 | 2.75 ± 0.09 | 3.09 ± 0.07 ϕ | 3.06 ± 0.05 ϕ |
| Large intestine 1/BW ratio (%) | 0.43 ± 0.01 | 0.42 ± 0.02 | 0.49 ± 0.01 | 0.41 ± 0.02 ϕ | 0.43 ± 0.02 |
| Small intestine 2/BW ratio (%) | 3.26 ± 0.07 | 3.45 ± 0.13 | 4.86 ± 0.11 *# | 3.51 ± 0.10 *ϕ | 5.09 ± 0.13 *#Δ |
| Large intestine length/BW (cm/g) | 44.43 ± 1.62 | 40.86 ± 1.37 | 39.13 ± 0.04 * | 36.47 ± 1.29 *# | 39.98 ± 0.93 Δ |
| Small intestine length/BW (cm/g) | 233.68 ± 8.80 | 227.92 ± 7.36 | 239.00 ± 0.01 | 211.55 ± 7.04 ϕ | 243.62 ± 6.04 Δ |
| Stomach/BW ratio (%) | 0.70 ± 0.02 | 0.66 ± 0.02 | 0.74 ± 0.11 | 0.67 ± 0.02 | 0.70 ± 0.02 |
| Clinical Outcome | Variable | RV | RV + PRE | RV + POST | RV + P/P |
|---|---|---|---|---|---|
| Incidence | |||||
| MDA | 58.33 | 39.13 | 34.78 | 45.83 | |
| MDAd | 8.00 | 7.00 | 7.00 | 7.00 | |
| daAUC | 166.67 | 159.69 | 86.23 | 222.92 | |
| MDF | 82.35 | 47.37 | 50.00 | 68.75 | |
| MDFd | 8.00 | 7.00 | 6.00 | 7.00 | |
| dfAUC | 258.35 | 232.49 | 146.01 | 315.60 | |
| Duration | |||||
| DDB | 6.78 ± 0.32 | 7.22 ± 0.98 | 7.00 ± 0.33 | 6.82 ± 0.88 | |
| DDE | 8.33 ± 0.24 | 7.78 ± 1.01 | 7.63 ± 0.38 | 8.18 ± 0.87 | |
| DP | 1.17 ± 0.34 | 0.56 ± 0.24 | 0.42 ± 0.26 | 1.25 ± 0.49 | |
| DwD | 1.75 ± 0.37 | 1.42 ± 0.31 | 0.92 ± 0.23 | 2.25 ± 0.43 Δ | |
| Severity | |||||
| MDI | 2.39 ± 0.15 | 2.02 ± 0.09 | 1.84 ± 0.15 # | 2.25 ± 0.10 Δ | |
| MDId | 7.48 ± 0.16 | 7.27 ± 0.43 | 7.18 ± 0.23 | 7.77 ± 0.61 | |
| sAUC | 3.73 ± 0.43 | 2.58 ± 0.23 # | 2.58 ± 0.23 # | 3.13 ± 0.25 |
| REF | RV | RV + PRE | RV + POST | RV + P/P | |
|---|---|---|---|---|---|
| SCFA (µmol/g) | 29,019.90 ± 4043.90 | 25,353.06 ± 2889.38 | 30,680.63 ± 2873.36 | 27,726.29 ± 3082.71 | 39,014.80 ± 7066.49 |
| Acetic acid (%) | 89.26 ± 1.29 | 89.35 ± 1.59 | 97.00 ± 0.52 *# | 91.47 ± 1.12 ϕ | 96.44 ± 0.52 *#Δ |
| Propionic acid (%) | 9.36 ± 1.11 | 9.09 ± 1.37 | 2.23 ± 0.44 *# | 6.91 ± 0.96 ϕ | 1.71 ± 0.29 *#Δ |
| Isobutyric acid (%) | 0.28 ± 0.04 | 0.19 ± 0.03 | 0.08 ± 0.02 *# | 0.22 ± 0.03 ϕ | 0.16 ± 0.04 |
| Butyric acid (%) | 0.86 ± 0.17 | 1.12 ± 0.32 | 0.56 ± 0.17 | 1.14 ± 0.28 ϕ | 1.50 ± 0.36 ϕ |
| Isovaleric acid (%) | 0.20 ± 0.03 | 0.19 ± 0.03 | 0.10 ± 0.01 *# | 0.21 ± 0.03 ϕ | 0.12 ± 0.02 Δ |
| Valeric acid (%) | 0.06 ± 0.02 | 0.07 ± 0.01 | 0.10 ± 0.02 | 0.05 ± 0.01 | 0.11 ± 0.02 *Δ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Ferré, C.; Azagra-Boronat, I.; Massot-Cladera, M.; Tims, S.; Knipping, K.; Garssen, J.; Knol, J.; Franch, À.; Castell, M.; Pérez-Cano, F.J.; et al. Preventive Effect of a Postbiotic and Prebiotic Mixture in a Rat Model of Early Life Rotavirus Induced-Diarrhea. Nutrients 2022, 14, 1163. https://doi.org/10.3390/nu14061163
Morales-Ferré C, Azagra-Boronat I, Massot-Cladera M, Tims S, Knipping K, Garssen J, Knol J, Franch À, Castell M, Pérez-Cano FJ, et al. Preventive Effect of a Postbiotic and Prebiotic Mixture in a Rat Model of Early Life Rotavirus Induced-Diarrhea. Nutrients. 2022; 14(6):1163. https://doi.org/10.3390/nu14061163
Chicago/Turabian StyleMorales-Ferré, Carla, Ignasi Azagra-Boronat, Malén Massot-Cladera, Sebastian Tims, Karen Knipping, Johan Garssen, Jan Knol, Àngels Franch, Margarida Castell, Francisco J. Pérez-Cano, and et al. 2022. "Preventive Effect of a Postbiotic and Prebiotic Mixture in a Rat Model of Early Life Rotavirus Induced-Diarrhea" Nutrients 14, no. 6: 1163. https://doi.org/10.3390/nu14061163