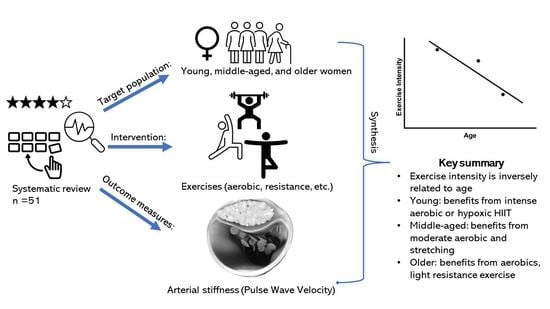
Effect of Exercise on Arterial Stiffness in Healthy Young, Middle-Aged and Older Women: A Systematic Review
Abstract
:1. Introduction
2. Methods
2.1. Data Selection
2.2. Studies Selection
2.3. Data Extraction and Studies Methodological Quality
3. Results and Discussions
3.1. Defining Exercise Intensity
3.2. Effect of Exercise on Arterial Stiffness in Young Women
3.3. Effect of Exercise on Arterial Stiffness in Middle-Aged Women
3.4. Effect of Exercise on Arterial Stiffness in Older Women
3.5. Possible Mechanisms Underlying the Effect of Exercise on Arterial Stiffness in Women
3.6. General Guidelines and Direction for Further Studies Investigating the Effect of Exercise on Arterial Stiffness in Women
4. Summary
4.1. What Is Already Known?
- Arterial stiffness is an independent predictor of cardiovascular disease.
- Arterial stiffness increases with age in women.
- Exercise can improve arterial stiffness in pathological states.
4.2. What Are the New Findings?
- The effect of exercise is dependent on age and arterial stiffness measure.
- Exercise intensity is inversely related to age and arterial stiffness measure.
- For young women, prolonged high-intensity aerobic exercise is recommended.
- For middle-aged women, moderate-intensity aerobic or stretching exercises are recommended.
- For older women, any intensity of aerobic exercise, such as daily walking and cycling, or light-intensity resistance training are recommended.
- To summarise, structured exercises can influence arterial stiffness positively. In addition, aerobic exercise is consistently found to be beneficial for woman, with an inverse relationship between age and recommended aerobic exercise intensity.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zieman, S.J.; Melenovsky, V.; Kass, D.A. Mechanisms, Pathophysiology, and Therapy of Arterial Stiffness. Arter. Thromb. Vasc. Biol. 2005, 25, 932–943. [Google Scholar] [CrossRef] [Green Version]
- Safar, M.E.; Asmar, R.; Benetos, A.; Blacher, J.; Boutouyrie, P.; Lacolley, P.; Laurent, S.; London, G.; Pannier, B.; Protogerou, A.; et al. Interaction Between Hypertension and Arterial Stiffness. Hypertension 2018, 72, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Hsu, B.-G.; Tsai, J.-P. Arterial stiffness: A brief review. Tzu-Chi Med. J. 2021, 33, 115–121. [Google Scholar] [CrossRef]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redán, J.; Zanchetti, A.; Böhm, M.; Christiaens, T.; Cifkova, R.; De Backer, G.; Dominiczak, A.; et al. 2013 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC): ESH/ESC Task Force for the Management of Arterial Hypertension. J. Hypertens. 2013, 31, 1925–1938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maas, A.; Appelman, Y. Gender differences in coronary heart disease. Neth. Heart J. 2010, 18, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Vogel, B.; Acevedo, M.; Appelman, Y.; Merz, C.N.B.; Chieffo, A.; Figtree, G.A.; Guerrero, M.; Kunadian, V.; Lam, C.S.P.; Maas, A.H.E.M.; et al. The Lancet women and cardiovascular disease Commission: Reducing the global burden by 2030. Lancet 2021, 397, 2385–2438. [Google Scholar] [CrossRef] [PubMed]
- Strong, J.P.; Malcom, G.T.; Mcmahan, C.A.; Tracy, R.E.; Newman, W.P., III; Herderick, E.E.; Cornhill, J.F. Prevalence and extent of atherosclerosis in adolescents and young adults: Implications for prevention from the Pathobiological Determinants of Atherosclerosis in Youth Study. JAMA 1999, 281, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Panchangam, C.; Merrill, E.D.; Raghuveer, G. Utility of arterial stiffness assessment in children. Cardiol. Young 2018, 28, 362–376. [Google Scholar] [CrossRef]
- DuPont, J.J.; Kenney, R.M.; Patel, A.R.; Jaffe, I.Z. Sex differences in mechanisms of arterial stiffness. Br. J. Pharmacol. 2019, 176, 4208–4225. [Google Scholar] [CrossRef]
- Pierce, D.R.; Doma, K.; Leicht, A.S. Acute Effects of exercise mode on arterial stiffness and wave reflection in healthy young adults: A systematic review and meta-analysis. Front. Physiol. 2018, 9, 73. [Google Scholar] [CrossRef]
- Lephart, E.D.; Naftolin, F. Menopause and the skin: Old favorites and new innovations in cosmeceuticals for estrogen-deficient skin. Dermatol. Ther. 2021, 11, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Hart, E.C.; Joyner, M.J. The curse of the sympathetic nervous system: Are men or women more unfortunate? J. Physiol. 2010, 588 Pt 22, 4345–4346. [Google Scholar] [CrossRef] [PubMed]
- Gerlo, E.A.M.; Schoors, D.F.; Dupont, A.G. Age- and sex-related differences for the urinary excretion of norepinephrine, epinephrine, and dopamine in adults. Clin. Chem. 1991, 37, 875–878. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, V.; Vickneson, K.; Kofidis, T.; Woo, C.C.; Lin, X.Y.; Foo, R.; Shanahan, C.M. Role of vascular smooth muscle cell plasticity and interactions in vessel wall inflammation. Front. Immunol. 2020, 11, 599415. [Google Scholar] [CrossRef]
- Semba, R.D.; Nicklett, E.J.; Ferrucci, L. Does accumulation of advanced glycation end products contribute to the aging phenotype? J. Gerontol. Ser. A 2010, 65A, 963–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Ling, W.; Teng, X.; Quan, C.; Cai, S.; Hu, S. Effect of advanced glycation end products, extracellular matrix metalloproteinase inducer and matrix metalloproteinases on type-I collagen metabolism. Biomed. Rep. 2016, 4, 691–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doonan, R.J.; Mutter, A.; Egiziano, G.; Gomez, Y.-H.; Daskalopoulou, S.S. Differences in arterial stiffness at rest and after acute exercise between young men and women. Hypertens. Res. 2013, 36, 226–231. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.-J.; Du, L.-F.; Luo, X.-H. Gender differences in ventricular-vascular coupling following exercise. Chin. Med. Sci. J. 2015, 30, 231–238. [Google Scholar] [CrossRef]
- Kioi, K.; Yamamoto, R.; Mori, K.; Nomura, T. Acute effect of resistance exercise on arterial stiffness in healthy young women. J. Allied Health Sci. 2018, 9, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Fjeldstad, A.S.; Bemben, M.G.; Bemben, D.A. Resistance training effects on arterial compliance in premenopausal women. Angiology 2009, 60, 750–756. [Google Scholar] [CrossRef]
- Rossow, L.M.; Fahs, C.A.; Thiebaud, R.S.; Loenneke, J.P.; Kim, D.; Mouser, J.G.; Shore, E.A.; Beck, T.W.; Bemben, D.A.; Bemben, M.G. Arterial stiffness and blood flow adaptations following eight weeks of resistance exercise training in young and older women. Exp. Gerontol. 2014, 53, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [Green Version]
- The Society for Adolescent Health and Medicine. Young adult health and well-being: A position statement of the Society for Adolescent Health and Medicine. J. Adolesc. Health 2017, 60, 758–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowal, P.; Dowd, J.E. Definition of an Older Person. Proposed Working Definition of an Older Person in Africa for the MDS Project; World Health Organization: Geneva, Switzerland, 2001; Volume 2, pp. 5188–9286.
