Adherence to the Mediterranean Diet and Risk of Gastric Cancer: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Search Strategy and Selection Criteria
2.3. Data Extraction and Quality Assessment
2.4. Statistical Analysis
3. Results
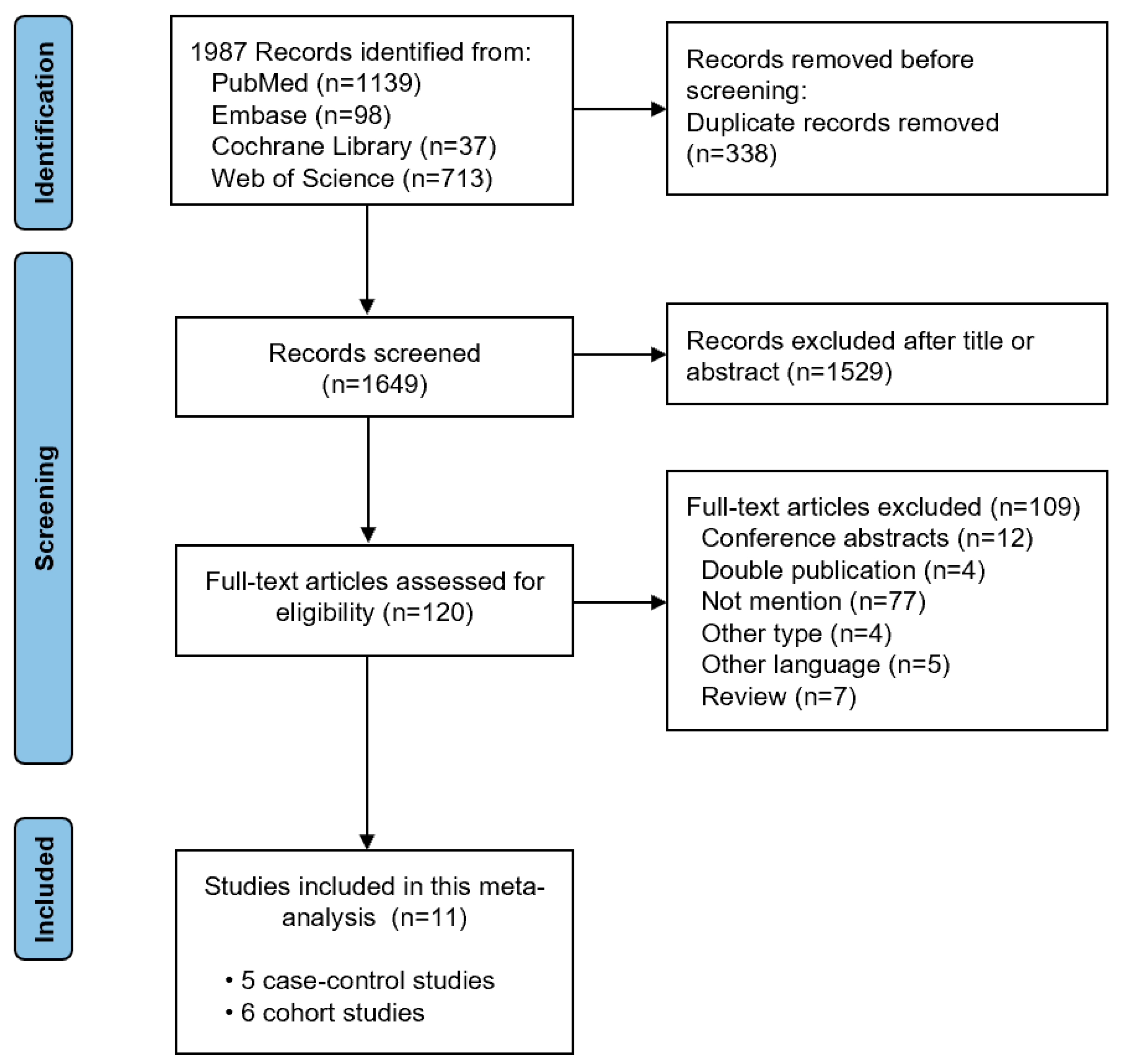
3.1. Literature Search and Study Characteristics
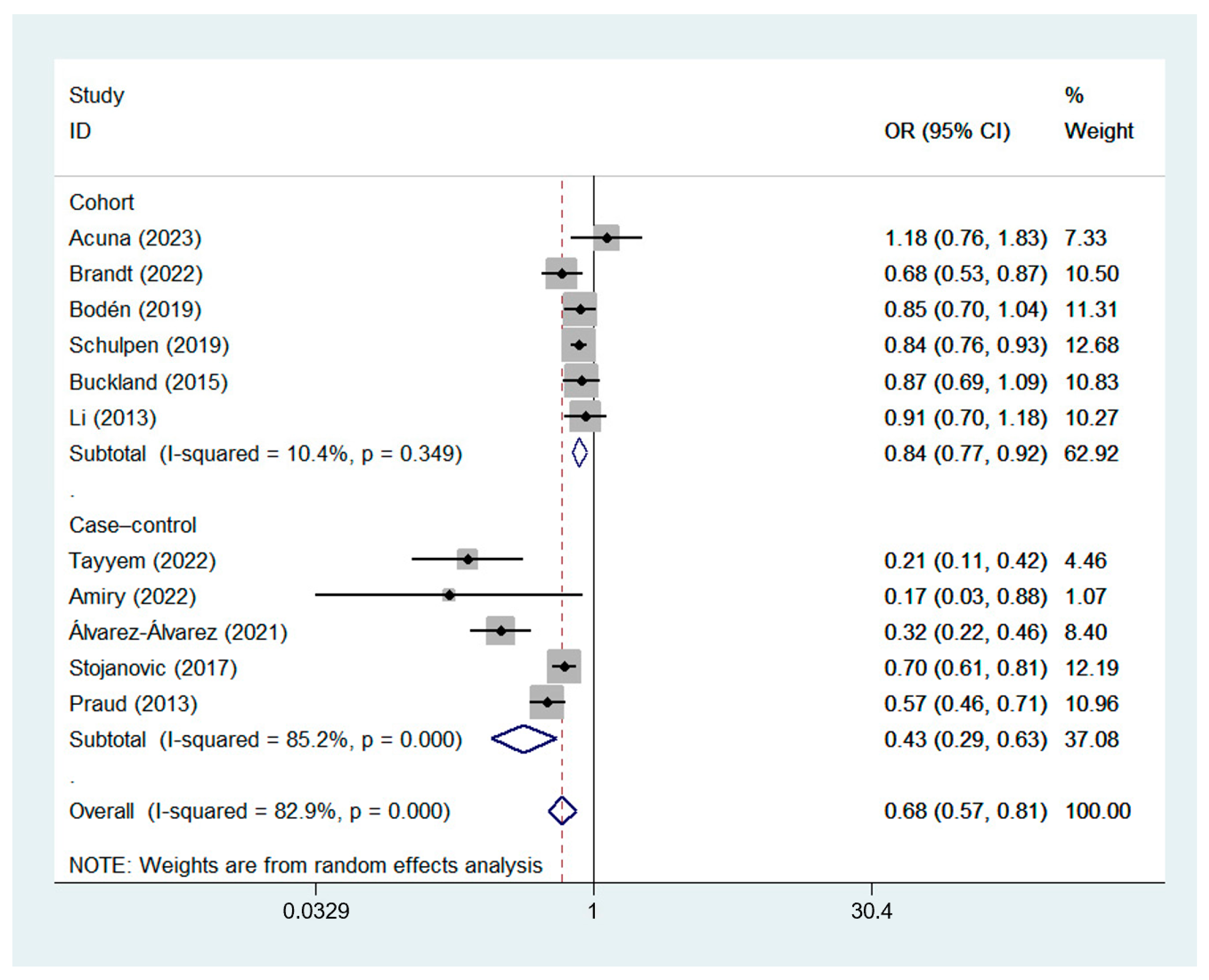
3.2. Effect of the MD on GC Risk
3.2.1. Estimates from Different Types of Studies
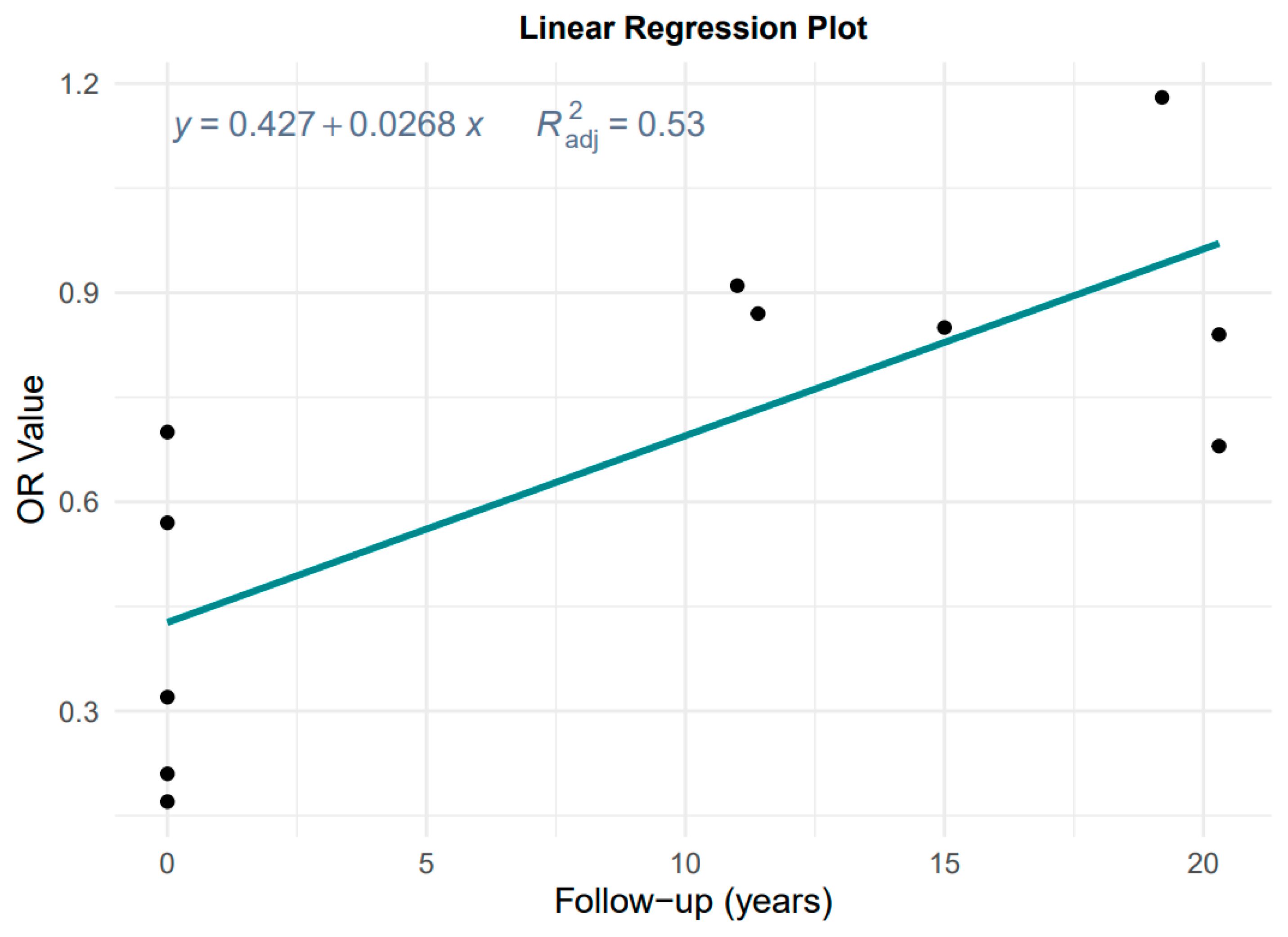
3.2.2. Effect of Follow-Up Time
3.3. Effect of the MD on GCA and GNCA Risk
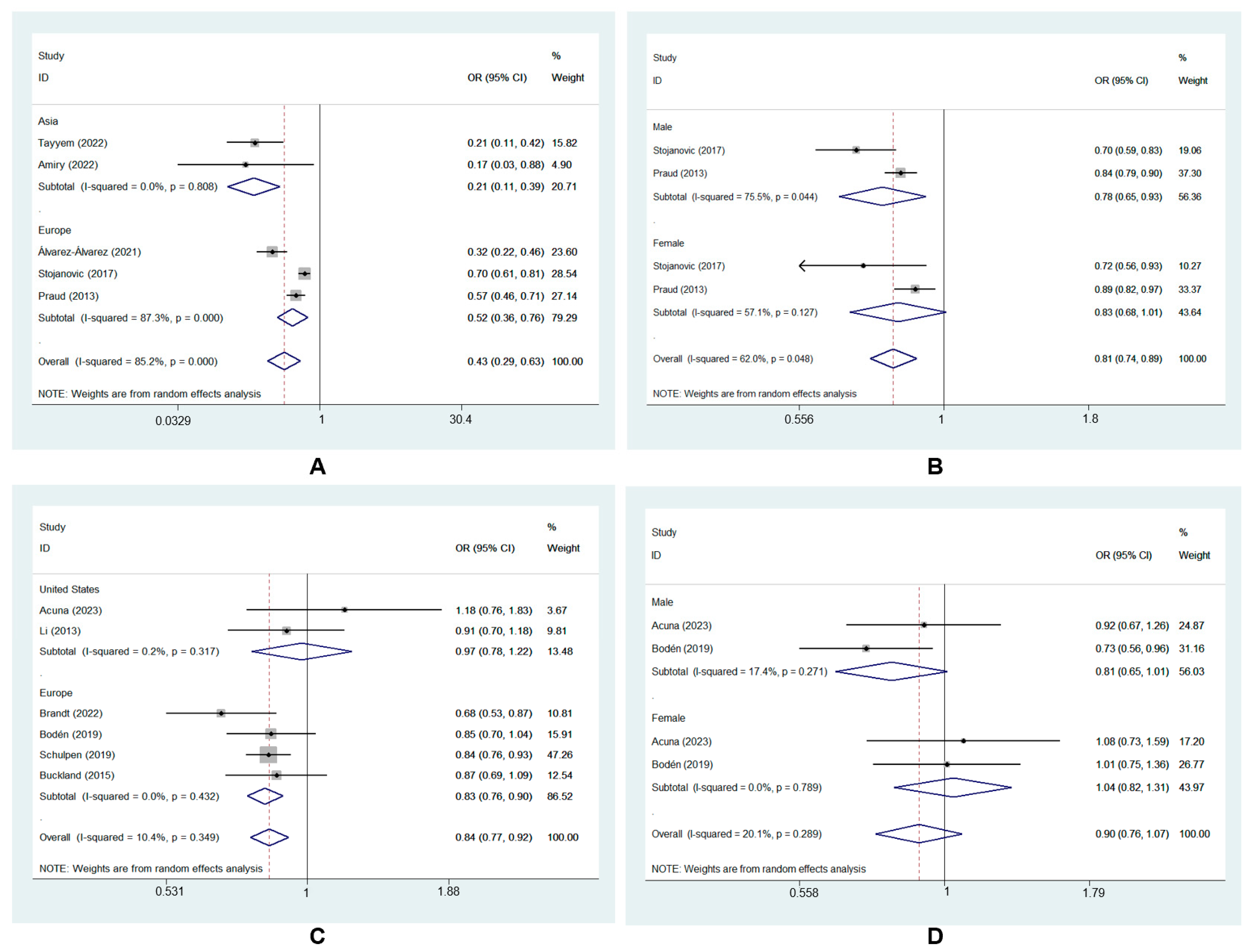
3.4. Subgroup Analysis of MD and GC Risk
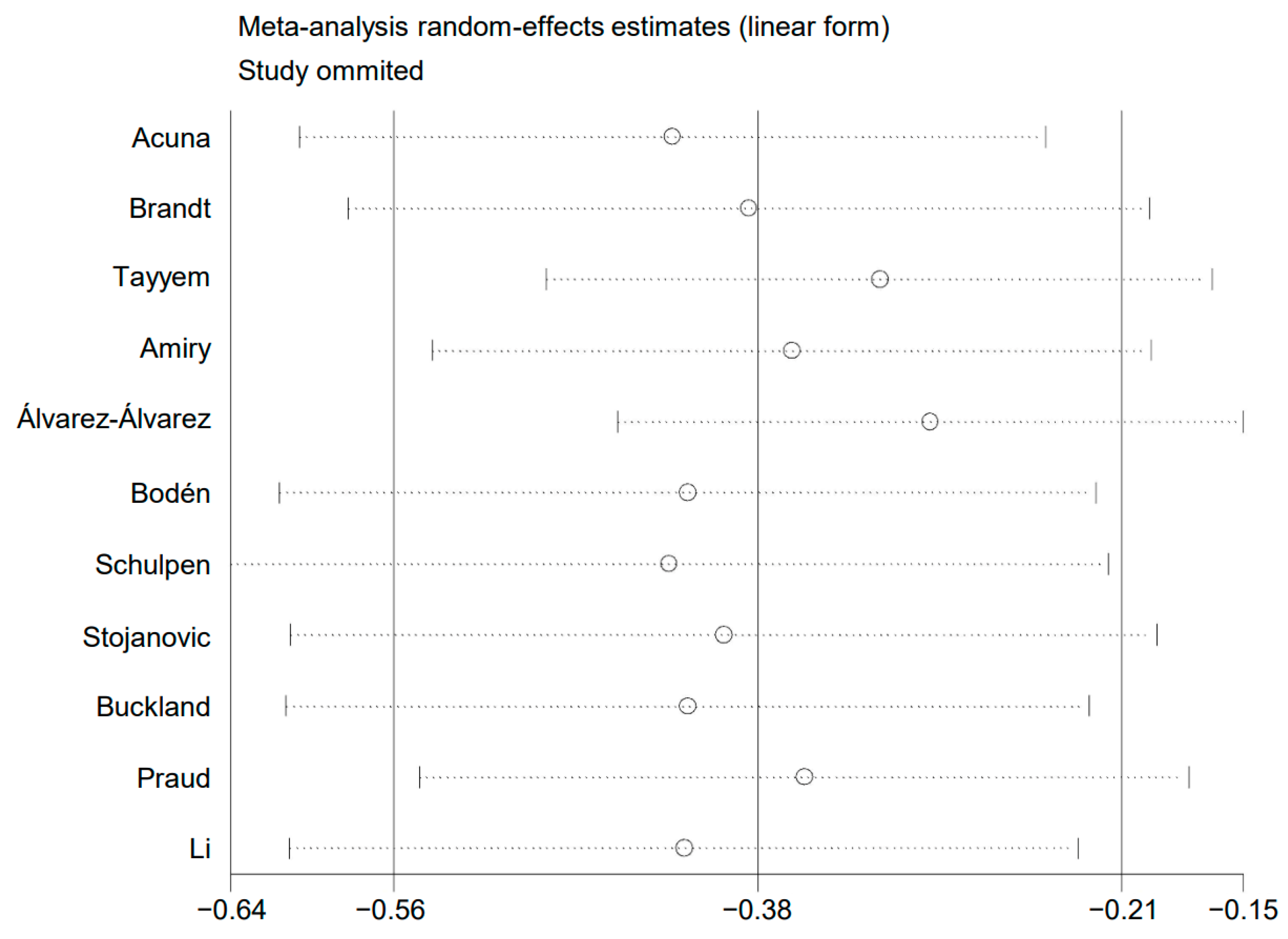
3.5. Sensitivity Analysis
3.6. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Machlowska, J.; Baj, J.; Sitarz, M.; Maciejewski, R.; Sitarz, R. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int. J. Mol. Sci. 2020, 21, 4012. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Song, M.; Eliassen, A.H.; Wang, M.; Fung, T.T.; Clinton, S.K.; Rimm, E.B.; Hu, F.B.; Willett, W.C.; Tabung, F.K.; et al. Optimal dietary patterns for prevention of chronic disease. Nat. Med. 2023, 29, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.S.; Tresserra-Rimbau, A.; Karavasiloglou, N.; Jennings, A.; Cantwell, M.; Hill, C.; Perez-Cornago, A.; Bondonno, N.P.; Murphy, N.; Rohrmann, S.; et al. Association of Healthful Plant-based Diet Adherence With Risk of Mortality and Major Chronic Diseases Among Adults in the UK. JAMA Netw. Open 2023, 6, e234714. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. New Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Poveda, B.; Torres-Vargas, J.A.; Ocaña, M.D.C.; García-Caballero, M.; Medina, M.; Quesada, A.R. The Mediterranean Diet, a Rich Source of Angiopreventive Compounds in Cancer. Nutrients 2019, 11, 2036. [Google Scholar] [CrossRef] [PubMed]
- Stojanovic, J.; Giraldi, L.; Arzani, D.; Pastorino, R.; Biondi, A.; Persiani, R.; Boccia, S.; Leoncini, E. Adherence to Mediterranean diet and risk of gastric cancer: Results of a case-control study in Italy. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ. (ECP) 2017, 26, 491–496. [Google Scholar] [CrossRef]
- Li, W.Q.; Park, Y.; Wu, J.W.; Ren, J.S.; Goldstein, A.M.; Taylor, P.R.; Hollenbeck, A.R.; Freedman, N.D.; Abnet, C.C. Index-based dietary patterns and risk of esophageal and gastric cancer in a large cohort study. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2013, 11, 1130–1136.e2. [Google Scholar] [CrossRef]
