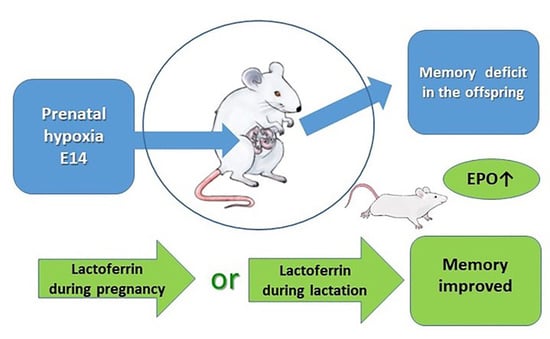
Lactoferrin Induces Erythropoietin Synthesis and Rescues Cognitive Functions in the Offspring of Rats Subjected to Prenatal Hypoxia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Animals
2.3. Model of Prenatal Normobaric Hypoxia
2.4. Collection of Milk Samples
2.5. Western Blotting
2.6. Novel Object Recognition Test (NOR Test)
2.7. Working Memory Analysis in the Eight-Arm Radial Maze
2.8. Statistical Analysis
3. Results
3.1. Lactoferrin-Induced Upregulation of EPO in Pregnant Rats and Their Pups
3.2. Effect of apo-rhLF Injections to Pregnant and Lactating Rats on the Memory of Their Offspring
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Nalivaeva, N.N.; Turner, A.J.; Zhuravin, I.A. Role of prenatal hypoxia in brain development, cognitive functions, and neurodegeneration. Front. Neurosci. 2018, 12, 825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rice, D.; Barone, S., Jr. Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Environ. Health Perspect. 2000, 108 (Suppl. 3), 511–533. [Google Scholar] [CrossRef] [PubMed]
- Vasilev, D.S.; Dubrovskaya, N.M.; Tumanova, N.L.; Zhuravin, I.A. Prenatal hypoxia in different periods of embryogenesis differentially affects cell migration, neuronal plasticity, and rat behaviour in postnatal ontogenesis. Front. Neurosci. 2016, 10, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuravin, I.A.; Dubrovskaya, N.M.; Tumanova, N.L. Postnatal physiological development of rats after acute prenatal hypoxia. Neurosci. Behav. Physiol. 2004, 34, 809–816. [Google Scholar] [CrossRef]
- Zhuravin, I.A.; Dubrovskaya, N.M.; Tumanova, N.L.; Vasilev, D.S.; Nalivaeva, N.N. Ontogenetic and phylogenetic approaches for studying the mechanisms of cognitive dysfunctions. In Evolutionary Physiology and Biochemistry: Advances and Perspectives; InTechOpen: London, UK, 2018; pp. 205–224. [Google Scholar] [CrossRef] [Green Version]
- Zhuravin, I.A.; Dubrovskaya, N.M.; Vasilev, D.S.; Kozlova, D.I.; Kochkina, E.G.; Tumanova, N.L.; Nalivaeva, N.N. Regulation of neprilysin activity and cognitive functions in rats after prenatal hypoxia. Neurochem. Res. 2019, 44, 1387–1398. [Google Scholar] [CrossRef]
- Kochkina, E.G.; Plesneva, S.A.; Zhuravin, I.A.; Turner, A.J.; Nalivaeva, N.N. The effect of hypoxia on cholinesterase activity in rat sensorimotor cortex. Zh. Evol. Biokhim. Fiziol. 2015, 51, 95–102. [Google Scholar] [CrossRef]
- Zhuravin, I.A.; Dubrovskaya, N.M.; Vasilev, D.S.; Postnikova, T.Y.; Zaitsev, A.V. Prenatal hypoxia produces memory deficits associated with impairment of long-term synaptic plasticity in young rats. Neurobiol. Learn. Mem. 2019, 164, 107066. [Google Scholar] [CrossRef]
- Brock, J.H. The physiology of lactoferrin. Biochem. Cell Biol. 2002, 80, 1–6. [Google Scholar] [CrossRef]
- Valenti, P.; Antonini, G. Lactoferrin: An important host defense against microbial and viral attack. Cell. Mol. Life Sci. 2005, 62, 2576–2587. [Google Scholar] [CrossRef]
- Puddu, P.; Valenti, P.; Gessani, S. Immunomodulating effects of lactoferrin on antigen presenting cells. Biochimie 2009, 91, 11–18. [Google Scholar] [CrossRef]