- Park, H.-Y.; Jung, W.-S.; Kim, S.-W.; Lim, K. Effects of interval training under hypoxia on the autonomic nervous system and arterial and hemorheological function in healthy women. Int. J. Women’s Health 2022, 14, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Merchant, N.; Bajdek, N. Racial differences in arterial stiffness following acute bouts of anaerobic exercise. Circulation. 2021, 144, A10585. [Google Scholar] [CrossRef]
- Lee, C.; Lee, S.; Lee, C.; Lee, S. Acute effects of foam rolling exercises on arterial stiffness, flexibility and autonomic nervous system function in young and middle-aged women. Exerc. Sci. 2021, 30, 491–500. [Google Scholar] [CrossRef]
- Marshall, E.M.; Parks, J.C.; Singer, T.J.; Tai, Y.L.; DeBord, A.R.; Humm, S.M.; Kingsley, J.D. Vascular responses to high-intensity battling rope exercise between the sexes. J. Sport. Sci. Med. 2021, 20, 349–356. [Google Scholar] [CrossRef]
- Lim, J.; Kim, H.; Hwang, C.; Yoo, J.; Perez, H.; Handberg, E.M.; Christou, D. Acute Effects of Aerobic Exercise on Arterial Stiffness and Wave Reflection in Young Men and Young Premenopausal Women. FASEB J. 2018, 32, 587. [Google Scholar] [CrossRef]
- Tomschi, F.; Köster, P.; Predel, H.-G.; Lay, D.; Bloch, W.; Grau, M. Acute effects of lower and upper body-resistance training on arterial stiffness, peripheral, and central blood pressure in young normotensive women. Sport Sci. Health 2018, 14, 357–363. [Google Scholar] [CrossRef]
- Augustine, J.A.; Lefferts, W.K.; Heffernan, K.S. Sex differences in aortic stiffness following acute resistance exercise. Artery Res. 2018, 23, 52–55. [Google Scholar] [CrossRef]
- Okamoto, T.; Kobayashi, R.; Sakamaki-Sunaga, M. Effect of resistance exercise on arterial stiffness during the follicular and luteal phases of the menstrual cycle. Int. J. Sport. Med. 2017, 38, 347–352. [Google Scholar] [CrossRef]
- Kingsley, J.D.; Tai, Y.L.; Mayo, X.; Glasgow, A.; Marshall, E. Free-weight resistance exercise on pulse wave reflection and arterial stiffness between sexes in young, resistance-trained adults. Eur. J. Sport Sci. 2017, 17, 1056–1064. [Google Scholar] [CrossRef]
- Perdomo, S.J.; Moody, A.M.; McCoy, S.M.; Barinas-Mitchell, E.; Jakicic, J.M.; Gibbs, B.B. Effects on carotid–femoral pulse wave velocity 24 h post exercise in young healthy adults. Hypertens. Res. 2016, 39, 435–439. [Google Scholar] [CrossRef]
- Lane, A.D.; Yan, H.; Ranadive, S.M.; Kappus, R.M.; Sun, P.; Cook, M.D.; Harvey, I.; Woods, J.; Wilund, K.; Fernhall, B. Sex differences in ventricular–vascular coupling following endurance training. Eur. J. Appl. Physiol. 2014, 114, 2597–2606. [Google Scholar] [CrossRef] [Green Version]
- Harris, E.; Rakobowchuk, M.; Birch, K.M. Sprint interval and sprint continuous training increases circulating CD34+ cells and cardio-respiratory fitness in young healthy women. PloS ONE 2014, 9(9), e108720. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Masuhara, M.; Ikuta, K. Effects of eccentric and concentric resistance training on arterial stiffness. J. Hum. Hypertens. 2006, 20, 348–354. [Google Scholar] [CrossRef]
- Cebrowska, K.; Minczykowski, A.; Krauze, T.; Guzik, P.; Wykrętowicz, A. Arterial stiffness increases in response to an acute arterial load challenge induced by an isometric handgrip in healthy individuals. Kardiol. Pol. 2022, 80, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Grigoriadis, G.; Rosenberg, A.J.; Lefferts, W.K.; Wee, S.O.; Schroeder, E.C.; Baynard, T. similar effects of acute resistance exercise on carotid stiffness in males and females. Int. J. Sport. Med. 2020, 41, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Chen, X.; Zeng, Z.; Li, S.; Wang, J.; Yu, F.; Liu, S.; Li, H.; Fernhall, B. Sex differences in lower-limb arterial stiffness following acute aerobic exercise. Sci. Sport. 2020, 35, e39–e48. [Google Scholar] [CrossRef]
- Augustine, N.K.N.; Heffernan, K.S. Menstrual phase and the vascular response to acute resistance exercise. Eur. J. Appl. Physiol. 2018, 118, 937–946. [Google Scholar] [CrossRef]
- Logan, J.G.; Kim, S.-S.; Lee, M.; Byon, H.D.; Yeo, S. Effects of static stretching exercise on lumbar flexibility and central arterial stiffness. J. Cardiovasc. Nurs. 2018, 33, 322–328. [Google Scholar] [CrossRef]
- Shinno, H.; Kurose, S.; Yamanaka, Y.; Higurashi, K.; Fukushima, Y.; Tsutsumi, H.; Kimura, Y. Evaluation of a static stretching intervention on vascular endothelial function and arterial stiffness. Eur. J. Sport Sci. 2017, 17, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Bemben, M.G.; Bemben, D.A. Effects of an 8-month yoga intervention on arterial compliance and muscle strength in premenopausal women. J. Sport. Sci. Med. 2012, 11, 322–330. [Google Scholar]
- Okamoto, T.; Masuhara, M.; Ikuta, K. Home-based resistance training improves arterial stiffness in healthy premenopausal women. Eur. J. Appl. Physiol. 2009, 107, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, M.; Maeda, S.; Miyaki, A.; Misono, M.; Saito, Y.; Tanabe, K.; Kuno, S.; Ajisaka, R. Effect of 12 weeks of moderate-intensity resistance training on arterial stiffness: A randomised controlled trial in women aged 32-59 years. Br. J. Sport. Med. 2009, 43, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Sabatier, M.J.; Schwark, E.H.; Lewis, R.; Sloan, G.; Cannon, J.; McCully, K. Femoral artery remodeling after aerobic exercise training without weight loss in women. Dyn. Med. 2008, 7, 13. [Google Scholar] [CrossRef] [Green Version]