- Bodén, S.; Myte, R.; Wennberg, M.; Harlid, S.; Johansson, I.; Shivappa, N.; Hébert, J.R.; Van Guelpen, B.; Nilsson, L.M. The inflammatory potential of diet in determining cancer risk; A prospective investigation of two dietary pattern scores. PLoS ONE 2019, 14, e0214551. [Google Scholar] [CrossRef]
- Thrift, A.P.; El-Serag, H.B. Burden of Gastric Cancer. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2020, 18, 534–542. [Google Scholar] [CrossRef]
- Karimi, P.; Islami, F.; Anandasabapathy, S.; Freedman, N.D.; Kamangar, F. Gastric cancer: Descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol. Biomark. Prev. A Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2014, 23, 700–713. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ (Clin. Res. Ed.) 2021, 372, n160. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Jackson, D.; White, I.R.; Thompson, S.G. Extending DerSimonian and Laird’s methodology to perform multivariate random effects meta-analyses. Stat. Med. 2010, 29, 1282–1297. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, A.; Grant, W.B. Vitamin D and Cancer: An Historical Overview of the Epidemiology and Mechanisms. Nutrients 2022, 14, 1448. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B. 25-hydroxyvitamin D and breast cancer, colorectal cancer, and colorectal adenomas: Case-control versus nested case-control studies. Anticancer Res. 2015, 35, 1153–1160. [Google Scholar]
- Greenland, S. Quantitative methods in the review of epidemiologic literature. Epidemiol. Rev. 1987, 9, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Briel, M.; Walter, S.D.; Guyatt, G.H. Is a subgroup effect believable? Updating criteria to evaluate the credibility of subgroup analyses. BMJ (Clin. Res. Ed.) 2010, 340, c117. [Google Scholar] [CrossRef] [PubMed]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clin. Res. Ed.) 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Acuna, N.; Park, S.Y.; Le Marchand, L.; Hébert, J.R.; Boushey, C.; Wilkens, L.R.; Wu, A.H.; Setiawan, V.W. Diet quality and risk of gastric adenocarcinoma: The Multiethnic Cohort. Am. J. Clin. Nutr. 2023, 117, 46–54. [Google Scholar] [CrossRef] [PubMed]
