Apolipoprotein A-IV-Deficient Mice in 129/SvJ Background Are Susceptible to Obesity and Glucose Intolerance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Body Composition
2.3. Lipid Absorption
2.4. Indirect Calorimetry
2.5. Locomotive Activity
2.6. Glucose and Insulin Tolerance Tests
2.7. Biochemical Analyses
2.8. Islet Morphology
2.9. Statistics
3. Results
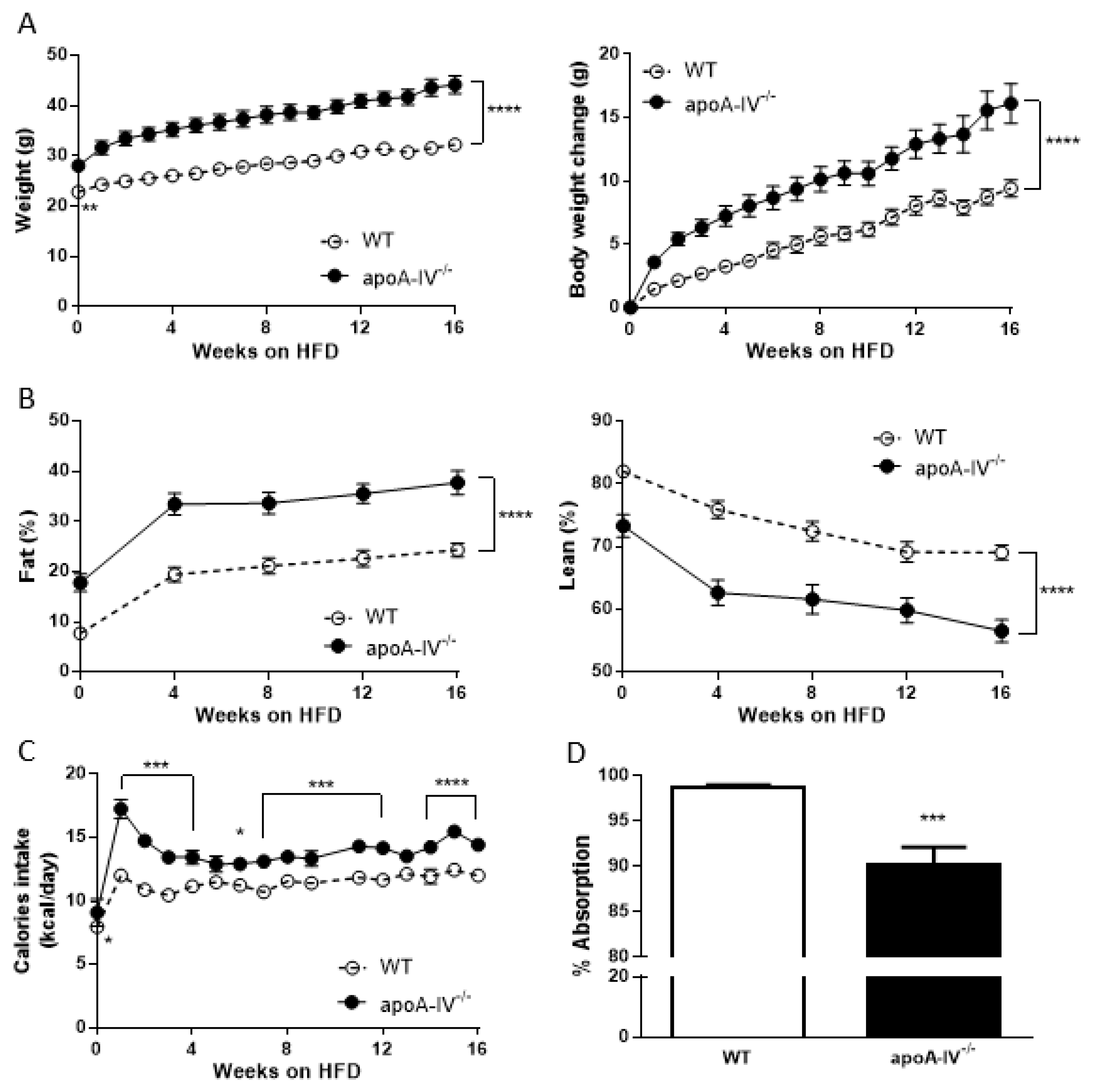
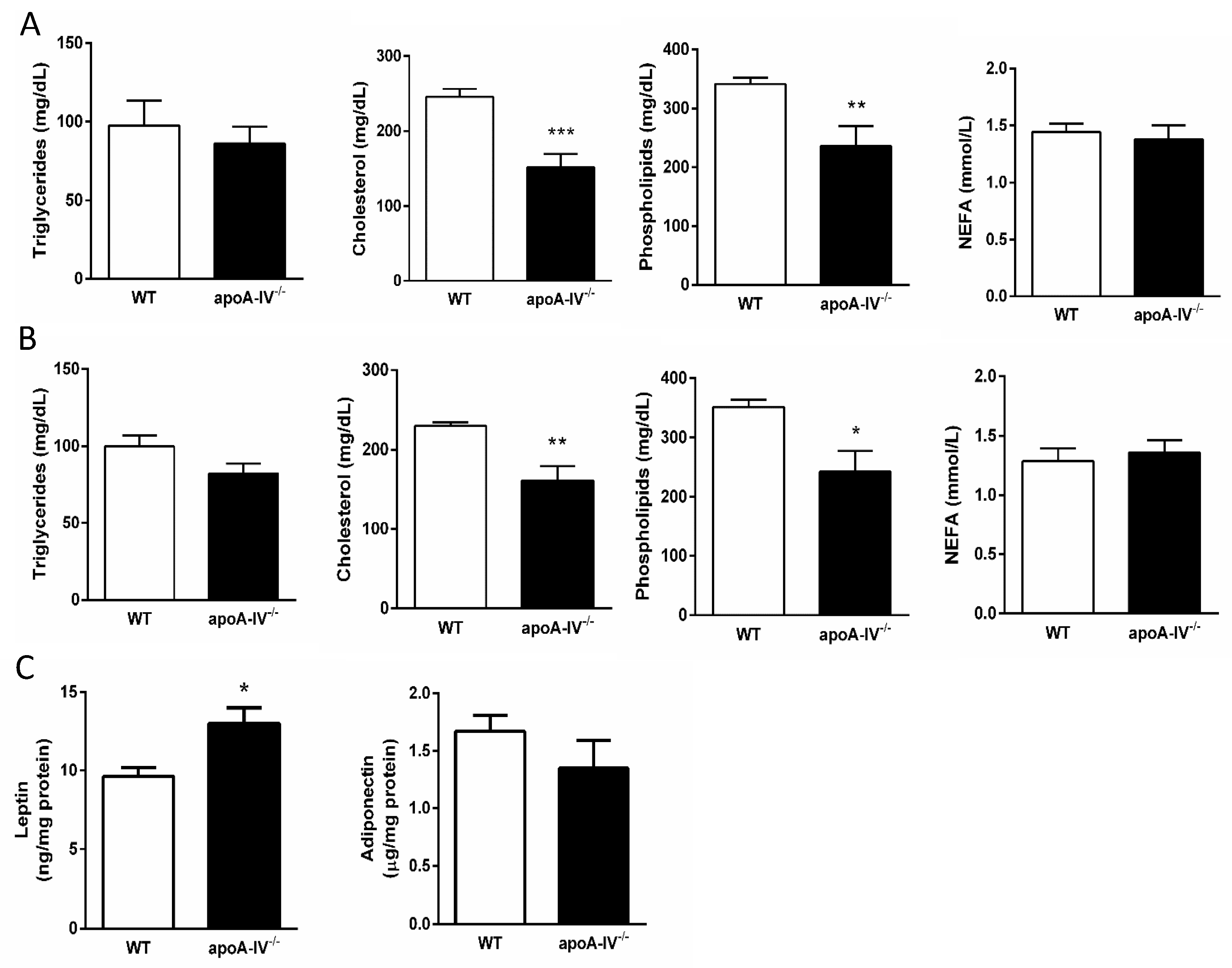
3.1. ApoA-IV−/− Mice Are Susceptible to Obesity
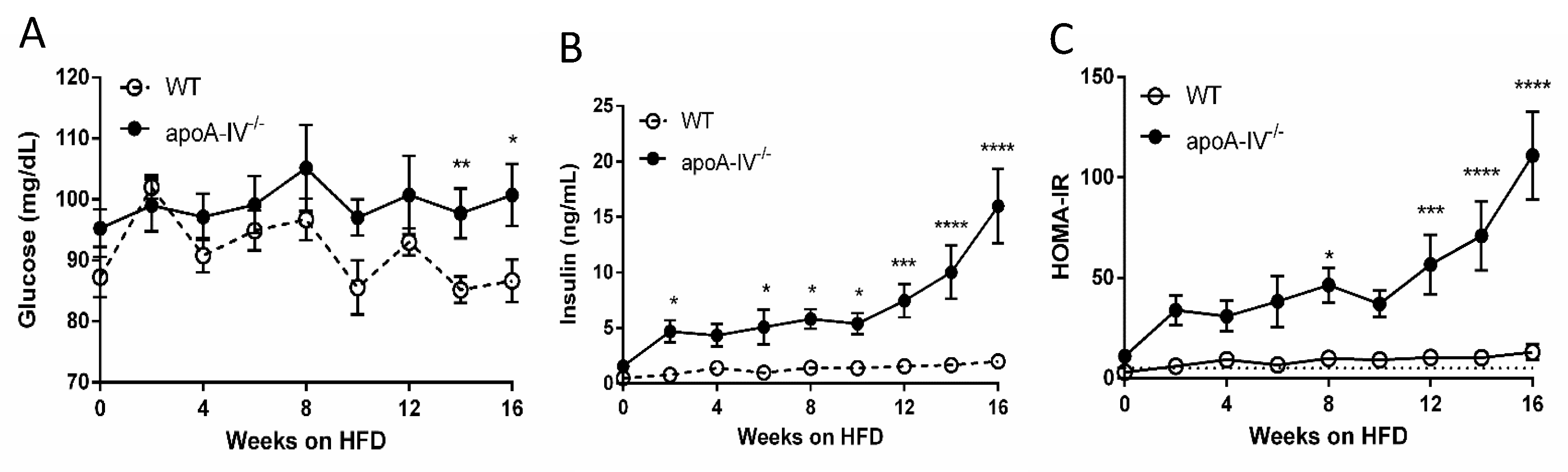
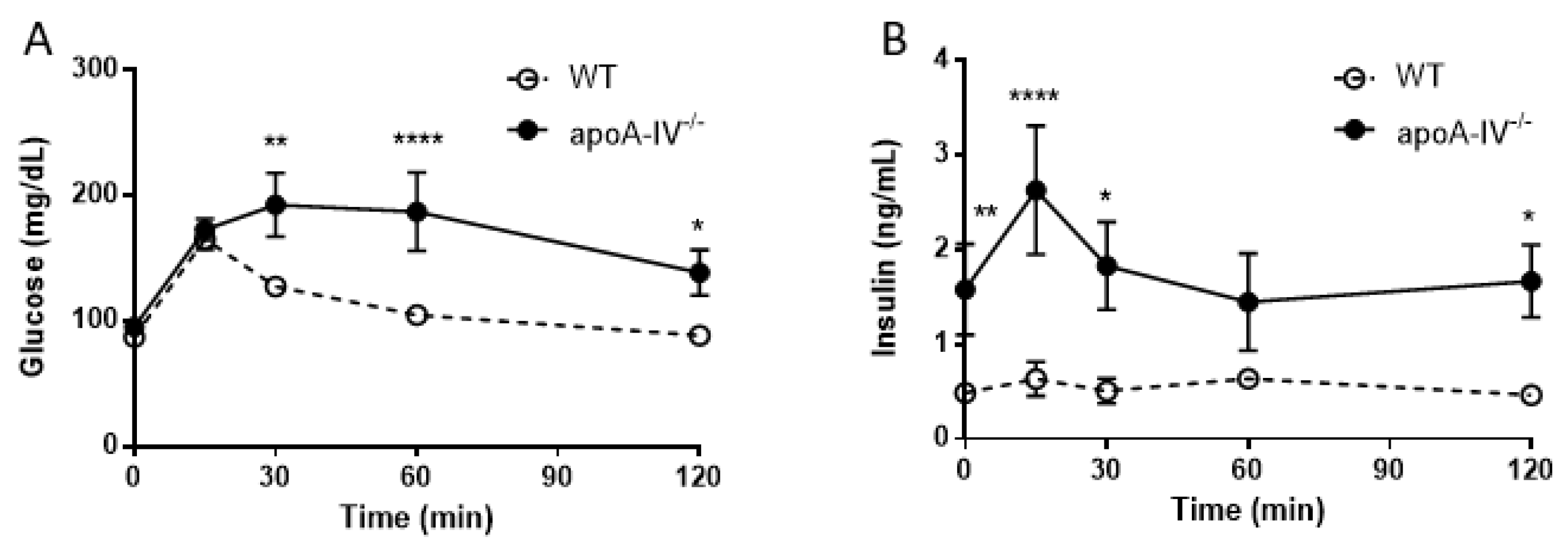
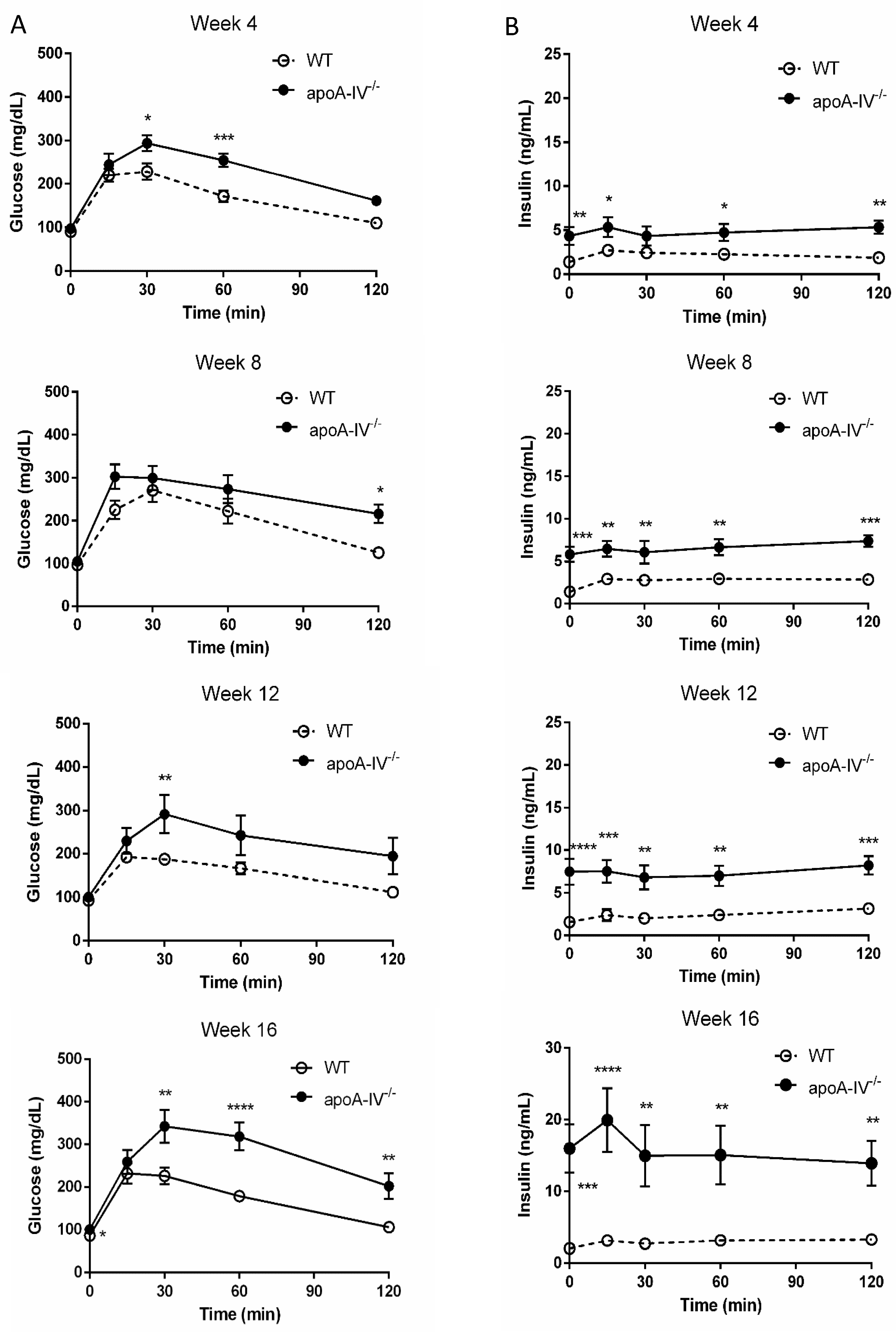
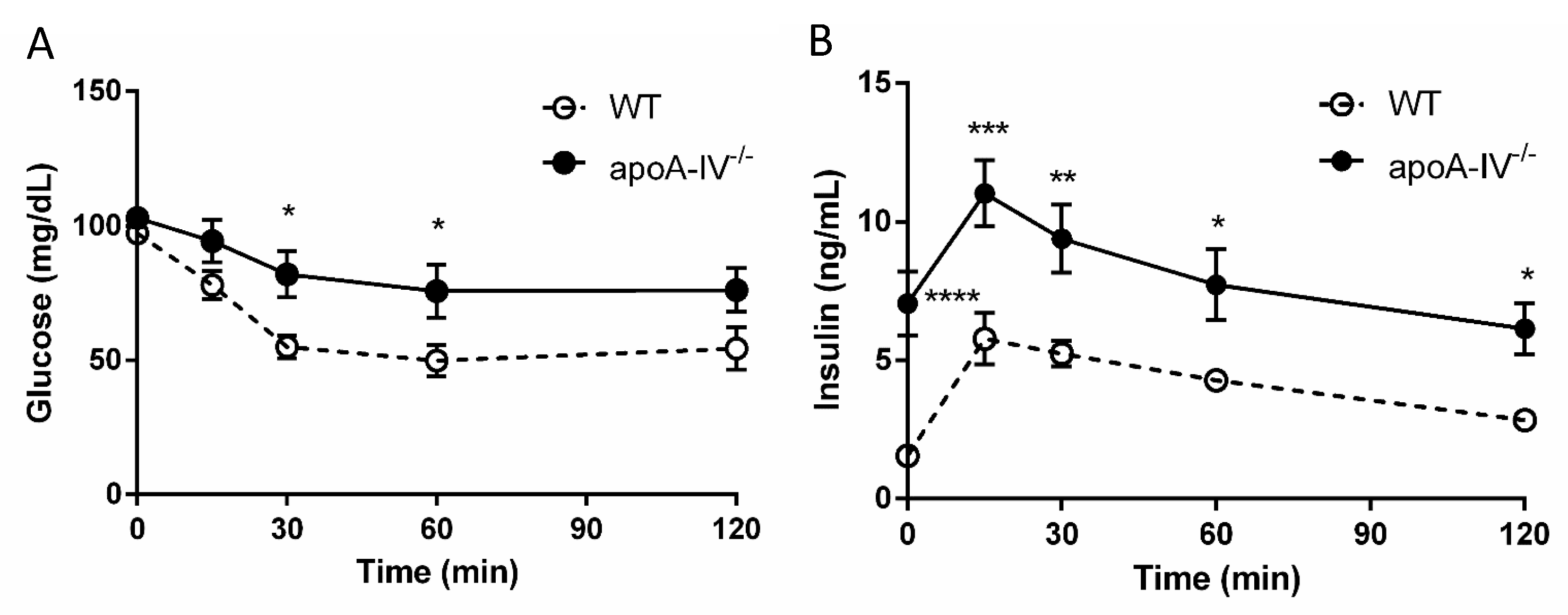
3.2. ApoA-IV−/− Mice Are Insulin-Resistant with Elevated Circulating Insulin Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Kahn, S.E. Clinical Review 135: The Importance of Beta-Cell Failure in the Development and Progression of Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2001, 86, 4047–4058. [Google Scholar] [CrossRef] [PubMed]
- Home, P.; Riddle, M.; Cefalu, W.T.; Bailey, C.J.; Bretzel, R.G.; Del Prato, S.; Leroith, D.; Schernthaner, G.; van Gaal, L.; Raz, I. Insulin Therapy in People with Type 2 Diabetes: Opportunities and Challenges? Diabetes Care 2014, 37, 1499–1508. [Google Scholar] [CrossRef] [PubMed]