- Cortez-Cooper, M.Y.; Devan, A.E.; Anton, M.M.; Farrar, R.P.; Beckwith, K.A.; Todd, J.S.; Tanaka, H. Effects of high intensity resistance training on arterial stiffness and wave reflection in women. Am. J. Hypertens. 2005, 18, 930–934. [Google Scholar] [CrossRef] [Green Version]
- Pekas, E.J.; Shin, J.; Son, W.-M.; Headid, R.J.; Park, S.-Y. Habitual combined exercise protects against age-associated decline in vascular function and lipid profiles in elderly postmenopausal women. Int. J. Environ. Res. Public Health 2020, 17, 3893. [Google Scholar] [CrossRef]
- Jaime, S.J.; Maharaj, A.; Alvarez-Alvarado, S.; Figueroa, A. Impact of low-intensity resistance and whole-body vibration training on aortic hemodynamics and vascular function in postmenopausal women. Hypertens. Res. 2019, 42, 1979–1988. [Google Scholar] [CrossRef]
- Molisz, A.; Schmederer, Z.; Siebert, J.; Kadamani, T.; Glasner, P.; Rosłonkiewicz, K.; Nowicka-Sauer, K.; Gutknecht, P.; Trzeciak, B.; Suchanowski, A. Haemodynamic parameters in postmenopausal women—Beneficial effect of moderate continuous exercise training. Ann. Agric. Environ. Med. 2019, 26, 425–428. [Google Scholar] [CrossRef]
- Kim, J.H.; Jung, Y.S.; Kim, J.W.; Ha, M.S.; Ha, S.M.; Kim, D.Y. Effects of aquatic and land-based exercises on amyloid beta, heat shock protein 27, and pulse wave velocity in elderly women. Exp. Gerontol. 2018, 108, 62–68. [Google Scholar] [CrossRef]
- Nishiwaki, M.; Fujibayashi, M.; Nanayama, C.; Ogawa, N.; Itakura, I.; Matsumoto, N. Increasing levels of daily physical activity for arterial stiffness reduction in older women: A community-based pilot study. J. Sport. Med. Phys. Fit. 2018, 58, 1701–1709. [Google Scholar] [CrossRef]
- Yasuda, T.; Fukumura, K.; Tomaru, T.; Nakajima, T. Thigh muscle size and vascular function after blood flow-restricted elastic band training in older women. Oncotarget 2016, 7, 33595–33607. [Google Scholar] [CrossRef] [Green Version]
- Yasuda, T.; Fukumura, K.; Iida, H.; Nakajima, T. Effects of detraining after blood flow-restricted low-load elastic band training on muscle size and arterial stiffness in older women. Springerplus 2015, 4, 348. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-Y.; Lee, D.-C. Cardiac and pulmonary benefits of forest walking versus city walking in elderly women: A randomised, controlled, open-label trial. Eur. J. Integr. Med. 2014, 6, 5–11. [Google Scholar] [CrossRef]
- Matsubara, T.; Miyaki, A.; Akazawa, N.; Choi, Y.; Ra, S.-G.; Tanahashi, K.; Kumagai, H.; Oikawa, S.; Maeda, S. Aerobic exercise training increases plasma Klotho levels and reduces arterial stiffness in postmenopausal women. Am. J. Physiol.-Heart Circ. Physiol. 2014, 306, H348–H355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanahashi, K.; Akazawa, N.; Miyaki, A.; Choi, Y.; Ra, S.-G.; Matsubara, T.; Kumagai, H.; Oikawa, S.; Maeda, S. aerobic exercise training decreases plasma asymmetric dimethylarginine concentrations with increase in arterial compliance in postmenopausal women. Am. J. Hypertens. 2014, 27, 415–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corrick, K.L.; Hunter, G.R.; Fisher, G.; Glasser, S.P. Changes in vascular hemodynamics in older women following 16 weeks of combined aerobic and resistance training. J. Clin. Hypertens. (Greenwich) 2013, 15, 241–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, X.; Hui-Chan, C.W.Y.; Tsang, W.W.N. Effects of Tai Chi training on arterial compliance and muscle strength in female seniors: A randomized clinical trial. Eur. J. Prev. Cardiol. 2013, 20, 238–245. [Google Scholar] [CrossRef]
- Williams, A.D.; Ahuja, K.; Almond, J.B.; Robertson, I.K.; Ball, M.J. Progressive resistance training might improve vascular function in older women but not in older men. J. Sci. Med. Sport 2013, 16, 76–81. [Google Scholar] [CrossRef]
- Miyaki, A.; Maeda, S.; Choi, Y.; Akazawa, N.; Tanabe, Y.; Ajisaka, R. Habitual aerobic exercise increases plasma pentraxin 3 levels in middle-aged and elderly women. Appl. Physiol. Nutr. Metab. 2012, 37, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Ohta, M.; Hirao, N.; Mori, Y.; Takigami, C.; Eguchi, M.; Tanaka, H.; Ikeda, M.; Yamato, H. Effects of bench step exercise on arterial stiffness in post-menopausal women: Contribution of IGF-1 bioactivity and nitric oxide production. Growth Horm. IGF Res. 2012, 22, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Coelho, J.M.O.; Sales, M.M.; Moraes, J.F.V.N.; Asana, R.Y.; Neto, W.B.; Santana, H.A.P.; Silva, C.B.; Moreira, S.R.; Simões, H.G.; Lima, R.M.; et al. Influence of the I/D polymorphism of the angiotensin converting enzyme gene and acute aerobic exercise in the ambulatory arterial stiffness index of elderly women. J. Exerc. Physiol. Online 2011, 14, 1–9. [Google Scholar]
- Figueroa, A.; Park, S.Y.; Seo, D.Y.; Sanchez-Gonzalez, M.A.; Baek, Y.H. Combined resistance and endurance exercise training improves arterial stiffness, blood pressure, and muscle strength in postmenopausal women. Menopause 2011, 18, 980–984. [Google Scholar] [CrossRef]
- Nishiwaki, M.; Kawakami, R.; Saito, K.; Tamaki, H.; Takekura, H.; Ogita, F. Vascular adaptations to hypobaric hypoxic training in postmenopausal women. J. Physiol. Sci. 2011, 61, 83–91. [Google Scholar] [CrossRef]
- Miura, H.; Nakagawa, E.; Takahashi, Y. Influence of group training frequency on arterial stiffness in elderly women. Eur. J. Appl. Physiol. 2008, 104, 1039–1044. [Google Scholar] [CrossRef]
