Ongoing Treatment with a Spore-Based Probiotic Containing Five Strains of Bacillus Improves Outcomes of Mild COVID-19
Abstract
:1. Introduction
2. Participants and Methods
2.1. Study Design
2.2. Participants
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Participants
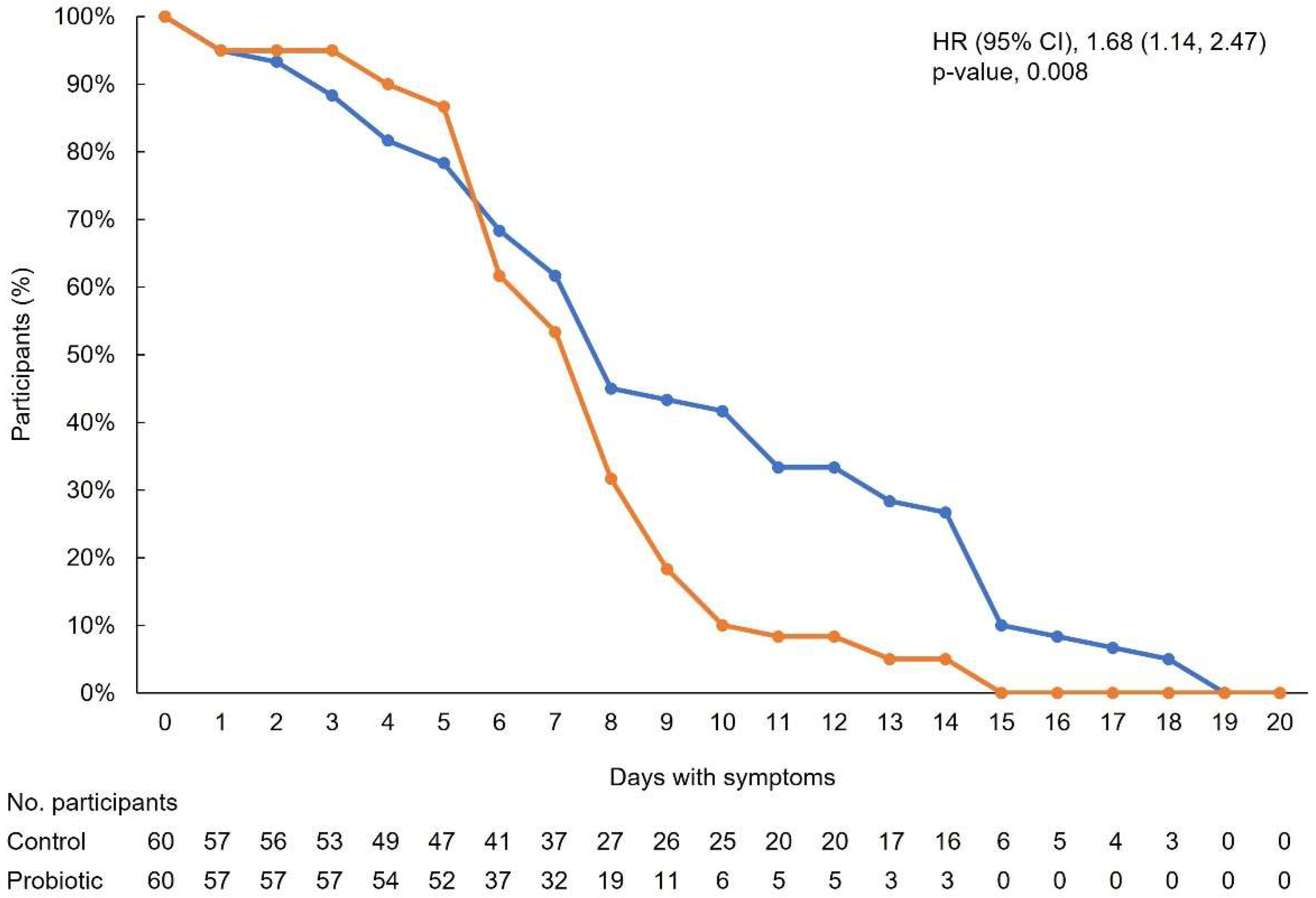