- Swaney, J.B.; Braithwaite, F.; Eder, H.A. Characterization of the Apolipoproteins of Rat Plasma Lipoproteins. Biochemistry 1977, 16, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Lenich, C.; Brecher, P.; Makrides, S.; Chobanian, A.; Zannis, V.I. Apolipoprotein Gene Expression in the Rabbit: Abundance, Size, and Distribution of Apolipoprotein MRNA Species in Different Tissues. J. Lipid Res. 1988, 29, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.; Cook, V.R.; Rao, A.; Weinberg, R.B. Impact of Murine Intestinal Apolipoprotein A-IV Expression on Regional Lipid Absorption, Gene Expression, and Growth. J. Lipid Res. 2011, 52, 1984–1994. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Yao, Y.; Meng, S.; Cheng, X.; Black, D.D. Overexpression of Apolipoprotein A-IV Enhances Lipid Transport in Newborn Swine Intestinal Epithelial Cells. J. Biol. Chem. 2002, 277, 31929–31937. [Google Scholar] [CrossRef]
- Lu, S.; Yao, Y.; Cheng, X.; Mitchell, S.; Leng, S.; Meng, S.; Gallagher, J.W.; Shelness, G.S.; Morris, G.S.; Mahan, J.; et al. Overexpression of Apolipoprotein A-IV Enhances Lipid Secretion in IPEC-1 Cells by Increasing Chylomicron Size. J. Biol. Chem. 2006, 281, 3473–3483. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-H.; Zhang, Y.; Chang, L.; Wei, Y.; Huang, N.; Zhou, J.