- Casey, D.P.; Pierce, G.L.; Howe, K.S.; Mering, M.C.; Braith, R.W. Effect of resistance training on arterial wave reflection and brachial artery reactivity in normotensive postmenopausal women. Eur. J. Appl. Physiol. 2007, 100, 403–408. [Google Scholar] [CrossRef]
- Sugawara, J.; Inoue, H.; Hayashi, K.; Yokoi, T.; Kono, I. Effect of low-intensity aerobic exercise training on arterial compliance in postmenopausal women. Hypertens. Res. 2004, 27, 897–901. [Google Scholar] [CrossRef] [Green Version]
- Canning, K.L.; Brown, R.E.; Jamnik, V.K.; Salmon, A.; Ardern, C.I.; Kuk, J.L. Individuals underestimate moderate and vigorous intensity physical activity. PLoS ONE 2014, 9, e97927. [Google Scholar] [CrossRef] [Green Version]
- Loprinzi, P.D.; Day, S.; Deming, R. Acute exercise intensity and memory function: Evaluation of the transient hypofrontality hypothesis. Medicina 2019, 55, 445. [Google Scholar] [CrossRef] [Green Version]
- Haff, G.G.; Triplett, N.T. Essentials of Strength Training and Conditioning, 4th ed.; Human Kinetics: Champaign, IL, USA, 2015. [Google Scholar]
- Larson-Meyer, D.E. A systematic review of the energy cost and metabolic intensity of yoga. Med. Sci. Sport. Exerc. 2016, 48, 1558–1569. [Google Scholar] [CrossRef] [PubMed]
- Kuehl, R.; Scharhag-Rosenberger, F.; Schommer, K.; Schmidt, M.E.; Dreger, P.; Huber, G.; Bohus, M.; Ulrich, C.M.; Wiskemann, J. Exercise intensity classification in cancer patients undergoing allogeneic HCT. Med. Sci. Sport. Exerc. 2015, 47, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Yoon, E.S.; Jung, S.J.; Cheun, S.K.; Oh, Y.S.; Kim, S.H.; Jae, S.Y. Effects of acute resistance exercise on arterial stiffness in young men. Korean Circ. J. 2010, 40, 16–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saz-Lara, A.; Cavero-Redondo, I.; Álvarez-Bueno, C.; Notario-Pacheco, B.; Ruiz-Grao, M.C.; Martínez-Vizcaíno, V. The acute effect of exercise on arterial stiffness in healthy subjects: A meta-analysis. J. Clin. Med. 2021, 10, 291. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, T.; Zhu, W.; Wu, H.; Yan, S. Acute effects of continuous and interval low-intensity exercise on arterial stiffness in healthy young men. Eur. J. Appl. Physiol. 2014, 114, 1385–1392. [Google Scholar] [CrossRef]
- Yamato, Y.; Hasegawa, N.; Sato, K.; Hamaoka, T.; Ogoh, S.; Iemitsu, M. Acute effect of static stretching exercise on arterial stiffness in healthy young adults. Am. J. Phys. Med. Rehabil. 2016, 95, 764–770. [Google Scholar] [CrossRef]
- Green, D.J.; Smith, K.J. Effects of exercise on vascular function, structure, and health in humans. Cold Spring Harb. Perspect. Med. 2018, 8, a029819. [Google Scholar] [CrossRef] [Green Version]
- Tomoto, T.; Sugawara, J.; Hirasawa, A.; Imai, T.; Maeda, S.; Ogoh, S. Impact of short-term training camp on arterial stiffness in endurance runners. J. Physiol. Sci. 2015, 65, 445–449. [Google Scholar] [CrossRef]
- Asano, R.Y.; Sales, M.M.; Coelho, J.M.; de Moraes, J.F.V.N.; Pereira, L.A.; Campbell, C.S.G.; Simoes, H.G. Exercise, nitric oxide, and endothelial dysfunction: A brief review. J. Exerc. Physiol. Online 2012, 15, 76–86. [Google Scholar]
- Houssiere, A.; Najem, B.; Ciarka, A.; Velez-Roa, S.; Naeije, R.; van de Borne, P. Chemoreflex and metaboreflex control during static hypoxic exercise. Am. J. Physiol.-Heart Circ. Physiol. 2005, 288, H1724–H1729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siasos, G.; Athanasiou, D.; Terzis, G.; Stasinaki, A.; Oikonomou, E.; Tsitkanou, S.; Kolokytha, T.; Spengos, K.; Papavassiliou, A.G.; Tousoulis, D. Acute effects of different types of aerobic exercise on endothelial function and arterial stiffness. Eur. J. Prev. Cardiol. 2016, 23, 1565–1572. [Google Scholar] [CrossRef]
- Mestek, M.L.; Westby, C.M.; Van Guilder, G.P.; Greiner, J.J.; Stauffer, B.; DeSouza, C.A. Regular aerobic exercise, without weight loss, improves endothelium-dependent vasodilation in overweight and obese adults. Obesity 2010, 18, 1667–1669. [Google Scholar] [CrossRef] [PubMed]
- Donley, D.A.; Fournier, S.B.; Reger, B.L.; DeVallance, E.; Bonner, D.E.; Olfert, I.M.; Frisbee, J.C.; Chantler, P.D. Aerobic exercise training reduces arterial stiffness in metabolic syndrome. J. Appl. Physiol. 2014, 116, 1396–1404. [Google Scholar] [CrossRef]
- Yokoyama, H.; Emoto, M.; Fujiwara, S.; Motoyama, K.; Morioka, T.; Koyama, H.; Shoji, T.; Inaba, M.; Nishizawa, Y. Short-term aerobic exercise improves arterial stiffness in type 2 diabetes. Diabetes Res. Clin. Pract. 2004, 65, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, N.T.; Martin, J.S.; Laughlin, M.H.; Padilla, J. Exercise-induced signals for vascular endothelial adaptations: Implications for cardiovascular disease. Curr. Cardiovasc. Risk Rep. 2012, 6, 331–346. [Google Scholar] [CrossRef] [Green Version]
- Drenth, M.H.; Zuidema, S.U.; Krijnen, W.P.; Bautmans, I.; Smit, A.J.; Van Der Schans, C.; Hobbelen, H. Advanced glycation end products are associated with physical activity and physical functioning in the older population. J. Gerontol. Ser. A 2018, 73, 1545–1551. [Google Scholar] [CrossRef]
- Ebert, H.; Lacruz, M.E.; Kluttig, A.; Simm, A.; Greiser, K.H.; Tiller, D.; Kartschmit, N.; Mikolajczyk, R. Advanced glycation end products and their ratio to soluble receptor are associated with limitations in physical functioning only in women: Results from the CARLA cohort. BMC Geriatr. 2019, 19, 299. [Google Scholar] [CrossRef]
