High Dietary Phosphorus Is Associated with Increased Breast Cancer Risk in a U.S. Cohort of Middle-Aged Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. Statistical Analysis
2.2. Dietary Assessment
3. Results
4. Discussion
- Strength of association: The magnitude of the relative risk of breast cancer incidence associated with high dietary P levels is up to 2.3 times greater than associations with low phosphorus levels. “As a measure of effect size, an RR value is generally considered clinically significant if it is less than 0.50 or more than 2.00; that is, if the risk is at least halved, or more than doubled” [60]. A recent review from the International Agency for Research on Cancer (IARC) found that most studies linking various cancers to occupational exposures known to be carcinogenic in humans reported relative risk values well below the 2.30 relative risk in the present study, and approximately one-third of the confidence intervals in the IARC review were not statistically significant [61];
- Specificity: the present study shows that P is a specific dietary factor in the association with breast cancer; notably, this does not preclude other risk factors that are associated with breast cancer;
- Temporality: exposure to high dietary P precedes breast cancer incidence, as revealed in the present nested case–control study’s longitudinal data;
- Biological gradient: compared to the lowest level of P intake, increasing levels of dietary P in the present study are associated with increasing risk of breast cancer;
- Coherence: dysregulated phosphate metabolism and phosphate toxicity fit the regulation-based model of cancer, which proposes that cancer is caused by dysregulated metabolic factors [8];
- Experimental evidence: Laboratory animal experiments confirm an association between high dietary P feeding and tumorigenesis [6,7]. Importantly, P from dietary sources in these animal experiments are not administered at the maximum tolerated dosages for chemical agents, which are often used in carcinogenic studies [66];
“The major disadvantage of nested case–control studies is that not all pertinent risk factors are likely to have been recorded. Furthermore, because many different healthcare professionals will be involved in patient care, risk factors and outcome(s) will probably not have been measured with the same accuracy and consistency throughout. It may also be problematic if the diagnosis of the disease or outcome changes with time”.[72]
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Xia, J.; Li, L.; Ke, Y.; Cheng, J.; Xie, Y.; Chu, W.; Cheung, P.; Kim, J.H.; Colditz, G.A.; et al. Associations between dietary patterns and the risk of breast cancer: A systematic review and meta-analysis of observational studies. Breast Cancer Res. 2019, 21, 16. [Google Scholar] [CrossRef] [PubMed]
- Clegg, D.J.; Gallant, K.M.H. Plant-based diets in CKD. Clin. J. Am. Soc. Nephrol. 2019, 14, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.B.; Razzaque, M.S. Endocrine Regulation of Phosphate Homeostasis. In Textbook of Nephro-Endocrinology, 2nd ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 539–548. [Google Scholar]
- Brown, R.B.; Razzaque, M.S. Phosphate toxicity and tumorigenesis. Biochim. Biophys. Acta (BBA) Rev. Cancer 2018, 1869, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Xu, C.-X.; Lim, H.-T.; Park, S.-J.; Shin, J.-Y.; Chung, Y.-S.; Park, S.-C.; Chang, S.-H.; Youn, H.-J.; Lee, K.-H. High dietary inorganic phosphate increases lung tumorigenesis and alters Akt signaling. Am. J. Respir. Crit. Care Med. 2009, 179, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Camalier, C.E.; Young, M.R.; Bobe, G.; Perella, C.M.; Colburn, N.H.; Beck, G.R. Elevated phosphate activates N-ras and promotes cell transformation and skin tumorigenesis. Cancer Prev. Res. 2010, 3, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Schipper, H.; Turley, E.A.; Baum, M. A new biological framework for cancer research. Lancet 1996, 348, 1149–1151. [Google Scholar] [CrossRef]
- Schmidt, D.R.; Patel, R.; Kirsch, D.G.; Lewis, C.A.; Vander Heiden, M.G.; Locasale, J.W. Metabolomics in cancer research and emerging applications in clinical oncology. CA Cancer J. Clin. 2021, 71, 333–358. [Google Scholar] [CrossRef]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef]
- Olanbiwonnu, T.; Holden, R.M. Inorganic phosphate as a potential risk factor for chronic disease. CMAJ 2018, 190, E784–E785. [Google Scholar] [CrossRef]
- Moshfegh, A.; Kovalchik, A.; Clemens, J. Phosphorus intake of Americans: What we eat in America, NHANES 2011-2012. Food Surveys Research Group: Dietary Data Brief. U.S. Department of Agriculture. 2016; Volume 15, pp. 1–5. Available online: https://www.ars.usda.gov/arsuserfiles/80400530/pdf/dbrief/15_phosphorus_intake_1112.pdf (accessed on 2 July 2023).