-T.; Cheng, C.; Zhang, J.; Xu, J.; Li, Z.; et al. Apolipoprotein A-IV Reduced Metabolic Inflammation in White Adipose Tissue by Inhibiting IKK and JNK Signaling in Adipocytes. Mol. Cell. Endocrinol. 2023, 559, 111813. [Google Scholar] [CrossRef]
- Geronimo, F.R.B.; Barter, P.J.; Rye, K.A.; Heather, A.K.; Shearston, K.D.; Rodgers, K.J. Plaque Stabilizing Effects of Apolipoprotein A-IV. Atherosclerosis 2016, 251, 39–46. [Google Scholar] [CrossRef]
- Wang, F.; Kohan, A.B.; Kindel, T.L.; Corbin, K.L.; Nunemaker, C.S.; Obici, S.; Woods, S.C.; Davidson, W.S.; Tso, P. Apolipoprotein A-IV Improves Glucose Homeostasis by Enhancing Insulin Secretion. Proc. Natl. Acad. Sci. USA 2012, 109, 9641–9646. [Google Scholar] [CrossRef]
- Li, X.; Xu, M.; Wang, F.; Kohan, A.B.; Haas, M.K.; Yang, Q.; Lou, D.; Obici, S.; Davidson, W.S.; Tso, P. Apolipoprotein A-IV Reduces Hepatic Gluconeogenesis through Nuclear Receptor NR1D1. J. Biol. Chem. 2014, 289, 2396–2404. [Google Scholar] [CrossRef] [PubMed]
- Black, B.L.; Croom, J.; Eisen, E.J.; Petro, A.E.; Edwards, C.L.; Surwit, R.S. Differential Effects of Fat and Sucrose on Body Composition in A/J and C57BL/6 Mice. Metabolism. 1998, 47, 1354–1359. [Google Scholar] [CrossRef] [PubMed]