- Gooding, H.C.; Gidding, S.S.; Moran, A.E.; Redmond, N.; Allen, N.B.; Bacha, F.; Burns, T.L.; Catov, J.M.; Grandner, M.A.; Harris, K.M.; et al. Challenges and opportunities for the prevention and treatment of cardiovascular disease among young adults: Report from a national heart, lung, and blood institute working group. J. Am. Heart Assoc. 2020, 9, e016115. [Google Scholar] [CrossRef]
- Xiong, J.; Qian, Y.; Yu, S.; Ji, H.; Teliewubai, J.; Chi, C.; Lu, Y.; Zhou, Y.; Fan, X.; Li, J.; et al. Somatotype and Its Impact on Asymptomatic Target Organ Damage in the Elderly Chinese: The Northern Shanghai Study. Clin. Interv. Aging 2021, 16, 887–895. [Google Scholar] [CrossRef]
- D’Elia, L.; Galletti, F.; La Fata, E.; Sabino, P.; Strazzullo, P. Effect of dietary sodium restriction on arterial stiffness: Systematic review and meta-analysis of the randomized controlled trials. J. Hypertens. 2018, 36, 734–743. [Google Scholar] [CrossRef] [PubMed]

| Filter: | (English) AND (in Title and Abstract) | Results |
|---|---|---|
| #1 | (exercise) and (women) and (arterial stiffness) | 549 |
| #2 | (exercise) and (female) and (arterial stiffness) | 182 |
| #3 | Total after merging duplicate articles from #1 and #2 | 677 |
| Reference | Subjects’ Characteristics | Exercise Prescription | Main Findings | ||
|---|---|---|---|---|---|
| Type | Duration/Frequency | Intensity | |||
| Park et al. (2022) [25] | Normoxic group; N = 10; Body Mass Index (BMI) = 19.1 ± 1.2 mkg−2 Hypoxic group; N = 10; BMI = 19.0 ± 1.1 mkg−2 All aged = 24.85 ± 3.84 yo | High-intensity interval Training (HIIT) | 90-min, 3 day/week for 6 weeks | 90–95% Heart rate (HR)max | Normoxic group showed no change in PWV. Hypoxic group showed reduction in PWV |
| Yan et al. (2021) [26] | African Americans (AA); N = 8; aged = 22 ± 1.0 yo; BMI = 24.7 ± 0.8 Caucasian Americans (CA); N = 12; aged = 22 ± 1.0 yo; BMI = 22.9 ± 0.7 | Anaerobic exercise | 30-min maximal anaerobic exercise (Exercise 1) with 30 min of recovery, following a maximal anaerobic exercise (exercise 2) | Maximal intensity | No changes in PWV. AA: Rest: 5.2 ± 0.2 m/s; 5 min post exercise 1: 5.0 ± 0.2 m/s; 15 min post exercise 1: 5.2 ± 0.3 m/s; 30 min post exercise 1: 5.1 ± 0.2 m/s; 5 min post exercise 2: 5.0 ± 0.3 m/s; 15 min post exercise 2: 5.2 ± 0.4 m/s; 30 min post exercise 2: 5.1 ± 0.3 m/s; CA: Rest: 4.9 ± 0.2 m/s; 5 min post exercise 1: 4.9 ± 0.2 m/s; 15 min post exercise 1: 5.2 ± 0.4 m/s; 30 min post exercise 1: 4.9 ± 0.4 m/s; 5 min post exercise 2: 4.9 ± 0.2 m/s; 15 min post exercise 2: 5.1 ± 0.3 m/s; 30 min post exercise 2: 4.9 ± 0.2 m/s |
| Lee & Lee (2021) [27] | N = 10; aged = 23.20 ± 0.59 yo; BMI = 25.2 ± 1.3 mkg−2 | Foam flexibility training | 30-min | Bodyweight to apply pressure | No changes in PWV. |
| Marshall et al. (2021) [28] | N = 10; aged = 22 ± 2.0 yo; BMI = 22.8 ± 2.2 mkg−2 | Resistance training (HI-BRE) | 6 set × 15 s | HR 180 bpm | Increase in PWV Rest: 5.4 ± 0.8 m/s; 10 min post exercise: 5.7 ± 1.0 m/s; 30 min post exercise: 5.4 ± 0.7 m/s; 60 min post exercise: 5.1 ± 0.5 m/s |
| Lim et al. (2018) [29] | N = 15; aged = 21.4 ± 0.7 yo; BMI = NA | HIIT vs. Aerobic exercise | HIIT: 40-min Aerobic: 47-min | HIIT at 90% HRmax Aerobic at 70% and 50% HRmax | HIIT group no change in PWV Aerobic: No change in PWV Rest: 5.5 ± 0.1 m/s; post-exercise: 5.5 ± 0.1 m/s |
| Tomschi et al. (2018) [30] | Upper body training group N = 10; aged = 21.5 ± 1.4 yo; BMI = 22.1 ± 2.0 mkg−2 Lower body training group N = 10; aged = 22.3 ± 1.30 yo; BMI = 23.3 ± 2.4 mkg−2 | Resistance exercise | Resistance exercise 4 movement × 3 set × 12 rep | At 70% of 1 RM | No change in PWV in both groups |
| Augustine et al. (2018) [31] | N = 13; aged = 24 ± 4.0; BMI = 22.0 ± 3.1 mkg−2 | Resistance training | 5 set × 5 rep bench press and 5 set × 10 rep bicep curl | 5 RM for bench press and 10 RM for bicep curl | Increase in PWV Rest: 5.1 ± 0.5 m/s; 10 min post exercise: 6.1 ± 0.8 m/s; 20 min post exercise: 5.7 ± 0.4 m/s |
| Okamoto et al. (2017) [32] | N = 9; aged = 21.30 ± 0.8 yo; BMI = NA | Resistance training | 5 set × 5 rep bench press and 5 set × 10 rep bicep curl | 80% 1 RM for bench press followed 70% 1 RM for bicep curl | Follicular phase showed an increase in PWV: increased from baseline by 10 ± 10% (Δ94 ± 91 cm/s) and 8 ± 9% (Δ74 ± 78 cm/s) at 30 and 60 min post exercise Luteal phase showed no change in PWV |
| Kingsley et al. (2017) [33] | N = 12: aged = 23 ± 4.0 yo; BMI = 24.3 ± 4.7 mkg−2 | Resistance training | 3 set × 10 rep of bench press and 3 set × 10 rep of dead lift | At 75% of 1 RM | Increase in PWV Rest: 5.1 ± 0.8 m/s; post exercise: 5.6 ± 0.7 m/s |
| Perdomo et al. (2016) [34] | N = 15; aged = 24.3 ± 3.0 yo; BMI = 23.4 ± 2.6 mkg−2 | Aerobic exercise | 30 min run | At 70–75% of HRmax | No difference in PWV Rest: 5.69 ± 0.64 m/s; post exercise: 5.62 ± 0.7 m/s |
| Lane et al. (2014) [35] | N = 25; aged = 24 ± 1.0 yo; BMI = 25.0 ± 4.0 mkg−2 | Aerobic exercise | 30–60 min for 3 days/week, 8 weeks | At 60–90% of HRmax | Decrease in arterial elastance Rest: 5.7 ± 1.2 m/s; post exercise: 5.3 ± 0.7 m/s |
| Harris et al. (2014) [36] | Interval training group; N = 6; BMI = 23.6 ± 1.8 mkg−2 Continuous training group; N = 6; BMI = 23.1 ± 2.6 mkg−2 All aged = 22 ± 2.0 yo; | Anaerobic sprint cycling | 3 days/week for 4 weeks | Maximum | No change in PWV Interval training group: Rest: 6.0 ± 0.8 m/s; post exercise: 6.2 ± 0.5 m/s Continuous training group: Rest: 6.6 ± 0.8 m/s; post exercise: 7.4 ± 0.7 m/s |