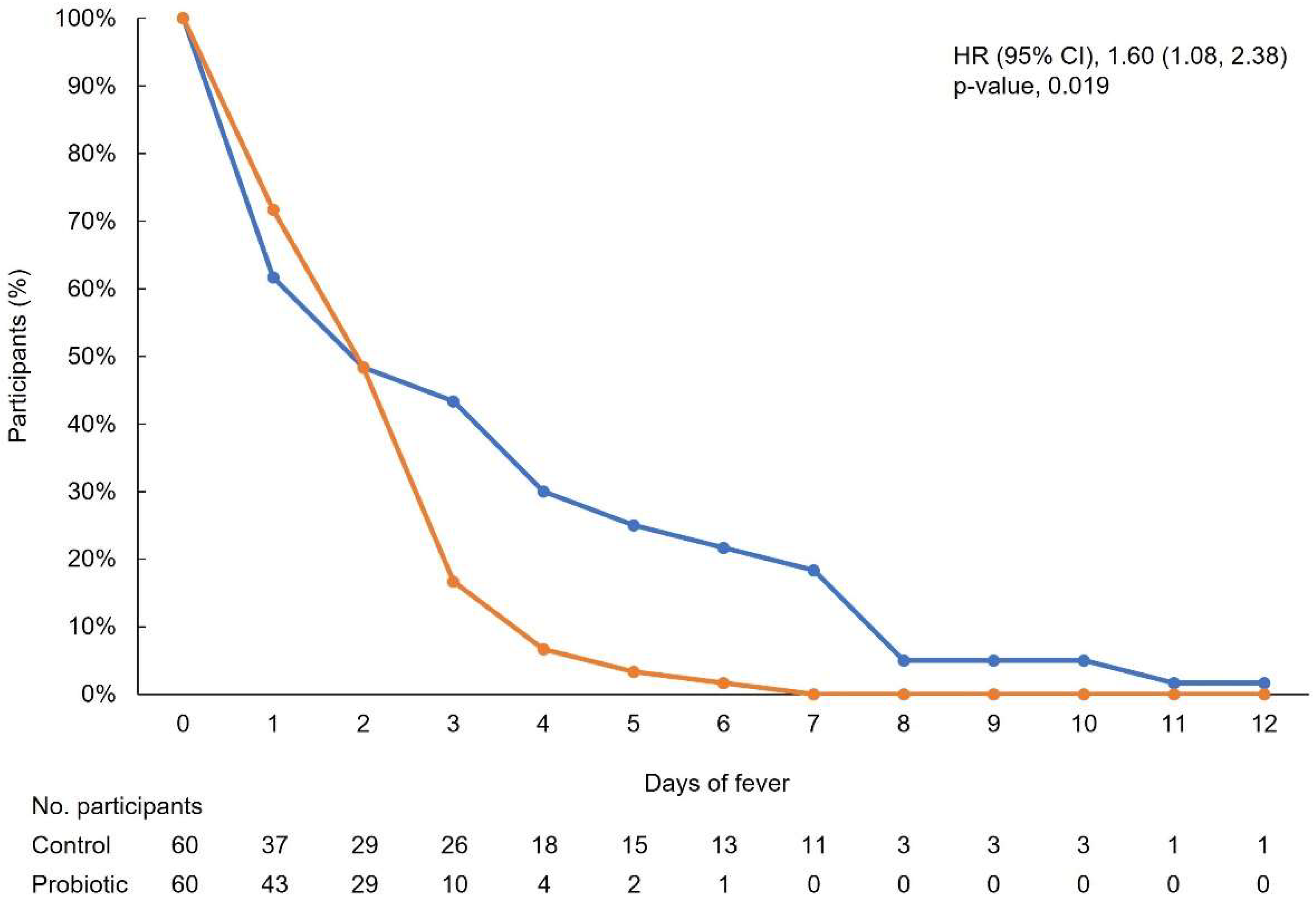
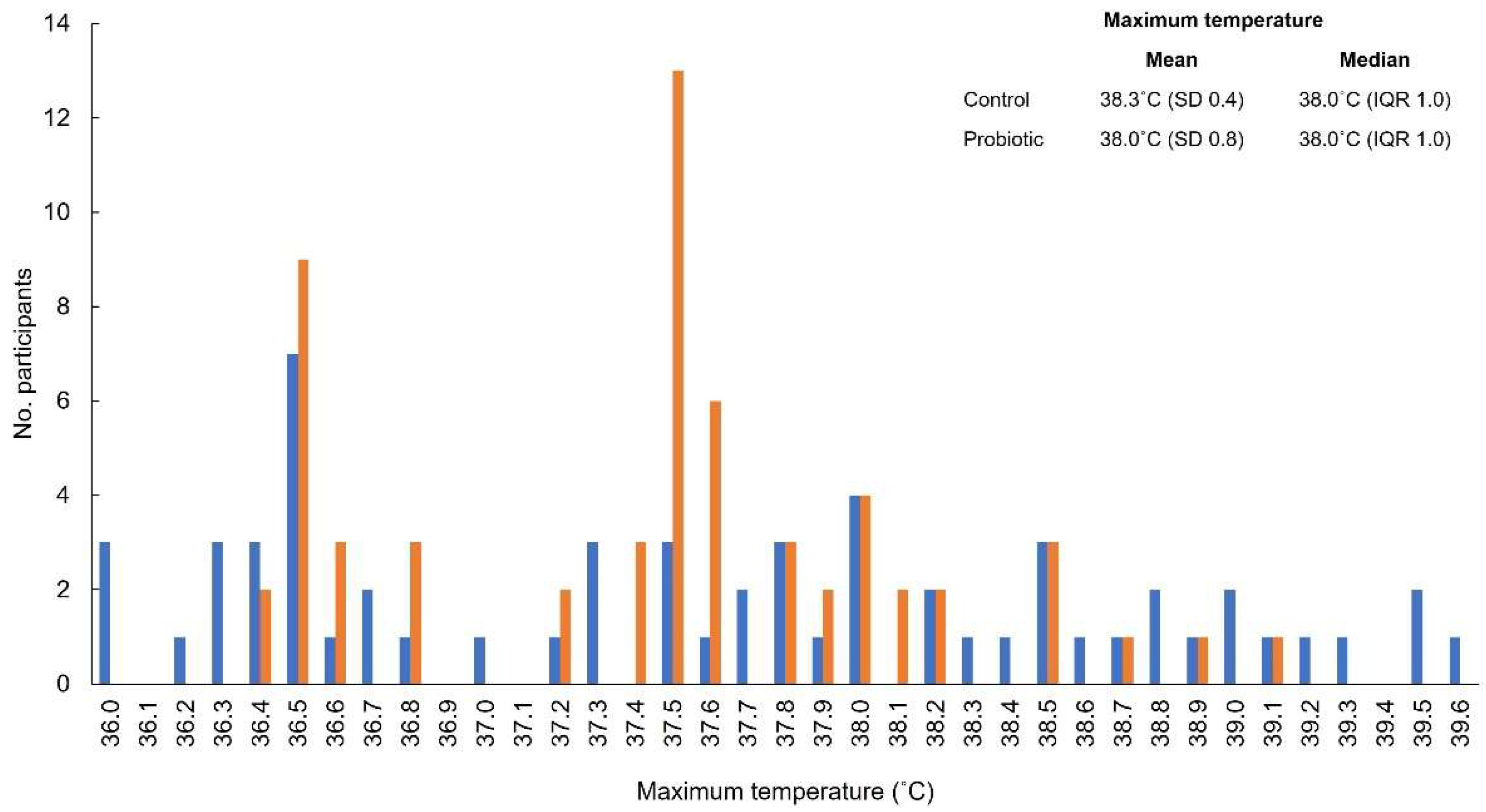
3.2. Primary Outcome
3.3. Secondary Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zuo, T.; Zhang, F.; Lui, G.C.Y.; Yeoh, Y.K.; Li, A.Y.L.; Zhan, H.; Wan, Y.; Chung, A.C.K.; Cheung, C.P.; Chen, N.; et al. Alterations in Gut Microbiota of Patients with COVID-19 during Time of Hospitalization. Gastroenterology 2020, 159, 944–955. [Google Scholar] [CrossRef] [PubMed]
- WHO. Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/#:~:text=Globally%2C%20as%20of%204%3A54pm,vaccine%20doses%20have%20been%20administered (accessed on 16 January 2023).