- Surwit, R.S.; Wang, S.; Petro, A.E.; Sanchis, D.; Raimbault, S.; Ricquier, D.; Collins, S. Diet-Induced Changes in Uncoupling Proteins in Obesity-Prone and Obesity-Resistant Strains of Mice. Proc. Natl. Acad. Sci. USA 1998, 95, 4061–4065. [Google Scholar] [CrossRef] [PubMed]
- Surwit, R.S.; Feinglos, M.N.; Rodin, J.; Sutherland, A.; Petro, A.E.; Opara, E.C.; Kuhn, C.M.; Rebuffé-Scrive, M. Differential Effects of Fat and Sucrose on the Development of Obesity and Diabetes in C57BL/6J and A/J Mice. Metabolism 1995, 44, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Tordoff, M.G.; Pilchak, D.M.; Williams, J.A.; McDaniel, A.H.; Bachmanov, A.A. The Maintenance Diets of C57BL/6J and 129X1/SvJ Mice Influence Their Taste Solution Preferences: Implications for Large-Scale Phenotyping Projects. J. Nutr. 2002, 132, 2288–2297. [Google Scholar] [CrossRef] [PubMed]
- Berglund, E.D.; Li, C.Y.; Poffenberger, G.; Ayala, J.E.; Fueger, P.T.; Willis, S.E.; Jewell, M.M.; Powers, A.C.; Wasserman, D.H. Glucose Metabolism in Vivo in Four Commonly Used Inbred Mouse Strains. Diabetes 2008, 57, 1790–1799. [Google Scholar] [CrossRef] [PubMed]