| Rossow et al. (2014) [21] | N = 16; aged = 22 ± 2.0 yo; BMI = NA | Resistance training | 6 movement × 3 set × 10 for 3 days/week, for 8 weeks | At 80% of 1 RM | No change in PWV |
| Doonan et al. (2013) [17] | N = 55; aged = 23.7 ± 4.80 yo; BMI = 21.7 ± 2.1 mkg−2 | Aerobic exercise | To exhaustion | Maximum, graded intensity (Bruce protocol) | No change in PWV |
| Okamoto et al. (2006) [37] | Sedentary group; N = 9; aged; 19.9 ± 1.20; BMI = 20.4 ± 3.1 mkg−2 Eccentric training group; N = 10; aged = 18.9 ± 0.30 yo; BMI = 21.7 ± 2.1 mkg−2 Concentric training group, N = 10; aged = 19.1 ± 0.30 yo; BMI = 21.9 ± 3.1 mkg−2 | Resistance training | 5 set × 10 rep of arm curl for 3 days/week 8 weeks | Eccentric group at 100% of 1 RM, and Concentric group at 80% of 1 RM | Eccentric group remain unchanged in PWV but increased after detraining. Concentric group increased in PWV and returned to bassline after detraining. |
| Reference | Subjects’ Characteristics | Exercise Prescription | Main Findings | ||
|---|---|---|---|---|---|
| Type | Time/Frequency | Intensity | |||
| Cebrowska et al. (2022) [38] | N = 22; aged = 35.4 ± 12.3 BMI = 26.0 ± 4.2 mkg−2 | Resistance training (IHG) | 3-min | 30% maximal IHG strength | Increase in ASI |
| Lee & Lee (2021) [27] | N = 10; aged = 44.50 ± 0.91 yo; BMI = 23.5 ± 0.8 mkg−2 | Flexibility training | 30-min | Bodyweight to apply pressure | No changes in PWV |
| Grigoriadis et al. (2020) [39] | N = 17,; aged = 25 ± 4 yo; BMI = 23.5 ± 4.2 mkg−2 | Resistance training | 3 set × 10 rep | Maximal isokinetic knee extension/flexion | Increase in PWV Rest: 5.1 ± 0.4 m/s; 5 min post exercise: 5.2 ± 0.4 m/s; 30 min post exercise: 5.2 ± 0.3 m/s |
| Sun et al. (2020) [40] | N = 29; aged = 27 ± 5 yo; BMI = 22.1 ± 2.8 mkg−2 | Aerobic exercise | 45-min | 70% HRR | Decrease in PWV; |
| Augustine et al. (2018) [41] | N = 18; aged = 28 ± 7 yo; BMI = 22.6 ± 2.9 mkg−2 | Resistance training | 5 set of 5 RM on the bench press then 5 set × 10 rep of biceps curl at different menstrual cycle phases | 5 RM and 10 RM | Increase in central PWV during early luteal phase (LP). Increase in peripheral PWV during follicular phase (FP). Decrease in central PWV during FP. Rest: 7.9 m/s; post exercise: 6.7 m/s Decrease in peripheral PWV during early LP. Rest: 7.9 m/s; post exercise: 6.7 m/s |
| Logan et al. (2018) [42] | N = 30; aged = 44.37 ± 10.8 yo; BMI = NA | Static Stretching | Repeated 3 to 5 times with 10 s of rest 30 min | “somewhat heavy” to “heavy” in Borg Scale | Decrease in PWV; Rest: 6.93 ± 1.54 m/s; post exercise: 6.29 ± 1.17 m/s |
| Shinno et al. (2017) [43] | N = 21; aged = 47.9 ± 2.2 yo; BMI = 21.6 ± 4.3 mkg−2 | Static Stretching | 20–30 s per site 7 days/week 6 months | Whole-body static stretching | Decrease in PWV; |
| Li et al. (2015) [18] | N = 18; aged = 25.5 ± 2.8; BMI = NA | Aerobic exercise | To exhaustion | Maximum, graded intensity (Bruce protocol) | Decrease in arterial elastance Rest: 8.68 ± 1.85 m/s; During exercise: 13.25 ± 3.92 m/s; post exercise: 10.70 ± 4.40 m/s |
| Kim et al. (2012) [44] | Exercise group; N = 16; aged = 45.7 ± 1.0 yo; BMI = 26.0 ± 1.0 mkg−2 Control group; N = 18; aged = 43.2 ± 1.0 yo BMI = 27.0 ± 1.0 mkg−2 | Stretching (Hata Yoga exercise) | 60-min; 2 days/week 8 months | 60–80% MHR | No change in arterial compliance |
| Fjeldstad et al. (2009) [20] | Training group; N = 21; aged = 33.2 ± 2.1 yo; BMI = 26.6 ± 1.4 mkg−2 Control group; N = 11; aged = 36.8 ± 3.2 yo; BMI = 24.4 ± 3.1 mkg−2 | Resistance training | 30 min of 7 movements × 2–3 sets (8 reps) 12 weeks | At 80% of 1 RM | No change in arterial compliance Rest: 7.6 ± 0.5 mL/mmHg × 100; post exercise: 7.8 ± 0.6 mL/mmHg × 100 |
| Okamoto et al. (2009) [45] | N = 12; aged = 42–55 yo; BMI = 23.6 ± 1.0 mkg−2 | Resistance training | 40-min, 6 movements × 2 sets (12–15 reps) × 2 days/week for 10 weeks. Between each set 10 min walk. | Body weight and light dumbbells (500–1000 g). | Decrease in PWV Rest: 1270 cm/s; post exercise: 1175 cm/s |
| Yoshizawa et al. (2009) [46] | N = 35, Resistance training group; Aged = 47 ± 2 yo; BMI = 21.6 ± 4.3 mkg−2; Aerobic exercise group; Aged = 47 ± 2 yo; BMI = 24.6 ± 1.1 mkg−2; Control group; Aged = 49 ± 3; BMI = 21.8 ± 1.0 mkg−2; | Resistance training Aerobic exercise | 6 movements × 3 sets (10 reps) × 2 days/week for 12 weeks. 30 min × 2 days/week cycling for 12 weeks. | Resistance: 60% 1 RM Aerobic: 60–70% VO2max | No change in PWV for Resistance group. Decrease in PWV for Aerobic group. |
| Sabatier et al. (2008) [47] | N = 13; aged = 33 ± 4 yo; BMI = 29.1 ± 9.1 mkg−2; High intensity group vs. Low intensity (cross-over) | Aerobic exercise | 50 min cycling × 2 days/week 14 weeks | High intensity: 75–90% HRR Low intensity: 55–65% HRR | No change in PWV for both intervention |
| Cortez-Cooper et al. (2005) [48] | N = 23; aged = 29 ± 1.0 yo; BMI = NA | Resistance training | 12 movements × 3–6 sets (5–10 reps) for 11 weeks | Light-day/heavy-day periodised approach (graded intensity) | Increase in PWV Rest: 791 ± 88 cm/s; post exercise: 833 ± 96 cm/s |
| Reference | Subjects’ Characteristics | Exercise Prescription | Main Findings | ||
|---|---|---|---|---|---|
| Type | Time/Frequency | Intensity | |||