- Puddu, P.; Lattore, D.; Carollo, M.; Catizone, V.; Ricci, G.; Valenti, P.; Gessani, S. Bovine lactoferrin counteracts toll-like receptor mediated activation signals in antigen presenting cells. PLoS ONE 2011, 6, e22504. [Google Scholar] [CrossRef] [PubMed]
- Brandl, N.; Zemann, A.; Kaupe, V.; Marlovits, S.; Huettinger, P.; Goldenberg, H.; Huettinger, M. Signal transduction and metabolism in chondrocytes is modulated by lactoferrin. Osteoarthr. Cartil. 2010, 18, 117–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Montoya, I.A.; Cendón, T.S.; Arévalo-Gallegos, S.; Rascón-Cruz, Q. Lactoferrin a multiple bioactive protein: An overview. Biochim. Biophys. Acta 2012, 1820, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Legrand, D. Lactoferrin, a key molecule in immune and inflammatory processes. Biochem. Cell Biol. 2012, 90, 252–268. [Google Scholar] [CrossRef] [PubMed]
- Vogel, H.J. Lactoferrin, a bird’s eye view. Biochem. Cell Biol. 2012, 90, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Mayeur, S.; Spahis, S.; Pouliot, Y.; Levy, E. Lactoferrin, a pleiotropic protein in health and disease. Antioxid. Redox. Signal. 2016, 24, 813–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aisen, P.; Harris, D.C. Physical biochemistry of the transferrins. In Iron Carriers and Iron Proteins; Loehr, T., Ed.; VCH Publishers: New York, NY, USA, 1989; pp. 241–351. [Google Scholar]
- Păduraru, D.I. The evidence for the benefits from breast milk in the neurodevelopment of premature babies. J. Mind. Med. Sci. 2018, 5, 151–157. [Google Scholar] [CrossRef]
- Wang, B. Molecular determinants of milk lactoferrin as a bioactive compound in early neurodevelopment and cognition. J. Pediatr. 2016, 173S, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Zakharova, E.T.; Sokolov, A.V.; Kostevich, V.A.; Vasilyev, V.B. Human apo-lactoferrin as a physiological mimetic of hypoxia stabilizes hypoxia-inducible factor-1 alpha. Biometals 2012, 25, 1247–1259. [Google Scholar] [CrossRef]
- Kostevich, V.A.; Sokolov, A.V.; Kozlov, S.O.; Vlasenko, A.Y.; Kolmakov, N.N.; Zakharova, E.T.; Vasilyev, V.B. Functional link between ferroxidase activity of ceruloplasmin and protective effect of apo-lactoferrin: Studying rats kept on a silver chloride diet. Biometals 2016, 26, 691–704. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Yanqi, L.; Sangild, P.T.; Bering, S.B.; Chatterton, D.E.W. Effects of bovine lactoferrin on the immature porcine intestine. Br. J. Nutr. 2014, 111, 321–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakharova, E.T.; Sokolov, A.V.; Pavlichenko, N.N.; Kostevich, V.A.; Abdurasulova, I.N.; Chechushkov, A.V.; Voynova, I.V.; Elizarova, A.Y.; Kolmakov, N.N.; Bass, M.G.; et al. Erythropoietin and Nrf2: Key factors in the neuroprotection provided by apo-lactoferrin. Biometals 2018, 31, 425–443. [Google Scholar] [CrossRef] [PubMed]