- Williams, C.; Ronco, C.; Kotanko, P. Whole Grains in the Renal Diet—Is It Time to Reevaluate Their Role? Blood Purif. 2013, 36, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Isaksen, I.M.; Dankel, S.N. Ultra-processed food consumption and cancer risk: A systematic review and meta-analysis. Clin. Nutr. 2023, 42, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Lian, Y.; Wang, G.-P.; Chen, G.-Q.; Chen, H.-N.; Zhang, G.-Y. Association between ultra-processed foods and risk of cancer: A systematic review and meta-analysis. Front. Nutr. 2023, 10, 1175994. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Gunter, M.J.; Rauber, F.; Levy, R.B.; Huybrechts, I.; Kliemann, N.; Millett, C.; Vamos, E.P. Ultra-processed food consumption, cancer risk and cancer mortality: A large-scale prospective analysis within the UK Biobank. eClinicalMedicine 2023, 56, 101840. [Google Scholar] [CrossRef]
- Willett, W. Nutritional Epidemiology; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- IOM. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride; Institute of Medicine Standing Committee on the Scientific Evaluation of Dietary Reference Intakes; National Academy of Sciences: Washington, DC, USA, 1997.
- nih.gov. Phosphorus—Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/Phosphorus-HealthProfessional/#en29 (accessed on 27 February 2022).
- Eknoyan, G.; Levin, A.; Levin, N. Bone metabolism and disease in chronic kidney disease. Am. J. Kidney Dis. 2003, 42, S1–S201. [Google Scholar] [CrossRef]
- Chang, A.R.; Lazo, M.; Appel, L.J.; Gutiérrez, O.M.; Grams, M.E. High dietary phosphorus intake is associated with all-cause mortality: Results from NHANES III. Am. J. Clin. Nutr. 2014, 99, 320–327. [Google Scholar] [CrossRef] [PubMed]
- myplate.gov. MyPlate Plan. Available online: https://www.myplate.gov/myplate-plan (accessed on 9 August 2021).
- fdc.nal.usda.gov. Milk, Nonfat, Fluid, without Added Vitamin A (Fat Free or Skim). Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/746776/nutrients (accessed on 1 March 2022).
- Fraser, G.E.; Jaceldo-Siegl, K.; Orlich, M.; Mashchak, A.; Sirirat, R.; Knutsen, S. Dairy, soy, and risk of breast cancer: Those confounded milks. Int. J. Epidemiol. 2020, 49, 1526–1537. [Google Scholar] [CrossRef]
- Michaëlsson, K.; Wolk, A.; Langenskiöld, S.; Basu, S.; Warensjö Lemming, E.; Melhus, H.; Byberg, L. Milk intake and risk of mortality and fractures in women and men: Cohort studies. BMJ Br. Med. J. 2014, 349, g6015. [Google Scholar] [CrossRef]
- Wang, R.; Wen, Z.Y.; Liu, F.H.; Wei, Y.F.; Xu, H.L.; Sun, M.L.; Zhao, Y.H.; Gong, T.T.; Wang, H.H.; Wu, Q.J. Association between dietary acid load and cancer risk and prognosis: An updated systematic review and meta-analysis of observational studies. Front. Nutr. 2022, 9, 891936. [Google Scholar] [CrossRef]
- Müller, A.; Zimmermann-Klemd, A.M.; Lederer, A.K.; Hannibal, L.; Kowarschik, S.; Huber, R.; Storz, M.A. A Vegan Diet Is Associated with a Significant Reduction in Dietary Acid Load: Post Hoc Analysis of a Randomized Controlled Trial in Healthy Individuals. Int. J. Environ. Res. Public Health 2021, 18, 9998. [Google Scholar] [CrossRef]