| Pekas et al. (2020) [49] | Combined resistance and aerobic exercise group; N = 57; aged = 75 yo; BMI = 23.0 ± 4.0 mkg−2; Sedentary group; N = 44; aged = 78; BMI = 25.0 ± 3.0 mkg−2; | Combined resistance training and aerobic exercise | 8 movements × 3 sets (10–15 reps) and 30 min walking/jogging/cycling × 3 days/week for 1 year. | Resistance training 12–15 RPE and 50–60% HRR for walking/jogging/cycling. | Decrease in PWV in exercise group. Exercise group: 12.1 ± 2.0 m/s; Sedentary group: 12.8 ± 1.8 m/s |
| Jaime et al. (2019) [50] | N = 33; aged = 65 ± 4.0 yo; BMI = 23.3 ± 2.6 mkg−2; Resistance training vs. Vibration training. | Resistance training vs. Vibration training | Resistance training; 4 movements × 1 set (15 rep) × daily for 12 weeks. Vibration training; 4 movements × 2–3 sets daily × 12 weeks. | Resistance training at 40% 1 RM, Vibration training at 24–40 Hz. | No change in PWV for both groups. Resistance: Rest: 11.7 ± 0.7 m/s; post exercise: 11.6 ± 0.7 m/s Vibration: Rest: 11.0 ± 0.4 m/s; post exercise: 10.6 ± 0.4 m/s |
| Molisz et al. (2019) [51] | Regular physical group; N = 38; aged = 59.4 yo; BMI = 24.1 mkg−2; and Control group; N = 17; aged = 62.4 yo; BMI = 24.9 mkg−2. | Aerobic exercise for the Regular physical group and no exercise for the Control group. | Retrospective record of physical activity; 10 months record; at least 2 days/week of 1 hr session. | Low intensity | Decrease in PWV for Regular physical group. Exercise group: 7.4 m/s; Control group: 8.4 m/s |
| Kim et al. (2018) [52] | Aquatic group N = 14; aged = 66.77 ± 3.1 yo; BMI = NA, vs. Land-based group N = 14; aged = 67.42 ± 1.8 yo; BMI = NA. | Aquatic and land-based aerobic exercise. | Aquarobic 60 min × 2 days/week, 16 weeks vs., Aerobic (land-based) 60 min × 2 days/week for 16 weeks. | Graded intensity: (From 40–50% HRR to 65–70% HRR) | Decrease in PWV for Aquatic and Land-based groups |
| Nishiwaki et al. (2018) [53] | N = 21; aged = 76 ± 1.0 yo; BMI = 22.2 ± 0.7 mkg−2 | Aerobic exercise, chair-based exercise. | 60 min × 1 day/week for 8 weeks. | 1.5–3.0 METs | Decrease in CAVI Rest: 9.2 ± 0.2 m/s; post exercise: 9.0 ± 0.2 m/s |
| Yasuda et al. (2016) [54] | Low-intensity elastic band BFR training group; N = 10; aged = 70 ± 6.0 yo; BMI = 20.8 ± 2.5 mkg−2; Middle-to high-intensity elastic band BFR training; N = 10; aged = 72 ± 7.0; BMI = 20.9 ± 2.1 mkg−2; Control group; N = 10; aged = 68 ± 6; BMI = 22.3 ± 2.8 mkg−2; | Resistance training with 50–200 mmHg blood flow restricted. | Low-intensity BFR group, 2 movements × 75 reps each. Middle–High intensity BFR group, 2 movements × 37–38 reps each × 2 days/week for 12 weeks. | Low-intensity BFR group used 5 OMNI resistance. Middle–High BFR group used 5.6 OMNI resistance. | No change in CAVI Rest: 8.4 ± 0.9 m/s; post exercise: 8.5 ± 0.8 m/s for both groups |
| Yasuda et al. (2015) [55] | N = 14; aged = 61–85 yo; BMI = NA | Resistance training (BFR) | 2 movements × 75 reps × 2 days/week for 12 weeks | Submaximal bilateral arm curl and triceps press down exercise | No change in CAVI Rest: 9.1 ± 1.3 m/s; post exercise: 9.3 ± 1.1 m/s |
| Lee & Lee (2014) [56] | City-walking group; N = 19; aged = 71.11 ± 5.8 yo; BMI = 23.18 ± 2.7 mkg−2; Forest-walking group; N = 43, aged = 70.19 ± 4.66 yo; BMI = 24.32 ± 4.75 mkg−2; | Aerobic exercise (Forest-walking and city-walking) | 60 min | Self-pace walking with normal breathing and without sweating, becoming over heated or experiencing palpitations | Forest-walking: Decrease in CAVI Rest: 8.32 ± 1.22 m/s; post exercise: 7.90 ± 1.09 m/s City-walking: no change in CAVI Rest: 8.59 ± 0.98 m/s; post exercise: 8.70 ± 0.86 m/s |
| Matsubara et al. (2014) [57] | Control group; N = 8; aged = 62 ± 3 yo; BMI = 23.0 ± 1.3 mkg−2; Aerobic exercise group; N = 11; aged = 62 ± 2 yo; BMI = 23.8 ± 0.6 mkg−2; | Aerobic exercise | 40–60 min cycling and walking, >3 days/week for 12 weeks | 70–80% HRmax | Increase in arterial compliance |
| Tanahashi et al. (2014) [58] | Exercise group; N = 20; aged = 62 ± 6 yo BMI = 22.5 ± 3.1 mkg−2; Control group; N = 10; aged = 61 ± 7 yo BMI = 23.1 ± 3.4 mkg−2; | Aerobic exercise | 40–60 min/day, 3–6 days/week), for 12 weeks | 65%–80% HRmax | Increase in arterial compliance |
| Rossow et al. (2014) [21] | N = 13; aged = 57 ± 3 yo; (postmenopausal) BMI = NA | Resistance training | 6 movements × 8–10 reps × 2 sets and final set till failure × 3 days/week for 8 weeks | At 80% of 1 RM | No change in PWV Rest: 7.9 ± 1.4 m/s; post exercise: 7.5 ± 1.0 m/s |
| Corrick et al. (2013) [59] | N = 79; aged = over 60; BMI = NA divided into Group 1, N = 27; aged = 65.6 ± 0.7 yo; Group 2, N = 30; aged = 63.7 ± 0.5 yo, Group 3, N = 22; aged = 64.8 ± 0.7 yo. | Combined resistance training and aerobic exercise | Aerobic training for 40 min of cycling or running. Resistance training with 10 movements × 2 sets × 10 reps Groups 1, 1 day/week, Groups 2, 2 days/week, Group 3, 3 days/week, All carried out for 16 weeks | 80% MHR and 80% 1 RM for aerobic and resistance training respectively. | Increase in arterial elasticity Group 1: Rest: 13.3 ± 0.9 m/s; post exercise: 15.3 ± 1.2 m/s Group 2: Rest: 13.8 ± 1.2 m/s; post exercise: 12.8 ± 0.7 m/s Group 3: Rest: 11.9 ± 0.7 m/s; post exercise: 13.1 ± 1.0 m/s |
| Lu et al. (2013) [60] | Interest class group; N = 16; aged = 68.9 ± 5. 8 yo; BMI = 24.8 ± 3.3 mkg−2; Tai Chi group; N = 15; aged = 73.9 ± 6.6 yo; BMI = 24.6 ± 3.1 mkg−2; | Aerobic exercise (Tai Chi training) | Interest class group; 60-min, 3 days/week, 16 weeks non-exercise activity. | NA | Increase in arterial compliance. Interest class group: Rest: 10.1 ± 2.7 mL/mmHg × 100; post exercise: 9.6 ± 3.8 mL/mmHg × 100 Tai Chi group: Rest: 10.3 ± 2.7 mL/mmHg × 100; post exercise: 13.0 ± 3.8 mL/mmHg × 100 |