- van den Brandt, P.A. The impact of a healthy lifestyle on the risk of esophageal and gastric cancer subtypes. Eur. J. Epidemiol. 2022, 37, 931–945. [Google Scholar] [CrossRef] [PubMed]
- Tayyem, R.; Al-Awwad, N.; Allehdan, S.; Ajeen, R.; Al-Jaberi, T.; Rayyan, Y.; Bawadi, H.; Hushki, A. Mediterranean Dietary Pattern is Associated with Lower Odds of Gastric Cancer: A Case-Control Study. Cancer Manag. Res. 2022, 14, 2017–2029. [Google Scholar] [CrossRef]
- Amiry, F.; Mousavi, S.M.; Barekzai, A.M.; Esmaillzadeh, A. Adherence to the Mediterranean Diet in Relation to Gastric Cancer in Afghanistan. Front. Nutr. 2022, 9, 830646. [Google Scholar] [CrossRef]
- Álvarez-Álvarez, L.; Vitelli-Storelli, F.; Rubín-García, M.; Aragonés, N.; Ardanaz, E.; Castaño-Vinyals, G.; Obón-Santacana, M.; Dierssen-Sotos, T.; Salas-Trejo, D.; Tardón, A.; et al. Relationship between the Risk of Gastric Cancer and Adherence to the Mediterranean Diet According to Different Estimators. MCC-Spain Study. Cancers 2021, 13, 5281. [Google Scholar] [CrossRef]
- Schulpen, M.; Peeters, P.H.; van den Brandt, P.A. Mediterranean diet adherence and risk of esophageal and gastric cancer subtypes in the Netherlands Cohort Study. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2019, 22, 663–674. [Google Scholar] [CrossRef]
- Buckland, G.; Travier, N.; Huerta, J.M.; Bueno-de-Mesquita, H.B.; Siersema, P.D.; Skeie, G.; Weiderpass, E.; Engeset, D.; Ericson, U.; Ohlsson, B.; et al. Healthy lifestyle index and risk of gastric adenocarcinoma in the EPIC cohort study. Int. J. Cancer 2015, 137, 598–606. [Google Scholar] [CrossRef]
- Praud, D.; Bertuccio, P.; Bosetti, C.; Turati, F.; Ferraroni, M.; La Vecchia, C. Adherence to the Mediterranean diet and gastric cancer risk in Italy. Int. J. Cancer 2014, 134, 2935–2941. [Google Scholar] [CrossRef]
- Collatuzzo, G.; Teglia, F.; Pelucchi, C.; Negri, E.; Rabkin, C.S.; Liao, L.M.; Sinha, R.; López-Carrillo, L.; Lunet, N.; Morais, S.; et al. Inverse Association between Dietary Iron Intake and Gastric Cancer: A Pooled Analysis of Case-Control Studies of the Stop Consortium. Nutrients 2022, 14, 2555. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, J.; Yu, F.; Tian, Y.; Wu, Y.; Cui, L.; Liu, L.E. The association between soy-based food and soy isoflavone intake and the risk of gastric cancer: A systematic review and meta-analysis. J. Sci. Food Agric. 2021, 101, 5314–5324. [Google Scholar] [CrossRef]