- Lees, J.S.; Elyan, B.M.P.; Herrmann, S.M.; Lang, N.N.; Jones, R.J.; Mark, P.B. The ‘other’ big complication: How chronic kidney disease impacts on cancer risks and outcomes. Nephrol. Dial. Transplant. 2022, 38, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.; Staplin, N.; Emberson, J.; Baigent, C.; Turner, R.; Chalmers, J.; Zoungas, S.; Pollock, C.; Cooper, B.; Harris, D.; et al. Chronic kidney disease and the risk of cancer: An individual patient data meta-analysis of 32,057 participants from six prospective studies. BMC Cancer 2016, 16, 488. [Google Scholar] [CrossRef] [PubMed]
- Stengel, B. Chronic kidney disease and cancer: A troubling connection. J. Nephrol. 2010, 23, 253–262. [Google Scholar]
- Tendulkar, K.K.; Cope, B.; Dong, J.; Plumb, T.J.; Campbell, W.S.; Ganti, A.K. Risk of malignancy in patients with chronic kidney disease. PLoS ONE 2022, 17, e0272910. [Google Scholar] [CrossRef] [PubMed]
- Kitchlu, A.; Reid, J.; Jeyakumar, N.; Dixon, S.N.; Munoz, A.M.; Silver, S.A.; Booth, C.M.; Chan, C.T.M.; Garg, A.X.; Amir, E.; et al. Cancer Risk and Mortality in Patients With Kidney Disease: A Population-Based Cohort Study. Am. J. Kidney Dis. 2022, 80, 436–448.e1. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Wang, Q.; Liu, B.; Ma, Q.; Zhang, T.; Huang, T.; Lv, Z.; Wang, R. Chronic Kidney Disease and Cancer: Inter-Relationships and Mechanisms. Front. Cell Dev. Biol. 2022, 10, 868715. [Google Scholar] [CrossRef]
- Movahhed, S.M.M.; Mousavi, S.S.B.; Hayati, F.; Shayanpour, S.; Halili, S.; Sabetnia, L.; Khazaei, Z. The relationship between chronic kidney disease and cancer. J. Nephropathol. 2018, 7, 115–116. [Google Scholar] [CrossRef]
- Wei, Y.-F.; Chen, J.-Y.; Lee, H.-S.; Wu, J.-T.; Hsu, C.-K.; Hsu, Y.-C. Association of chronic kidney disease with mortality risk in patients with lung cancer: A nationwide Taiwan population-based cohort study. BMJ Open 2018, 8, e019661. [Google Scholar] [CrossRef]
- Guo, K.; Wang, Z.; Luo, R.; Cheng, Y.; Ge, S.; Xu, G. Association between chronic kidney disease and cancer including the mortality of cancer patients: National health and nutrition examination survey 1999-2014. Am. J. Transl. Res. 2022, 14, 2356–2366. [Google Scholar]
- Na, S.Y.; Sung, J.Y.; Chang, J.H.; Kim, S.; Lee, H.H.; Park, Y.H.; Chung, W.; Oh, K.-H.; Jung, J.Y. Chronic Kidney Disease in Cancer Patients: An Independent Predictor of Cancer-Specific Mortality. Am. J. Nephrol. 2011, 33, 121–130. [Google Scholar] [CrossRef]
- Yu, T.M.; Chuang, Y.W.; Yu, M.C.; Chen, C.H.; Yang, C.K.; Huang, S.T.; Lin, C.L.; Shu, K.H.; Kao, C.H. Risk of cancer in patients with polycystic kidney disease: A propensity-score matched analysis of a nationwide, population-based cohort study. Lancet Oncol. 2016, 17, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Bacchetta, J.; Bernardor, J.; Garnier, C.; Naud, C.; Ranchin, B. Hyperphosphatemia and Chronic Kidney Disease: A Major Daily Concern Both in Adults and in Children. Calcif. Tissue Int. 2021, 108, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Aoyagi, H. Understudied Hyperphosphatemia (Chronic Kidney Disease) Treatment Targets and New Biological Approaches. Medicina 2023, 59, 959. [Google Scholar] [CrossRef]
- Sowers, M.F.R.; Crawford, S.L.; Sternfeld, B.; Morganstein, D.; Gold, E.B.; Greendale, G.A.; Evans, D.A.; Neer, R.; Matthews, K.A.; Sherman, S. SWAN: A Multicenter, Multiethnic, Community-Based Cohort Study of Women and the Menopausal Transition. In Menopause: Biology and Pathobiology; Academic Press: San Diego, CA, USA, 2000; pp. 175–188. [Google Scholar]
- swanstudy.org. SWAN: Study of Women’s Health Across the Nation. Available online: https://www.swanstudy.org/ (accessed on 2 July 2023).