- Clemente, J.C.; Manasson, J.; Scher, J.U. The role of the gut microbiome in systemic inflammatory disease. BMJ 2018, 360, j5145. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, E.; Shimokawa, C.; Steimle, A.; Desai, M.S.; Ohno, H. The impact of the gut microbiome on extra-intestinal autoimmune diseases. Nat. Rev. Immunol. 2023, 23, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.J.; Wu, E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 2012, 3, 4–14. [Google Scholar] [CrossRef] [Green Version]
- Han, S.K.; Shin, Y.J.; Lee, D.Y.; Kim, K.M.; Yang, S.J.; Kim, D.S.; Choi, J.W.; Lee, S.; Kim, D.H. Lactobacillus rhamnosus HDB1258 modulates gut microbiota-mediated immune response in mice with or without lipopolysaccharide-induced systemic inflammation. BMC Microbiol. 2021, 21, 146. [Google Scholar] [CrossRef]
- Li, W.; Qiao, J.; You, Q.; Zong, S.; Peng, Q.; Liu, Y.; Hu, S.; Liu, W.; Li, S.; Shu, X.; et al. SARS-CoV-2 Nsp5 Activates NF-kappaB Pathway by Upregulating SUMOylation of MAVS. Front. Immunol. 2021, 12, 750969. [Google Scholar] [CrossRef]
- Akour, A. Probiotics and COVID-19: Is there any link? Lett. Appl. Microbiol. 2020, 71, 229–234. [Google Scholar] [CrossRef]
- Safiabadi Tali, S.H.; LeBlanc, J.J.; Sadiq, Z.; Oyewunmi, O.D.; Camargo, C.; Nikpour, B.; Armanfard, N.; Sagan, S.M.; Jahanshahi-Anbuhi, S. Tools and Techniques for Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)/COVID-19 Detection. Clin. Microbiol. Rev. 2021, 34, e00228-20. [Google Scholar] [CrossRef]
- Blackett, J.W.; Wainberg, M.; Elkind, M.S.V.; Freedberg, D.E. Potential long coronavirus disease 2019 gastrointestinal symptoms 6 months after coronavirus infection are associated with mental health symptoms. Gastroenterology 2022, 162, 648–650.e2. [Google Scholar] [CrossRef]
- Pan, L.; Mu, M.; Yang, P.; Sun, Y.; Wang, R.; Yan, J.; Li, P.; Hu, B.; Wang, J.; Hu, C.; et al. Clinical Characteristics of COVID-19 Patients With Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. Am. J. Gastroenterol. 2020, 115, 766–773. [Google Scholar] [CrossRef]
- Nguyen, Q.V.; Chong, L.C.; Hor, Y.Y.; Lew, L.C.; Rather, I.A.; Choi, S.B. Role of Probiotics in the Management of COVID-19: A Computational Perspective. Nutrients 2022, 14, 274. [Google Scholar] [CrossRef] [PubMed]
- Rossini, V.; Tolosa-Enguis, V.; Frances-Cuesta, C.; Sanz, Y. Gut microbiome and anti-viral immunity in COVID-19. Crit. Rev. Food Sci. Nutr. 2022, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kurian, S.J.; Unnikrishnan, M.K.; Miraj, S.S.; Bagchi, D.; Banerjee, M.; Reddy, B.S.; Rodrigues, G.S.; Manu, M.K.; Saravu, K.; Mukhopadhyay, C.; et al. Probiotics in Prevention and Treatment of COVID-19: Current Perspective and Future Prospects. Arch. Med. Res. 2021, 52, 582–594. [Google Scholar] [CrossRef] [PubMed]
- Williams, N.T. Probiotics. Am. J. Health Syst. Pharm. 2010, 67, 449–458. [Google Scholar] [CrossRef]