- D’Elia, L.; Rossi, G.; Ippolito, R.; Cappuccio, F.P.; Strazzullo, P. Habitual salt intake and risk of gastric cancer: A meta-analysis of prospective studies. Clin. Nutr. (Edinb. Scotl.) 2012, 31, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Kim, K.; Lee, S.A.; Kwon, S.O.; Lee, J.K.; Keum, N.; Park, S.M. Effect of Red, Processed, and White Meat Consumption on the Risk of Gastric Cancer: An Overall and Dose-Response Meta-Analysis. Nutrients 2019, 11, 826. [Google Scholar] [CrossRef] [PubMed]
- Turati, F.; Pelucchi, C.; Guercio, V.; La Vecchia, C.; Galeone, C. Allium vegetable intake and gastric cancer: A case-control study and meta-analysis. Mol. Nutr. Food Res. 2015, 59, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Dai, C.; Zhou, L.; Li, Y.; Liu, K.; Deng, Y.J.; Li, N.; Zheng, Y.; Hao, Q.; Yang, S.; et al. Meta-analysis of the association between nut consumption and the risks of cancer incidence and cancer-specific mortality. Aging 2020, 12, 10772–10794. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Adherence to Mediterranean diet and risk of cancer: A systematic review and meta-analysis of observational studies. Int. J. Cancer 2014, 135, 1884–1897. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Schwedhelm, C.; Galbete, C.; Hoffmann, G. Adherence to Mediterranean Diet and Risk of Cancer: An Updated Systematic Review and Meta-Analysis. Nutrients 2017, 9, 1063. [Google Scholar] [CrossRef]
- Casas, R.; Sacanella, E.; Estruch, R. The immune protective effect of the Mediterranean diet against chronic low-grade inflammatory diseases. Endocr. Metab. Immune Disord. Drug Targets 2014, 14, 245–254. [Google Scholar] [CrossRef]
- Russo, G.I.; Solinas, T.; Urzì, D.; Privitera, S.; Campisi, D.; Cocci, A.; Carini, M.; Madonia, M.; Cimino, S.; Morgia, G. Adherence to Mediterranean diet and prostate cancer risk in Sicily: Population-based case-control study. Int. J. Impot. Res. 2019, 31, 269–275. [Google Scholar] [CrossRef]
- Jenab, M.; Bueno-de-Mesquita, H.B.; Ferrari, P.; van Duijnhoven, F.J.; Norat, T.; Pischon, T.; Jansen, E.H.; Slimani, N.; Byrnes, G.; Rinaldi, S.; et al. Association between pre-diagnostic circulating vitamin D concentration and risk of colorectal cancer in European populations:a nested case-control study. BMJ (Clin. Res. Ed.) 2010, 340, b5500. [Google Scholar] [CrossRef]