- Santoro, N.; Sutton-Tyrrell, K. The SWAN song: Study of Women’s Health Across the Nation’s recurring themes. Obstet. Gynecol. Clin. N. Am. 2011, 38, 417–423. [Google Scholar] [CrossRef] [PubMed]
- swanstudy.org. About SWAN. Available online: https://www.swanstudy.org/about/about-swan/ (accessed on 2 July 2023).
- Grimes, D.A.; Schulz, K.F. Compared to what? Finding controls for case-control studies. Lancet 2005, 365, 1429–1433. [Google Scholar] [CrossRef] [PubMed]
- Labrecque, J.A.; Hunink, M.M.G.; Ikram, M.A.; Ikram, M.K. Do Case-Control Studies Always Estimate Odds Ratios? Am. J. Epidemiol. 2021, 190, 318–321. [Google Scholar] [CrossRef]
- cdc.gov. Principles of Epidemiology in Public Health Practice—Lesson 3: Measures of Risk. Available online: https://www.cdc.gov/csels/dsepd/ss1978/lesson3/ (accessed on 6 July 2023).
- Knol, M.J.; Le Cessie, S.; Algra, A.; Vandenbroucke, J.P.; Groenwold, R.H. Overestimation of risk ratios by odds ratios in trials and cohort studies: Alternatives to logistic regression. CMAJ 2012, 184, 895–899. [Google Scholar] [CrossRef]
- medcalc.org. Relative Risk Calculator—Version 20.027. Available online: https://www.medcalc.org/calc/relative_risk.php (accessed on 17 March 2022).
- Wallace, T.C.; Jun, S.; Zou, P.; McCabe, G.P.; Craig, B.A.; Cauley, J.A.; Weaver, C.M.; Bailey, R.L. Dairy intake is not associated with improvements in bone mineral density or risk of fractures across the menopause transition: Data from the Study of Women’s Health Across the Nation. Menopause 2020, 27, 879–886. [Google Scholar] [CrossRef]
- Kong, J.S.; Kim, Y.M.; Woo, H.W.; Shin, M.H.; Koh, S.B.; Kim, H.C.; Shin, J.H.; Kim, M.K. Prospective Associations between Cumulative Average Intake of Flavonoids and Hypertension Risk in the CArdioVascular Disease Association Study (CAVAS). Nutrients 2023, 15, 1186. [Google Scholar] [CrossRef]
- Blankers, M.; Koeter, M.W.J.; Schippers, G.M. Missing data approaches in eHealth research: Simulation study and a tutorial for nonmathematically inclined researchers. J. Med. Internet Res. 2010, 12, e54. [Google Scholar] [CrossRef]
- Lachin, J.M. Fallacies of last observation carried forward analyses. Clin. Trials 2016, 13, 161–168. [Google Scholar] [CrossRef] [PubMed]
- dietassessmentprimer.cancer.gov. Learn More about Energy Adjustment. Available online: https://dietassessmentprimer.cancer.gov/learn/adjustment.html (accessed on 13 August 2021).