- Rodriguez, J.A.M.; Bifano, M.; Roca Goma, E.; Plasencia, C.M.; Torralba, A.O.; Font, M.S.; Millan, P.R. Effect and Tolerability of a Nutritional Supplement Based on a Synergistic Combination of beta-Glucans and Selenium-and Zinc-Enriched Saccharomyces cerevisiae (ABB C1((R))) in Volunteers Receiving the Influenza or the COVID-19 Vaccine: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2021, 13, 4347. [Google Scholar] [PubMed]
- Leal-Martinez, F.; Abarca-Bernal, L.; Garcia-Perez, A.; Gonzalez-Tolosa, D.; Cruz-Cazares, G.; Montell-Garcia, M.; Ibarra, A. Effect of a Nutritional Support System to Increase Survival and Reduce Mortality in Patients with COVID-19 in Stage III and Comorbidities: A Blinded Randomized Controlled Clinical Trial. Int. J. Environ. Res. Public Health 2022, 19, 1172. [Google Scholar] [CrossRef]
- De Boeck, I.; Cauwenberghs, E.; Spacova, I.; Gehrmann, T.; Eilers, T.; Delanghe, L.; Wittouck, S.; Bron, P.A.; Henkens, T.; Gamgami, I.; et al. Randomized, Double-Blind, Placebo-Controlled Trial of a Throat Spray with Selected Lactobacilli in COVID-19 Outpatients. Microbiol. Spectr. 2022, 10, e0168222. [Google Scholar] [CrossRef]
- Marzorati, M.; Abbeele, P.V.D.; Bubeck, S.S.; Bayne, T.; Krishnan, K.; Young, A.; Mehta, D.; DeSouza, A. Bacillus subtilis HU58 and Bacillus coagulans SC208 Probiotics Reduced the Effects of Antibiotic-Induced Gut Microbiome Dysbiosis in an M-SHIME® Model. Microorganisms 2020, 8, 1028. [Google Scholar] [CrossRef]
- Dound, Y.A.; Jadhav, S.S.; Devale, M.; Bayne, T.; Krishnan, K.; Mehta, D.S. The effect of Probiotic Bacillus Subtilis HU58 on Immune Function in Healthy Human. Indian Pract. 2019, 70, 15–20. [Google Scholar]
- McFarlin, B.K.; Henning, A.L.; Bowman, E.M.; Gary, M.A.; Carbajal, K.M. Oral spore-based probiotic supplementation was associated with reduced incidence of post-prandial dietary endotoxin, triglycerides, and disease risk biomarkers. World J. Gastrointest Pathophysiol. 2017, 8, 117–126. [Google Scholar] [CrossRef]
- Marzorati, M.; Bubeck, S.; Bayne, T.; Krishnan, K.; Giusto, M. Effects of combined prebiotic, probiotic, IgG and amino acid supplementation on the gut microbiome of patients with inflammatory bowel disease. Future Microbiol. 2022, 17, 1307–1324. [Google Scholar] [CrossRef]
- Marzorati, M.; Van den Abbeele, P.; Bubeck, S.; Bayne, T.; Krishnan, K.; Young, A. Treatment with a spore-based probiotic containing five strains of Bacillus induced changes in the metabolic activity and community composition of the gut microbiota in a SHIME(R) model of the human gastrointestinal system. Food Res. Int. 2021, 149, 110676. [Google Scholar] [CrossRef] [PubMed]
- Kalman, D.S.; Schwartz, H.I.; Alvarez, P.; Feldman, S.; Pezzullo, J.C.; Krieger, D.R. A prospective, randomized, double-blind, placebo-controlled parallel-group dual site trial to evaluate the effects of a Bacillus coagulans-based product on functional intestinal gas symptoms. BMC Gastroenterol. 2009, 9, 85. [Google Scholar] [CrossRef] [Green Version]