- Heaney, R.P. Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutr. Rev. 2014, 72, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Bakhti, S.Z.; Latifi-Navid, S. Oral microbiota and Helicobacter pylori in gastric carcinogenesis: What do we know and where next? BMC Microbiol. 2021, 21, 71. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ying, X.; Liu, S.; Lyu, G.; Xu, Z.; Zhang, X.; Li, H.; Li, Q.; Wang, N.; Ji, J. Gastric cancer: Epidemiology, risk factors and prevention strategies. Chin. J. Cancer Res.= Chung-Kuo Yen Cheng Yen Chiu 2020, 32, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Bakhti, S.Z.; Latifi-Navid, S.; Zahri, S.; Yazdanbod, A. Inverse association of Helicobacter pylori cagPAI genotypes with risk of cardia and non-cardia gastric adenocarcinoma. Cancer Med. 2019, 8, 4928–4937. [Google Scholar] [CrossRef]
- Goldbohm, R.A.; van den Brandt, P.A.; Brants, H.A.; van’t Veer, P.; Al, M.; Sturmans, F.; Hermus, R.J. Validation of a dietary questionnaire used in a large-scale prospective cohort study on diet and cancer. Eur. J. Clin. Nutr. 1994, 48, 253–265. [Google Scholar]
- Trichopoulou, A.; Orfanos, P.; Norat, T.; Bueno-de-Mesquita, B.; Ocké, M.C.; Peeters, P.H.; van der Schouw, Y.T.; Boeing, H.; Hoffmann, K.; Boffetta, P.; et al. Modified Mediterranean diet and survival: EPIC-elderly prospective cohort study. BMJ (Clin. Res. Ed.) 2005, 330, 991. [Google Scholar] [CrossRef]
- Tognon, G.; Nilsson, L.M.; Lissner, L.; Johansson, I.; Hallmans, G.; Lindahl, B.; Winkvist, A. The Mediterranean diet score and mortality are inversely associated in adults living in the subarctic region. J. Nutr. 2012, 142, 1547–1553. [Google Scholar] [CrossRef]
- Fung, T.T.; McCullough, M.L.; Newby, P.K.; Manson, J.E.; Meigs, J.B.; Rifai, N.; Willett, W.C.; Hu, F.B. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am. J. Clin. Nutr. 2005, 82, 163–173. [Google Scholar] [CrossRef]
- Buckland, G.; Agudo, A.; Luján, L.; Jakszyn, P.; Bueno-de-Mesquita, H.B.; Palli, D.; Boeing, H.; Carneiro, F.; Krogh, V.; Sacerdote, C.; et al. Adherence to a Mediterranean diet and risk of gastric adenocarcinoma within the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort study. Am. J. Clin. Nutr. 2010, 91, 381–390. [Google Scholar] [CrossRef]
- Castelló, A.; Fernández de Larrea, N.; Martín, V.; Dávila-Batista, V.; Boldo, E.; Guevara, M.; Moreno, V.; Castaño-Vinyals, G.; Gómez-Acebo, I.; Fernández-Tardón, G.; et al. High adherence to the Western, Prudent, and Mediterranean dietary patterns and risk of gastric adenocarcinoma: MCC-Spain study. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2018, 21, 372–382. [Google Scholar] [CrossRef]