- Semak, I.; Budzevich, A.; Maliushkova, E.; Kuzniatsova, V.; Popkov, N.; Zalutsky, I.; Ivashkevich, O. Development of dairy herd of transgenic goats as biofactory for large-scale production of biologically active recombinant human lactoferrin. Transgenic Res. 2019, 28, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, A.V.; Zakahrova, E.T.; Kostevich, V.A.; Samygina, V.R.; Vasilyev, V.B. Lactoferrin, myeloperoxidase, and ceruloplasmin: Complementary gearwheels cranking physiological and pathological processes. Biometals 2014, 27, 815–828. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Fling, S.P.; Gregerson, D.S. Peptide and protein molecular weight determination by electrophoresis using a high-molarity tris buffer system without urea. Anal. Biochem. 1986, 155, 83–88. [Google Scholar] [CrossRef]
- Anderson, N.L.; Nance, S.L.; Pearson, T.W.; Anderson, N.G. Specific antiserum staining of two-dimensional electrophoretic patterns of human plasma proteins immobilized on nitrocellulose. Electrophoresis 1982, 3, 135–142. [Google Scholar] [CrossRef]
- Ennaceur, A.; Delacour, J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav. Brain Res. 1988, 31, 47–59. [Google Scholar] [CrossRef]
- Mello, P.B.; Benetti, F.; Cammarota, M.; Izquierdo, I. Effects of acute and chronic physical exercise and stress on different types of memory in rats. An. Acad. Bras. Cienc. 2008, 80, 301–309. [Google Scholar] [CrossRef]
- Dubrovskaya, N.M.; Nalivaeva, N.N.; Turner, A.J.; Zhuravin, I.A. Effects of an inhibitor of α-secretase, which metabolizes the amyloid peptide precursor, on memory formation in rats. Neurosci. Behav. Physiol. 2006, 36, 911–913. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, T.J.; Sizonenko, S.V. Lactoferrin and prematurity: A promising milk protein? Biochem. Cell Biol. 2017, 95, 22–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanches, E.F.; van de Looij, Y.; Toulotte, A.; Sizonenko, S.V.; Lei, V. Mild Neonatal Brain hypoxia-ischemia in very immature rats causes long-term behavioural and cerebellar abnormalities at adulthood. Front. Physiol. 2019, 10, 634. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-G.; Moon, J.-H.; Park, S.-Y. Lactoferrin from bovine colostrum regulates prolyl hydroxylase 2 activity and prevents prion protein-mediated neuronal cell damage via cellular prion protein. Neuroscience 2014, 274, 187–197. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, M.; Chen, C.; Tong, X.; Li, Y.; Yang, K.; Lv, H.; Xu, J.; Qin, L. Holo-lactoferrin: The link between ferroptosis and radiotherapy in triple-negative breast cancer. Theranostics 2021, 11, 3167–3182. [Google Scholar] [CrossRef]
- Jelkmann, W. Molecular biology of erythropoietin. Intern. Med. 2004, 43, 649–659. [Google Scholar] [CrossRef] [Green Version]
- Jelkmann, W. Erythropoietin after a century of research: Younger than ever. Eur. J. Haematol. 2007, 78, 183–205. [Google Scholar] [CrossRef]
- van der Kooij, M.A.; Groenendaal, F.; Kavelaars, A.; Heijnen, C.J.; van Bel, F. Neuroprotective properties and mechanisms of erythropoietin in vitro and in vivo experimental models for hypoxia/ischemia. Brain Res. Rev. 2008, 59, 22–33. [Google Scholar] [CrossRef]