- Jandacek, R.J.; Heubi, J.E.; Tso, P. A Novel, Noninvasive Method for the Measurement of Intestinal Fat Absorption. Gastroenterology 2004, 127, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, L.; Zhang, Z.; Feng, L.; Song, X.; Wu, J. Apolipoprotein A-IV Involves in Glucose and Lipid Metabolism of Rat. Nutr. Metab. 2019, 16, 41. [Google Scholar] [CrossRef]
- Lo, C.M.; Zhang, D.M.; Pearson, K.; Ma, L.; Sun, W.; Sakai, R.R.; Davidson, W.S.; Liu, M.; Raybould, H.E.; Woods, S.C.; et al. Interaction of Apolipoprotein AIV with Cholecystokinin on the Control of Food Intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R1490–R1494. [Google Scholar] [CrossRef]
- Fujimoto, K.; Fukagawa, K.; Sakata, T.; Tso, P. Suppression of Food Intake by Apolipoprotein A-IV Is Mediated through the Central Nervous System in Rats. J. Clin. Investig. 1993, 91, 1830–1833. [Google Scholar] [CrossRef]
- Weinstock, P.H.; Bisgaier, C.L.; Hayek, T.; Aalto-Setala, K.; Sehayek, E.; Wu, L.; Sheiffele, P.; Merkel, M.; Essenburg, A.D.; Breslow, J.L. Decreased HDL Cholesterol Levels but Normal Lipid Absorption, Growth, and Feeding Behavior in Apolipoprotein A-IV Knockout Mice. J. Lipid Res. 1997, 38, 1782–1794. [Google Scholar] [CrossRef] [PubMed]
- Pence, S.; Zhu, Q.; Binne, E.; Liu, M.; Shi, H.; Lo, C.C. Reduced Diet-Induced Thermogenesis in Apolipoprotein A-IV Deficient Mice. Int. J. Mol. Sci. 2019, 20, 3176. [Google Scholar] [CrossRef] [PubMed]