- Castelló, A.; Buijsse, B.; Martín, M.; Ruiz, A.; Casas, A.M.; Baena-Cañada, J.M.; Pastor-Barriuso, R.; Antolín, S.; Ramos, M.; Muñoz, M.; et al. Evaluating the Applicability of Data-Driven Dietary Patterns to Independent Samples with a Focus on Measurement Tools for Pattern Similarity. J. Acad. Nutr. Diet. 2016, 116, 1914–1924.e6. [Google Scholar] [CrossRef] [PubMed]
- Castelló, A.; Pollán, M.; Buijsse, B.; Ruiz, A.; Casas, A.M.; Baena-Cañada, J.M.; Lope, V.; Antolín, S.; Ramos, M.; Muñoz, M.; et al. Spanish Mediterranean diet and other dietary patterns and breast cancer risk: Case-control EpiGEICAM study. Br. J. Cancer 2014, 111, 1454–1462. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Dinu, M.; Pagliai, G.; Marcucci, R.; Casini, A. Validation of a literature-based adherence score to Mediterranean diet: The MEDI-LITE score. Int. J. Food Sci. Nutr. 2017, 68, 757–762. [Google Scholar] [CrossRef] [PubMed]


| First Author [Ref], Year | Region | Design | Population; Follow-Up or Cases/Controls | Age (Years) | Gender | Components of Score | Adjustment | Multivariate-Adjusted OR/HR (95% CI) | NOS |
|---|---|---|---|---|---|---|---|---|---|
| Acuna [22], 2023 | United states | Prospective cohort | 176,752; 19.2 y | 45–75 | M/F | 1. ↑ vegetables (including potatoes); 2. ↑ fruits; 3. ↑ nuts; 4. ↑ legumes; 5. ↑ fish; 6. ↑ whole grains; 7. ↑ MUFA/SFA ratio; 8. ↔ alcohol; 9. ↓ red and processed meat | Age, sex, race, smoking status, pack years of cigarette smoking, BMI, total energy intake | GCA: HR: 1.56 (0.95, 2.54); GNCA: HR: 0.99 (0.78, 1.26) | 8 |
| Brandt [23], 2022 | The Netherlands | Prospective cohort | 120,852; 20.3 y | 55–69 | M/F | 1. ↑ vegetables (without potatoes); 2. ↑ legumes; 3. ↑ fruits; 4. ↑ nuts; 5. ↑ whole grains; 6. ↑ fish; 7. ↑ MUFA/SFA ratio; 8. ↓ red and processed meat | Age, sex, smoking frequency and duration, highest level of education, family history, chronic diseases, energy intake, BMI, physical activity, alcohol intake | GCA: HR: 0.57 (0.34, 0.96) for highest score category (6–8) versus lowest score category (0–3); GNCA: HR: 0.72 (0.55, 0.96) for highest score category (6–8) versus lowest score category (0–3) | 9 |