- Genc, K.; Egrilmez, M.Y.; Genc, S. Erythropoietin induces nuclear translocation of Nrf2 and heme oxygenase-1 expression in SH-SY5Y cells. Cell Biochem. Funct. 2010, 28, 197–201. [Google Scholar] [CrossRef]
- van de Looij, Y.; Chatagner, A.; Quairiaux, C.; Gruetter, R.; Hüppi, P.S.; Sizonenko, S.V. Multi-modal assessment of long-term erythropoietin treatment after neonatal hypoxic-ischemic injury in rat brain. PLoS ONE 2014, 9, e95643. [Google Scholar] [CrossRef]
- Ma, C.; Cheng, F.; Wang, X.; Zhai, C.; Yue, W.; Lian, Y.; Wang, Q. Erythropoietin pathway: A potential target for the treatment of depression. Int. J. Mol. Sci. 2016, 17, 677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, C.T. Lactoferrin gene expression and regulation: An overview. Biochem. Cell Biol. 2002, 80, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Telang, S. Lactoferrin: A critical player in neonatal host defense. Nutrients 2018, 10, 1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van de Looij, Y.; Ginet, V.; Chatagner, A.; Toulotte, A.; Somm, E.; Hüppi, P.S.; Sizonenko, S.V. Lactoferrin during lactation protects the immature hypoxic-ischemic rat brain. Ann. Clin. Transl. Neurol. 2014, 1, 955–967. [Google Scholar] [CrossRef]
- van de Looij, Y.; Larpin, C.; Cabungcal, J.-H.; Sanches, E.F.; Toulotte, A.; Do, K.Q.; Sizonenko, S.V. Nutritional intervention for developmental brain damage: Effects of lactoferrin supplementation in hypocaloric induced intrauterine growth restriction rat pups. Front. Endocrinol. 2019, 10, 46. [Google Scholar] [CrossRef] [Green Version]
- Sanches, E.; van de Looij, Y.; Sow, S.; Toulotte, A.; da Silva, A.; Modernell, L.; Sizonenko, S. Dose-dependent neuroprotective effects of bovine lactoferrin following neonatal hypoxia–ischemia in the immaturerat brain. Nutrients 2021, 13, 3880. [Google Scholar] [CrossRef]
- Adamcio, B.; Sperling, S.; Hagemeyer, N.; Walkinshaw, G. Hypoxia inducible factor stabilization leads to lasting improvement of hippocampal memory in healthy mice. Behav. Brain Res. 2009, 208, 80–84. [Google Scholar] [CrossRef]
- Myata, T.; Takizawa, S.; van Ypersele de Strihou, C. Hypoxia. 1. Intracellular sensors for oxygen and oxidative stress: Novel therapeutic targets. Am. J. Physiol. Cell. Physiol. 2011, 300, C226–C231. [Google Scholar] [CrossRef]
- Lepanto, M.S.; Rosa, L.; Paesano, R.; Valenti, P.; Cutone, A. Lactoferrin in Aseptic and Septic Inflammation. Molecules 2019, 24, 1323. [Google Scholar] [CrossRef] [Green Version]





| Rat Groups (N) | Substance Injected | Biochemical Tests | Behavioral Tests |
|---|---|---|---|
| Control with saline | Saline (0.5 mL; E9, 11, 13, 15) | 2 females and 10 embryos (E14); WB-EPO | NOR (P22, n = 17 and P90, n = 15) 8 arm-maze, (P90, n = 7) |
| Hypoxia (E14) with saline | Saline (0.5 mL; E9, 11, 13, 15) | 2 females and 16 embryos (E14); WB-EPO 4 females and 11 pups (P14); WB: EPO, HIF1α and HIF2α | NOR (P22, n = 17 and P90, n = 15) 8 arm-maze, (P90, n = 7) |
| Hypoxia (E14) with apo-rhLF during gestation—Hyp+LF1 | Apo-rhLF (10 mg in 0.5 mL of saline; E9, E11, E13, E15) | 2 females and 12 embryos (E14); WB: EPO | NOR (P22, n = 15 and P90, n = 7) 8 arm-maze, (P90, n = 7) |