- Fulgoni, K.; Fulgoni, V.L., 3rd. Trends in Total, Added, and Natural Phosphorus Intake in Adult Americans, NHANES 1988-1994 to NHANES 2015–2016. Nutrients 2021, 13, 2249. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, G.M.; Feinn, R. Using Effect Size-or Why the P Value Is Not Enough. J. Grad. Med. Educ. 2012, 4, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Nestle, M. Perspective: Challenges and Controversial Issues in the Dietary Guidelines for Americans, 1980–2015. Adv. Nutr. 2018, 9, 148–150. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Fedak, K.M.; Bernal, A.; Capshaw, Z.A.; Gross, S. Applying the Bradford Hill criteria in the 21st century: How data integration has changed causal inference in molecular epidemiology. Emerg. Themes Epidemiol. 2015, 12, 14. [Google Scholar] [CrossRef]
- Andrade, C. Understanding relative risk, odds ratio, and related terms: As simple as it can get. J. Clin. Psychiatry 2015, 76, e857–e861. [Google Scholar] [CrossRef]
- Marant Micallef, C.; Shield, K.D.; Baldi, I.; Charbotel, B.; Fervers, B.; Gilg Soit Ilg, A.; Guénel, P.; Olsson, A.; Rushton, L.; Hutchings, S.J.; et al. Occupational exposures and cancer: A review of agents and relative risk estimates. Occup. Environ. Med. 2018, 75, 604–614. [Google Scholar] [CrossRef]
- Brown, R.B.; Bigelow, P.; Dubin, J.A.; Neiterman, E. Breast cancer, alcohol, and phosphate toxicity. J. Appl. Toxicol. 2023. [Google Scholar] [CrossRef]
- Kuang, Y.; Nagy, J.D.; Elser, J.J. Biological stoichiometry of tumor dynamics: Mathematical models and analysis. Discrete Contin. Dyn. Syst. Ser. B 2004, 4, 221–240. [Google Scholar]
- Brown, R.B. Vitamin D, cancer, and dysregulated phosphate metabolism. Endocrine 2019, 65, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.B. Cancer Cachexia and Dysregulated Phosphate Metabolism: Insights from Mutant p53 and Mutant Klotho Mouse Models. Metabolites 2022, 12, 1284. [Google Scholar] [CrossRef] [PubMed]
- HASEMAN, J.K. Issues in Carcinogenicity Testing: Dose Selection. Toxicol. Sci. 1985, 5, 66–78. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Pal, D.B. Chapter 11—Nutrients contamination and eutrophication in the river ecosystem. In Ecological Significance of River Ecosystems; Madhav, S., Kanhaiya, S., Srivastav, A., Singh, V., Singh, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 203–216. [Google Scholar] [CrossRef]
- Elser, J.J.; Nagy, J.D.; Kuang, Y. Biological Stoichiometry: An Ecological Perspective on Tumor Dynamics. BioScience 2003, 53, 1112–1120. [Google Scholar] [CrossRef]
- whi.org. Women’s Health Initiative. Available online: https://www.whi.org/ (accessed on 6 June 2023).
- nursesheatlhstudy.org. The Nurses’ Health Study and Nurses’ Health Study II Are among the Largest Investigations into the Risk Factors for Major Chronic Diseases in Women. Available online: https://nurseshealthstudy.org/ (accessed on 6 June 2023).
- Avis, N.E.; Levine, B.; Goyal, N.; Crawford, S.L.; Hess, R.; Colvin, A.; Bromberger, J.T.; Greendale, G.A. Health-related quality of life among breast cancer survivors and noncancer controls over 10 years: Pink SWAN. Cancer 2020, 126, 2296–2304. [Google Scholar] [CrossRef] [PubMed]
- Sedgwick, P. Nested case-control studies: Advantages and disadvantages. BMJ Br. Med. J. 2014, 348, g1532. [Google Scholar] [CrossRef]
- Partlett, C.; Hall, N.J.; Leaf, A.; Juszczak, E.; Linsell, L. Application of the matched nested case-control design to the secondary analysis of trial data. BMC Med. Res. Methodol. 2020, 20, 117. [Google Scholar] [CrossRef]
- Morimoto, Y.; Sakuma, M.; Ohta, H.; Suzuki, A.; Matsushita, A.; Umeda, M.; Ishikawa, M.; Taketani, Y.; Takeda, E.; Arai, H. Estimate of dietary phosphorus intake using 24-h urine collection. J. Clin. Biochem. Nutr. 2014, 55, 62–66. [Google Scholar] [CrossRef]
- Cui, Q.; Xia, Y.; Wu, Q.; Chang, Q.; Niu, K.; Zhao, Y. A meta-analysis of the reproducibility of food frequency questionnaires in nutritional epidemiological studies. Int. J. Behav. Nutr. Phys. Act. 2021, 18, 12. [Google Scholar] [CrossRef]
- cdc.gov. What Are the Risk Factors for Breast Cancer? Available online: https://www.cdc.gov/cancer/breast/basic_info/risk_factors.htm (accessed on 1 June 2023).