| Buckland [28], 2015 | Europe | Prospective cohort | 461,550; 11.4 y | 25–70 | M/F | 1. ↑ vegetables; 2. ↑ legumes; 3. ↑ fruit (including nuts and seeds); 4. ↑ cereals; 5. ↑ fish and seafood; 6. ↑ olive oil; 7. ↓ meat; 8. ↓ dairy products | Total energy intake, education level, BMI, physical activity level | HR: 0.87 (0.69, 1.09) for highest score category (≥8) versus lowest score category (<8) | 7 |
| Bodén [9], 2019 | Sweden | Prospective cohort | 100,881; 15.0 y | 30–60 | M/F | 1. ↑ vegetables and potatoes; 2. ↑ fruit and juices; 3. ↑ whole-grain cereals; 4. ↑ fish and fish products; 5. ↑ MUFA+PUFA/SFA ratio; 6. ↔ alcohol intake; 7. ↓ meat and meat products; 8. ↓ dairy products | Energy intake, BMI, physical activity, smoking, educational status | HR: 0.85 (0.69, 1.03) per one tertile increase | 6 |
| Li [8], 2013 | United States | Prospective cohort | 49,468; 11.0 y | 51–70 | M/F | 1. ↑ vegetables; 2. ↑ legumes; 3. ↑ fruit; 4. ↑ nuts; 5. ↑ whole grains; 6. ↑ fish; 7. ↑ MUFA/SFA ratio; 8. ↓ red and processed meat; 9. ↔ alcohol | Age, sex, race, smoking, education, BMI, vigorous physical activity, usual activity, total energy intake | GCA: HR: 1.10 (0.76, 1.61) for fifth versus first quintile; GNCA: HR: 0.75 (0.52, 1.09) for fifth versus first quintile | 8 |
| Schulpen [27], 2019 | The Netherlands | Case–cohort | 120,852; 20.3 y | 55–69 | M/F | 1. ↑ vegetables; 2. ↑ fruits; 3. ↑ nuts; 4. ↑ legumes; 5. ↑ fish; 6. ↑ whole grains; 7. ↑ MUFA/SFA ratio; 8. ↓ red and processed meat | Age at baseline, sex, cigarette smoking status, cigarette smoking frequency, cigarette smoking duration, BMI, total daily energy intake, alcohol consumption, highest level of education, non-occupational physical activity, and family history of gastric cancer | GCA: HR: 0.86 (0.71, 1.04); GNCA: HR: 0.83 (0.73, 0.93) | 7 |
| Tayyem [24], 2022 | Jordan | Case–control | 172/314 | Case: 54.1 ± 1.0; Control: 54.0 ± 0.7 | M/F | 1. ↑ fruits and juices; 2. ↑ vegetables; 3. ↑ lentils; 4. ↑ dairy products; 5. ↑ olive oil; 6. ↓ meat and meat products; 7. ↓ drinks and snacks | Age, gender, BMI, smoking, marital status, total energy intake, education level, family history, physical activity | OR: 0.212 (0.107, 0.419) for forth versus first quartile | 6 |