| Hypoxia (E14) with apo-rhLF during lactation—Hyp+LF2 | Apo-rhLF (10 mg in 0.5 mL of saline; daily from P0 to P15 days after birth) | 4 females and 16 pups (P14); WB: EPO, HIF1α and HIF2α | NOR (P22, n = 17 and P90, n = 7) 8 arm-maze, (P90, n = 7) |
| Control with apo-rhLF during lactation | Apo-rhLF (10 mg in 0.5 mL of saline; daily from P0 to P15 days after birth) | 3 females and 9 pups (P14); WB: EPO, HIF1α and HIF2α; | |
| Control with apo-rhLF during lactation | Apo-rhLF (single injection 10 mg in 0.5 mL of saline on P14) | 3 females and 6 pups (P14) WB: LF in dam milk and in the pup gastric content |
| Groups | Analyzed Part of Pregnant Rats or Fetus | ||||
|---|---|---|---|---|---|
| Brain of Pregnant Females | Placenta | Brain of the Embryos | Torso of the Embryos | ||
| Embryos (n = 10) from control pregnant rats (n = 2) with i.p. saline injections during gestation | EPO presence | 0 | 0 | 0 | 0 |
| EPO absence | 2 | 10 | 10 | 10 | |
| Embryos (n = 16) from pregnant rats (n = 2) with hypoxia and i.p. saline injections during gestation | EPO presence | 0 | 0 | 0 | 0 |
| EPO absence | 2 | 16 | 16 | 16 | |
| Embryos (n = 12) from pregnant rats (n = 2) with hypoxia and i.p. apo-rhLF injections during gestation | EPO presence | 2 | 0 | 12 | 12 |
| EPO absence | 0 | 12 | 0 | 0 | |
| Organ | Pups (n = 9) of Control Rats with i.p. apo-rhLF Injections during Lactation (n = 3) | Pups (n = 11)of Rats after Hypoxia and i.p. Saline Injections during Lactation (n = 4) | Pups (n = 16)of Rats after Hypoxia and i.p. apo-rhLF Injections during Lactation (n = 4) | |||
|---|---|---|---|---|---|---|
| HIF1α HIF2α and EPO Presence | HIF1α HIF2α and EPO Absence | HIF1α HIF2α and EPO Presence | HIF1α HIF2α and EPO Absence | HIF1α HIF2α and EPO Presence | HIF1α HIF2α and EPO Absence | |
| Brain | 9 | 0 | 0 | 11 | 16 | 0 |
| Liver | 9 | 0 | 0 | 11 | 16 | 0 |
| Spleen | 9 | 0 | 0 | 11 | 16 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokolov, A.V.; Dubrovskaya, N.M.; Kostevich, V.A.; Vasilev, D.S.; Voynova, I.V.; Zakharova, E.T.; Runova, O.L.; Semak, I.V.; Budevich, A.I.; Nalivaeva, N.N.; et al. Lactoferrin Induces Erythropoietin Synthesis and Rescues Cognitive Functions in the Offspring of Rats Subjected to Prenatal Hypoxia. Nutrients 2022, 14, 1399. https://doi.org/10.3390/nu14071399
Sokolov AV, Dubrovskaya NM, Kostevich VA, Vasilev DS, Voynova IV, Zakharova ET, Runova OL, Semak IV, Budevich AI, Nalivaeva NN, et al. Lactoferrin Induces Erythropoietin Synthesis and Rescues Cognitive Functions in the Offspring of Rats Subjected to Prenatal Hypoxia. Nutrients. 2022; 14(7):1399. https://doi.org/10.3390/nu14071399
Chicago/Turabian StyleSokolov, Alexey V., Nadezhda M. Dubrovskaya, Valeria A. Kostevich, Dmitrii S. Vasilev, Irina V. Voynova, Elena T. Zakharova, Olga L. Runova, Igor V. Semak, Alexander I. Budevich, Natalia N. Nalivaeva, and et al. 2022. "Lactoferrin Induces Erythropoietin Synthesis and Rescues Cognitive Functions in the Offspring of Rats Subjected to Prenatal Hypoxia" Nutrients 14, no. 7: 1399. https://doi.org/10.3390/nu14071399