- Beyerstedt, S.; Casaro, E.B.; Rangel, E.B. COVID-19: Angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 905–919. [Google Scholar] [CrossRef]
- Hamming, I.; Timens, W.; Bulthuis, M.L.; Lely, A.T.; Navis, G.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef]
- Tanikawa, T.; Kiba, Y.; Yu, J.; Hsu, K.; Chen, S.; Ishii, A.; Yokogawa, T.; Suzuki, R.; Inoue, Y.; Kitamura, M. Degradative Effect of Nattokinase on Spike Protein of SARS-CoV-2. Molecules 2022, 27, 5405. [Google Scholar] [CrossRef]
- Oba, M.; Rongduo, W.; Saito, A.; Okabayashi, T.; Yokota, T.; Yasuoka, J.; Sato, Y.; Nishifuji, K.; Wake, H.; Nibu, Y.; et al. Natto extract, a Japanese fermented soybean food, directly inhibits viral infections including SARS-CoV-2 in vitro. Biochem. Biophys. Res. Commun. 2021, 570, 21–25. [Google Scholar] [CrossRef]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Mao, R.; Qiu, Y.; He, J.S.; Tan, J.Y.; Li, X.H.; Liang, J.; Shen, J.; Zhu, L.R.; Chen, Y.; Iacucci, M.; et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 667–678. [Google Scholar] [CrossRef]
- Qian, Y.; Lei, T.; Patel, P.S.; Lee, C.H.; Monaghan-Nichols, P.; Xin, H.B.; Qiu, J.; Fu, M. Direct Activation of Endothelial Cells by SARS-CoV-2 Nucleocapsid Protein Is Blocked by Simvastatin. J. Virol. 2021, 95, e0139621. [Google Scholar] [CrossRef] [PubMed]
- Catinean, A.; Neag, M.A.; Krishnan, K.; Muntean, D.M.; Bocsan, C.I.; Pop, R.M.; Mitre, A.O.; Melincovici, C.S.; Buzoianu, A.D. Probiotic Bacillus Spores Together with Amino Acids and Immunoglobulins Exert Protective Effects on a Rat Model of Ulcerative Colitis. Nutrients 2020, 12, 3607. [Google Scholar] [CrossRef]
- El-Radhi, A.S. Pathogenesis of fever. In Clinical Manual of Fever in Children; Springer: Berlin/Heidelberg, Germany, 2019; pp. 53–68. [Google Scholar]
- Belon, L.; Skidmore, P.; Mehra, R.; Walter, E. Effect of a fever in viral infections—The ‘Goldilocks’ phenomenon? World J. Clin. Cases 2021, 9, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Teijaro, J.R. Type I interferons in viral control and immune regulation. Curr. Opin. Virol. 2016, 16, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, W.J.; Upton, J.W.; Mocarski, E.S. Viral modulation of programmed necrosis. Curr. Opin. Virol. 2013, 3, 296–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Xie, X.; Tu, Z.; Fu, J.; Xu, D.; Zhou, Y. The signal pathways and treatment of cytokine storm in COVID-19. Signal Transduct. Target. Ther. 2021, 6, 255. [Google Scholar] [CrossRef] [PubMed]
- Leon, L.R.; White, A.A.; Kluger, M.J. Role of IL-6 and TNF in thermoregulation and survival during sepsis in mice. Am. J. Physiol. 1998, 275, R269–R277. [Google Scholar] [CrossRef]