- Woods, S.C.; Seeley, R.J.; Porte, D.J.; Schwartz, M.W. Signals That Regulate Food Intake and Energy Homeostasis. Science 1998, 280, 1378–1383. [Google Scholar] [CrossRef] [PubMed]
- Cone, R.D. The Central Melanocortin System and Energy Homeostasis. Trends Endocrinol. Metab. 1999, 10, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.W.; Woods, S.C.; Porte, D.J.; Seeley, R.J.; Baskin, D.G. Central Nervous System Control of Food Intake. Nature 2000, 404, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; He, Y.; Xu, Y.; Shu, G.; Wang, C.; Yang, Y.; Saito, K.; Xu, P.; Hinton, A.O.J.; Yan, X.; et al. Apolipoprotein A-IV Inhibits AgRP/NPY Neurons and Activates Pro-Opiomelanocortin Neurons in the Arcuate Nucleus. Neuroendocrinology 2016, 103, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Saleh, J.; Sniderman, A.D.; Cianflone, K. Regulation of Plasma Fatty Acid Metabolism. Clin. Chim. Acta 1999, 286, 163–180. [Google Scholar] [CrossRef] [PubMed]
- Klop, B.; Wouter Jukema, J.; Rabelink, T.J.; Castro Cabezas, M. A Physician’s Guide for the Management of Hypertriglyceridemia: The Etiology of Hypertriglyceridemia Determines Treatment Strategy. Panminerva Med. 2012, 54, 91–103. [Google Scholar]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms Linking Obesity to Insulin Resistance and Type 2 Diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef]