- Zhang, D.; Maalouf, N.M.; Adams-Huet, B.; Moe, O.W.; Sakhaee, K. Effects of sex and postmenopausal estrogen use on serum phosphorus levels: A cross-sectional study of the National Health and Nutrition Examination Survey (NHANES) 2003–2006. Am. J. Kidney Dis. 2014, 63, 198–205. [Google Scholar] [CrossRef]
- Surakasula, A.; Nagarjunapu, G.C.; Raghavaiah, K.V. A comparative study of pre- and post-menopausal breast cancer: Risk factors, presentation, characteristics and management. J. Res. Pharm. Pract. 2014, 3, 12–18. [Google Scholar] [CrossRef]
- Toyama, T.; Kitagawa, K.; Oshima, M.; Kitajima, S.; Hara, A.; Iwata, Y.; Sakai, N.; Shimizu, M.; Hashiba, A.; Furuichi, K.; et al. Age differences in the relationships between risk factors and loss of kidney function: A general population cohort study. BMC Nephrol. 2020, 21, 477. [Google Scholar] [CrossRef]
- cancer.gov. Diet. National Cancer Institute. Available online: https://www.cancer.gov/about-cancer/causes-prevention/risk/diet (accessed on 3 August 2023).


| Group | Mean | SD | Min. | Max. |
|---|---|---|---|---|
| Cases, unadjusted (N = 74) | 1120 | 330 | 360 | 1850 |
| Cases, standardized | 1390 | 340 | 770 | 2180 |
| Controls, unadjusted (N = 296) | 1150 | 380 | 330 | 2620 |
| Controls, standardized | 1320 | 290 | 570 | 2450 |
| Dietary P | Breast Cancer Cases | Controls | Risk | Relative Risk |
|---|---|---|---|---|
| 800–1000 mg P | 6 | 34 | 0.15 | Reference |
| >1000–1200 mg P | 13 | 58 | 0.18 | 1.22 (95% CI 0.50–2.96) p = 0.66 |
| >1200–1400 mg P | 20 | 93 | 0.18 | 1.18 (95% CI 0.51–2.72) p = 0.70 |
| >1400–1600 mg P | 14 | 62 | 0.18 | 1.23 (95% CI 0.51–2.95) p = 0.65 |
| >1600–1800 mg P | 9 | 22 | 0.29 | 1.94 (95% CI 0.77–4.86) p = 0.16 |
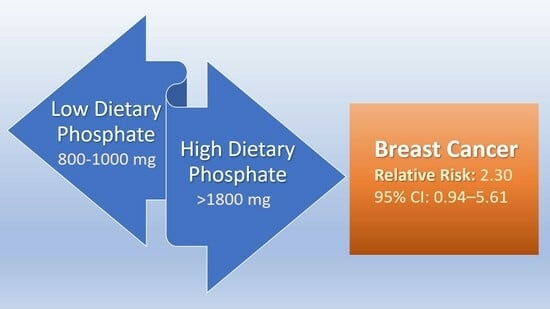
| >1800 mg P | 10 | 19 | 0.34 | 2.30 (95% CI 0.94–5.61) p = 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, R.B.; Bigelow, P.; Dubin, J.A.; Mielke, J.G. High Dietary Phosphorus Is Associated with Increased Breast Cancer Risk in a U.S. Cohort of Middle-Aged Women. Nutrients 2023, 15, 3735. https://doi.org/10.3390/nu15173735
Brown RB, Bigelow P, Dubin JA, Mielke JG. High Dietary Phosphorus Is Associated with Increased Breast Cancer Risk in a U.S. Cohort of Middle-Aged Women. Nutrients. 2023; 15(17):3735. https://doi.org/10.3390/nu15173735
Chicago/Turabian StyleBrown, Ronald B., Philip Bigelow, Joel A. Dubin, and John G. Mielke. 2023. "High Dietary Phosphorus Is Associated with Increased Breast Cancer Risk in a U.S. Cohort of Middle-Aged Women" Nutrients 15, no. 17: 3735. https://doi.org/10.3390/nu15173735