| Amiry [25], 2022 | Afghanistan | Case–control | 90/180 | 20–75 | M/F | 1. ↑ vegetables; 2. ↑ fruits; 3. ↑ nuts; 4. ↑ legumes; 5. ↑ fish; 6. ↑ whole grains; 7. ↑ MUFA/SFA ratio; 8. ↓ red and processed meat; 9. ↓ dairy products | Age, sex, physical activity, marriage status, smoking usage, toothbrushing, job, education, alcohol usage, BMI | OR: 0.17 (0.03, 0.80) for highest tertile versus lowest tertile | 7 |
| Álvarez-Álvarez [26], 2021 | Spain | Case–control | 354/3040 | 20–85 | M/F | 1. ↑ fruits; 2. ↑ vegetables (leafy, fruiting root, other); 3. ↑ legumes; 4. ↑ boiled potatoes; 5. ↑ fish (white and oily); 6. ↑ seafood/shellfish; 7. ↑ olives and vegetable oil; 8. ↓ juices | Sex, age, education, family history of gastric cancer, tobacco status, total energy consumed, BMI, NSAIDs intake, physical activity | OR: 0.32 (0.22, 0.46) for highest tertile versus lowest tertile | 8 |
| Stojanovic [7], 2017 | Italy | Case–control | 223/223 | NA | M/F | 1. ↑ fruit; 2. ↑ vegetables; 3. ↑ legumes; 4. ↑ fish; 5. ↓ meat and meat products; 6. ↔ alcohol | Sex, tobacco smoking, total energy intake | OR: 0.70 (0.61, 0.81) | 6 |
| Praud [29], 2013 | Italy | Case–control | 999/2628 | 19–80 | M/F | 1. ↑ cereals; 2. ↑ fruit; 3. ↑ vegetables; 4. ↑ legumes; 5. ↑ fish; 6. ↑ MUFA/SFA ratio; 7. ↓ milk (including dairy products); 8. ↓ meat (including meat products); 9. ↔ alcohol | Age, sex, study, year of interview, education, BMI, tobacco smoking, family history, total energy intake | OR: 0.57 (0.45, 0.70) for score ≥ 6 versus score ≤ 3 | 7 |
| Subgroups | No. of Studies | OR (95% CI) | Heterogeneity Test | |
|---|---|---|---|---|
| I2 | p Value | |||
| Geographic location | ||||
| Asia | 2 | 0.21 (0.11, 0.39) | 0.0% | 0.81 |
| Europe | 3 | 0.52 (0.36, 0.76) | 87.3% | <0.001 |
| Gender | ||||
| Male | 2 | 0.78 (0.65, 0.93) | 75.5% | 0.04 |
| Female | 2 | 0.83 (0.68, 1.01) | 57.1% | 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, X.; Li, X.; Ding, S.; Dai, D. Adherence to the Mediterranean Diet and Risk of Gastric Cancer: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 3826. https://doi.org/10.3390/nu15173826
Bai X, Li X, Ding S, Dai D. Adherence to the Mediterranean Diet and Risk of Gastric Cancer: A Systematic Review and Meta-Analysis. Nutrients. 2023; 15(17):3826. https://doi.org/10.3390/nu15173826
Chicago/Turabian StyleBai, Xiao, Xue Li, Siqi Ding, and Dongqiu Dai. 2023. "Adherence to the Mediterranean Diet and Risk of Gastric Cancer: A Systematic Review and Meta-Analysis" Nutrients 15, no. 17: 3826. https://doi.org/10.3390/nu15173826