- Shah, P.; Vella, A.; Basu, A.; Basu, R.; Adkins, A.; Schwenk, W.F.; Johnson, C.M.; Nair, K.S.; Jensen, M.D.; Rizza, R.A. Effects of Free Fatty Acids and Glycerol on Splanchnic Glucose Metabolism and Insulin Extraction in Nondiabetic Humans. Diabetes 2002, 51, 301–310. [Google Scholar] [CrossRef]
- Ginsberg, H.N. Insulin Resistance and Cardiovascular Disease. J. Clin. Investig. 2000, 106, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Guilherme, A.; Virbasius, J.V.; Puri, V.; Czech, M.P. Adipocyte Dysfunctions Linking Obesity to Insulin Resistance and Type 2 Diabetes. Nat. Rev. Mol. Cell Biol. 2008, 9, 367–377. [Google Scholar] [CrossRef]
- Roden, M.; Price, T.B.; Perseghin, G.; Petersen, K.F.; Rothman, D.L.; Cline, G.W.; Shulman, G.I. Mechanism of Free Fatty Acid-Induced Insulin Resistance in Humans. J. Clin. Investig. 1996, 97, 2859–2865. [Google Scholar] [CrossRef]
- Arita, Y.; Kihara, S.; Ouchi, N.; Takahashi, M.; Maeda, K.; Miyagawa, J.; Hotta, K.; Shimomura, I.; Nakamura, T.; Miyaoka, K.; et al. Paradoxical Decrease of an Adipose-Specific Protein, Adiponectin, in Obesity. Biochem. Biophys. Res. Commun. 1999, 257, 79–83. [Google Scholar] [CrossRef]