- Kageyama, Y.; Nishizaki, Y.; Aida, K.; Yayama, K.; Ebisui, T.; Akiyama, T.; Nakamura, T. Lactobacillus plantarum induces innate cytokine responses that potentially provide a protective benefit against COVID-19: A single-arm, double-blind, prospective trial combined with an in vitro cytokine response assay. Exp. Ther. Med. 2022, 23, 20. [Google Scholar] [CrossRef]
- Xu, J.; Ren, Z.; Cao, K.; Li, X.; Yang, J.; Luo, X.; Zhu, L.; Wang, X.; Ding, L.; Liang, J.; et al. Boosting Vaccine-Elicited Respiratory Mucosal and Systemic COVID-19 Immunity in Mice with the Oral Lactobacillus Plantarum. Front. Nutr. 2021, 8, 789242. [Google Scholar] [CrossRef]
- Lau, H.C.; Ng, S.C.; Yu, J. Targeting the Gut Microbiota in Coronavirus Disease 2019: Hype or Hope? Gastroenterology 2022, 162, 9–16. [Google Scholar] [CrossRef]
- Venegas-Borsellino, C.; Sankararaman, S.; Roche, K.; Burns, J.; Landis, R.M. Impact of COVID-19 on the Intestinal Microbiome. Curr. Nutr. Rep. 2021, 10, 300–306. [Google Scholar] [CrossRef]
- Probiotics in Food: Health and Nutritional Properties and Guidelines for Evaluation. Available online: https://www.fao.org/3/a0512e/a0512e.pdf (accessed on 28 September 2022).
- Gu, S.; Chen, Y.; Wu, Z.; Chen, Y.; Gao, H.; Lv, L.; Guo, F.; Zhang, X.; Luo, R.; Huang, C.; et al. Alterations of the Gut Microbiota in Patients with Coronavirus Disease 2019 or H1N1 Influenza. Clin. Infect. Dis. 2020, 71, 2669–2678. [Google Scholar] [CrossRef]
- Marsland, B.J.; Trompette, A.; Gollwitzer, E.S. The Gut-Lung Axis in Respiratory Disease. Ann. Am. Thorac. Soc. 2015, 12, S150–S156. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Giudici, F.; Fiorindi, C.; Ficari, F.; Scaringi, S.; Amedei, A. Immunomodulating Activity and Therapeutic Effects of Short Chain Fatty Acids and Tryptophan Post-Biotics in Inflammatory Bowel Disease. Front. Immunol. 2019, 10, 2754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deleu, S.; Machiels, K.; Raes, J.; Verbeke, K.; Vermeire, S. Short chain fatty acids and its producing organisms: An overlooked therapy for IBD? EBioMedicine 2021, 66, 103293. [Google Scholar] [CrossRef]
- Geva-Zatorsky, N.; Sefik, E.; Kua, L.; Pasman, L.; Tan, T.G.; Ortiz-Lopez, A.; Yanortsang, T.B.; Yang, L.; Jupp, R.; Mathis, D.; et al. Mining the Human Gut Microbiota for Immunomodulatory Organisms. Cell 2017, 168, 928–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vollenbroich, D.; Ozel, M.; Vater, J.; Kamp, R.M.; Pauli, G. Mechanism of inactivation of enveloped viruses by the biosurfactant surfactin from Bacillus subtilis. Biologicals 1997, 25, 289–297. [Google Scholar] [CrossRef] [PubMed]
- McElvaney, O.J.; McEvoy, N.L.; McElvaney, O.F.; Carroll, T.P.; Murphy, M.P.; Dunlea, D.M.; Ni Choileain, O.; Clarke, J.; O’Connor, E.; Hogan, G.; et al. Characterization of the Inflammatory Response to Severe COVID-19 Illness. Am. J. Respir. Crit. Care Med. 2020, 202, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Carsetti, R.; Zaffina, S.; Piano Mortari, E.; Terreri, S.; Corrente, F.; Capponi, C.; Palomba, P.; Mirabella, M.; Cascioli, S.; Palange, P.; et al. Different Innate and Adaptive Immune Responses to SARS-CoV-2 Infection of Asymptomatic, Mild, and Severe Cases. Front. Immunol. 2020, 11, 610300. [Google Scholar] [CrossRef]