| Williams et al. (2013) [61] | Cross-over design, N = 22; aged = 66.7 ± 4.3 yo; BMI = 28.0 ± 4.6 mkg−2. | Resistance training and Flexibility training | 4–5 movements × 2–3 sets × 8–12 reps × 2 days/week for 16 weeks. For stretching, 12 movements × 2 sessions/weeks for 16 weeks. | Resistance training: 8–12 RM | Female decrease in arterial stiffness |
| Miyaki et al. (2012) [62] | Total of, N = 22; Exercise group; Aged = 60 ± 6 yo; BMI = 22.2 ± 2.0 mkg−2; Control group; Aged = 60 ± 7 yo; BMI = 22.4 ± 2.6 mkg−2; | Aerobic exercise | 30–45 min × 5 days/week for 2 months | 60–75% HRmax | Decrease in β-stiffness index Exercise group: Rest: 8.72 ± 2.05; post exercise: 7.76 ± 1.97 Control group: Rest: 7.58 ± 1.34; post exercise: 7.71 ± 1.51 |
| Ohta et al. (2012) [63] | Bench step exercise group; N = 13; aged = 72.2 ± 4.2 yo; BMI = 23.0 ± 2.6 mkg−2; Control group; N = 13; aged = 71.5 ± 7.4; BMI = 21.8 ± 2.6 mkg−2; | Aerobic exercise (Bench step exercise) | 10–20 min × 40 steps/min with 10 steps increment every min × 3 times/day × 3 days/week for 12 weeks. | Lactate threshold. | Decrease in PWV Decreased by 206 ± 165.5 cm/s in exercise group. |
| Coelho et al. (2011) [64] | rs4646994 gene deletion/deletion group; N = 10; aged = 70.6 ± 5.8; BMI = 25.4 ± 3.2 mkg−2; rs4646994 gene insertion/insertion + insertion/deletion group; I/I + I/D group; N = 15; aged = 71.1 ± 6.5; BMI = 25.1 ± 2.4 mkg−2. | Aerobic exercise vs. no exercise. | 20 min cycling session. | 90% anaerobic threshold. | Lower AASI in the rs4646994 gene deletion/deletion group after cycling. |
| Figueroa et al. (2011) [65] | Exercise group; N = 12; aged = 54 ± 2 (postmenopausal) BMI = 24.2 ± 0.7 mkg−2. Control group; N = 12; aged = 54 ± 1; (postmenopausal) BMI = 23.1 ± 0.7 mkg−2. | Combined resistance training and aerobic exercise. | 40 min total; for resistance 9 movements × 12 reps for 20 min; and for aerobic exercise; treadmill waling for 20-min. All exercises were carried out 3 days/week for 12 weeks. | 60% 1 RM for Resistance training and 60% HRmax for aerobic exercise. | Decrease in PWV in exercise group. Decreased 0.8 ± 0.2 m/s in exercise group. |
| Nishiwaki et al. (2011) [66] | N = 16; aged = 56 ± 1 (postmenopausal) BMI = NA. | Aquatic aerobic with normoxic (749.3–750.0 mmHg) and hypoxic (600.1–603.8 mmHg) conditions. | 30 min exercise × 4 days/week for 8 weeks. | 50% VO2peak | Normoxic: No changes in PWV. Hypoxic: Decrease in PWV. |
| Miura et al. (2008) [67] | Control group; N = 23; aged = 68.9 ± 7.5; BMI = 23.7 ± 3.0 mkg−2; Exercise with 1 day/week group; N = 29; aged = 69.0 ± 6.5; BMI = 22.8 ± 2.4 mkg−2; Exercise with 2 day/week group; N = 25; aged= 69.5 ± 7.0; BMI = 23.5 ± 2.7 mkg−2; | Combined resistance training and aerobic exercise | 90 min exercise × 1 day/week and another group 2 days/week for 12 weeks. For resistance, 6–8 movements × 15–20 reps × 3–5 sets for 12 weeks. For aerobic, 20 min cycling; and Chair-based exercise for 30-min. | Resistance training, lightweight dumbbells (500–1000 g), and Aerobic exercise at 70–75% HRmax. | 1 day/week: group showed no change in PWV. Rest: 1597.6 ± 201.5 cm/s; post exercise: 1570.5 ± 208.1 cm/s 2 day/week group showed a decreased PWV. Rest: 1598.2 ± 165.6 cm/s; post exercise: 1473.1 ± 188.4 cm/s |
| Casey et al. (2007) [68] | Resistance training group; N = 13; aged = 58.7 ± 4.5 (postmenopausal) BMI = 25.5 ± 3.2 mkg−2; Aerobic exercise group; N = 10; aged = 59.7 ± 6.5 (postmenopausal) BMI = 27.1 ± 4.9 mkg−2; | Resistance training or Aerobic exercise. | 40 min × 2 day/week for 18 weeks. For resistance training, 10 movements × 12 reps × 1 set. For aerobic, treadmill walking. | Resistance training at 50% 1 RM. Aerobic exercise at 65–80% HRR. | Resistance training showed no change in aortic augmentation index (AIa), Rest: 28.9 ± 1.9; post exercise: 28.5 ± 1.9% Aerobic training showed decreased AIa. Rest: 28.8 ± 2.1; post exercise: 25.1 ± 1.4% |
| Sugawara et al. (2004) [69] | Total of N = 15 Low-intensity exercise training group; Aged = 58.0 ± 4.0 (postmenopausal) BMI = NA. Moderate-intensity exercise training group; Aged = 59.0 ± 6.0 (postmenopausal) BMI = NA | Aerobic cycling exercise | 3–5 days/week 12 weeks. | Low-intensity group at 40% HRR, and Moderate-intensity group at 70% HRR. | Increase in arterial compliance in both groups. Low-intensity exercise training group: Rest: 0.7 ± 0.32 mm2/mmHg × 10−1; post exercise: 1.06 ± 0.55 mm2/mmHg × 10−1 Moderate-intensity exercise training group: Rest: 0.82 ± 0.37 mm2/mmHg × 10−1; post exercise: 1.14 ± 0.39 mm2/mmHg × 10−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, Y.S.; Khong, T.K.; Yusof, A. Effect of Exercise on Arterial Stiffness in Healthy Young, Middle-Aged and Older Women: A Systematic Review. Nutrients 2023, 15, 308. https://doi.org/10.3390/nu15020308
Lan YS, Khong TK, Yusof A. Effect of Exercise on Arterial Stiffness in Healthy Young, Middle-Aged and Older Women: A Systematic Review. Nutrients. 2023; 15(2):308. https://doi.org/10.3390/nu15020308
Chicago/Turabian StyleLan, Yong Sheng, Teng Keen Khong, and Ashril Yusof. 2023. "Effect of Exercise on Arterial Stiffness in Healthy Young, Middle-Aged and Older Women: A Systematic Review" Nutrients 15, no. 2: 308. https://doi.org/10.3390/nu15020308