- Li, S.; Shin, H.J.; Ding, E.L.; van Dam, R.M. Adiponectin Levels and Risk of Type 2 Diabetes: A Systematic Review and Meta-Analysis. JAMA 2009, 302, 179–188. [Google Scholar] [CrossRef]
- Weinberg, R.B. Apolipoprotein A-IV Polymorphisms and Diet-Gene Interactions. Curr. Opin. Lipidol. 2002, 13, 125–134. [Google Scholar] [CrossRef]
- Tuttle, A.H.; Philip, V.M.; Chesler, E.J.; Mogil, J.S. Comparing Phenotypic Variation between Inbred and Outbred Mice. Nat. Methods 2018, 15, 994–996. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Ko, C.-W.; Qu, J.; Wu, D.; Zhu, Q.; Liu, M.; Tso, P. Apolipoprotein A-IV-Deficient Mice in 129/SvJ Background Are Susceptible to Obesity and Glucose Intolerance. Nutrients 2023, 15, 4840. https://doi.org/10.3390/nu15224840
Wang F, Ko C-W, Qu J, Wu D, Zhu Q, Liu M, Tso P. Apolipoprotein A-IV-Deficient Mice in 129/SvJ Background Are Susceptible to Obesity and Glucose Intolerance. Nutrients. 2023; 15(22):4840. https://doi.org/10.3390/nu15224840
Chicago/Turabian StyleWang, Fei, Chih-Wei Ko, Jie Qu, Dong Wu, Qi Zhu, Min Liu, and Patrick Tso. 2023. "Apolipoprotein A-IV-Deficient Mice in 129/SvJ Background Are Susceptible to Obesity and Glucose Intolerance" Nutrients 15, no. 22: 4840. https://doi.org/10.3390/nu15224840