- Copaescu, A.; Smibert, O.; Gibson, A.; Phillips, E.J.; Trubiano, J.A. The role of IL-6 and other mediators in the cytokine storm associated with SARS-CoV-2 infection. J. Allergy Clin. Immunol. 2020, 146, 518–534. [Google Scholar] [CrossRef]
- Tan, M.; Liu, Y.; Zhou, R.; Deng, X.; Li, F.; Liang, K.; Shi, Y. Immunopathological characteristics of coronavirus disease 2019 cases in Guangzhou, China. Immunology 2020, 160, 261–268. [Google Scholar] [CrossRef]
- Magleby, R.; Westblade, L.F.; Trzebucki, A.; Simon, M.S.; Rajan, M.; Park, J.; Goyal, P.; Safford, M.M.; Satlin, M.J. Impact of Severe Acute Respiratory Syndrome Coronavirus 2 Viral Load on Risk of Intubation and Mortality among Hospitalized Patients with Coronavirus Disease 2019. Clin. Infect. Dis. 2021, 73, e4197–e4205. [Google Scholar] [CrossRef] [PubMed]
- Garbers, C.; Heink, S.; Korn, T.; Rose-John, S. Interleukin-6: Designing specific therapeutics for a complex cytokine. Nat. Rev. Drug Discov. 2018, 17, 395–412. [Google Scholar] [CrossRef] [PubMed]
- Umakanthan, S.; Sahu, P.; Ranade, A.V.; Bukelo, M.M.; Rao, J.S.; Abrahao-Machado, L.F.; Dahal, S.; Kumar, H.; Kv, D. Origin, transmission, diagnosis and management of coronavirus disease 2019 (COVID-19). Postgrad. Med. J. 2020, 96, 753–758. [Google Scholar] [PubMed]

| Control Group (n = 60) | Probiotic Group (n = 60) | p-Value | |
|---|---|---|---|
| Age, years, mean (SD) | 41.5 (13.6) | 41.4 (12.1) | 0.99 a |
| BMI, mean (SD) | 26.9 (4.8) | 26.0 (5.5) | 0.28 b |
| Sex, male/female, n (%) | 18/36 (33.3/66.7) | 27/31 (46.6/53.5) | 0.15 c |
| SIBO, n (%) | 0 | 10 (10.7) | <0.002 d |
| AHT, n (%) | 13 (21.7) | 9 (15.0) | 0.35 c |
| Type 2 diabetes, n (%) | 5 (8.3) | 3 (5.0) | 0.72 d |
| IBS, n (%) | 0 | 23 (38.33) | <0.001 d |
| CD, n (%) | 0 | 2 (3.33) | 0.50 d |
| UC, n (%) | 0 | 4 (6.66) | 0.12 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catinean, A.; Sida, A.; Silvestru, C.; Balan, G.G. Ongoing Treatment with a Spore-Based Probiotic Containing Five Strains of Bacillus Improves Outcomes of Mild COVID-19. Nutrients 2023, 15, 488. https://doi.org/10.3390/nu15030488
Catinean A, Sida A, Silvestru C, Balan GG. Ongoing Treatment with a Spore-Based Probiotic Containing Five Strains of Bacillus Improves Outcomes of Mild COVID-19. Nutrients. 2023; 15(3):488. https://doi.org/10.3390/nu15030488
Chicago/Turabian StyleCatinean, Adrian, Anamaria Sida, Celina Silvestru, and Gheorghe G. Balan. 2023. "Ongoing Treatment with a Spore-Based Probiotic Containing Five Strains of Bacillus Improves Outcomes of Mild COVID-19" Nutrients 15, no. 3: 488. https://doi.org/10.3390/nu15030488





