The Hidden Dangers of Plant-Based Diets Affecting Bone Health: A Cross-Sectional Study with U.S. National Health and Nutrition Examination Survey (NHANES) Data from 2005–2018
Abstract
:1. Introduction
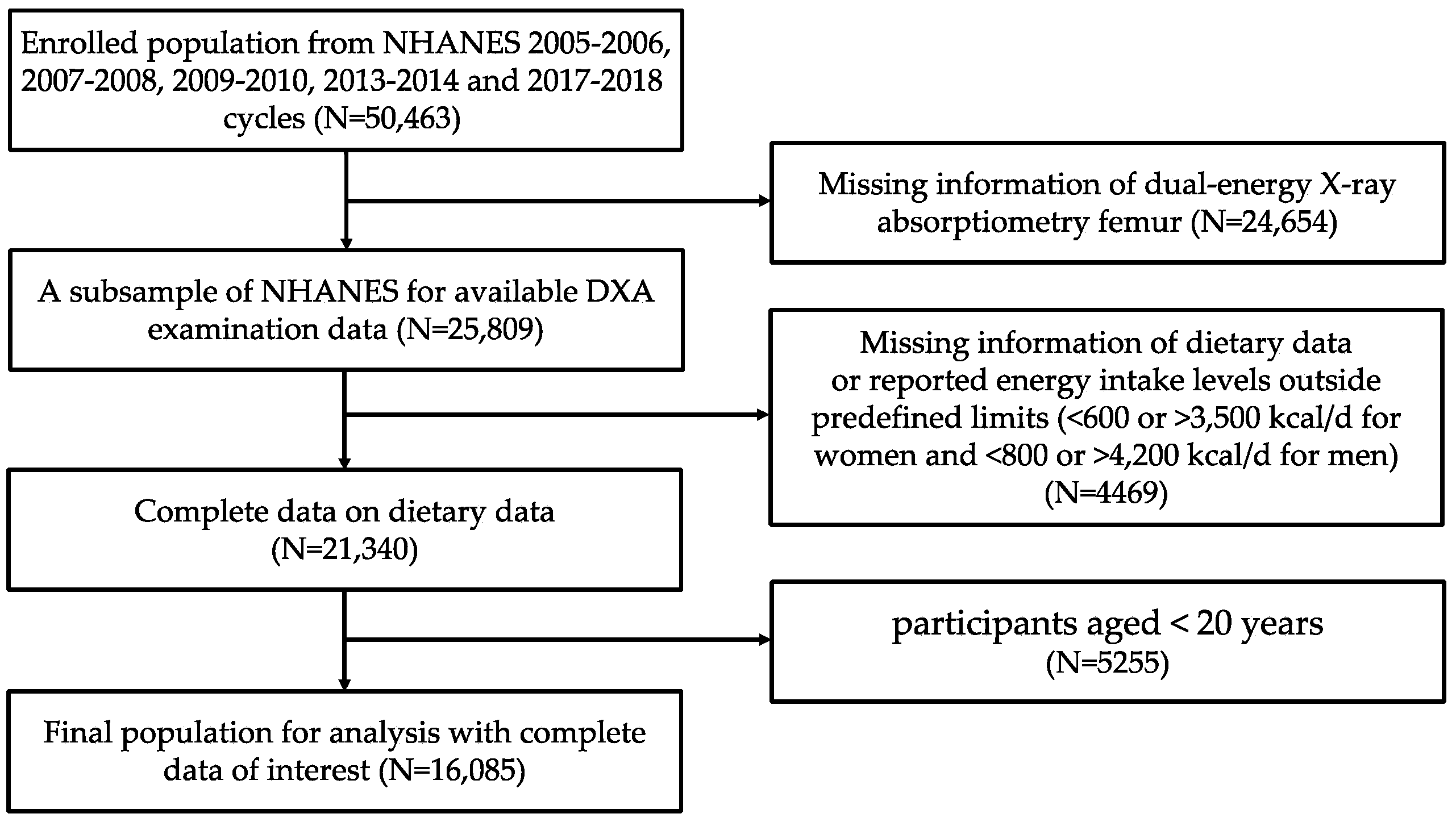
2. Materials and Methods
2.1. Study Population
2.2. Bone Mineral Density Assessment
2.3. Plant-Based Diet Index
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Participants
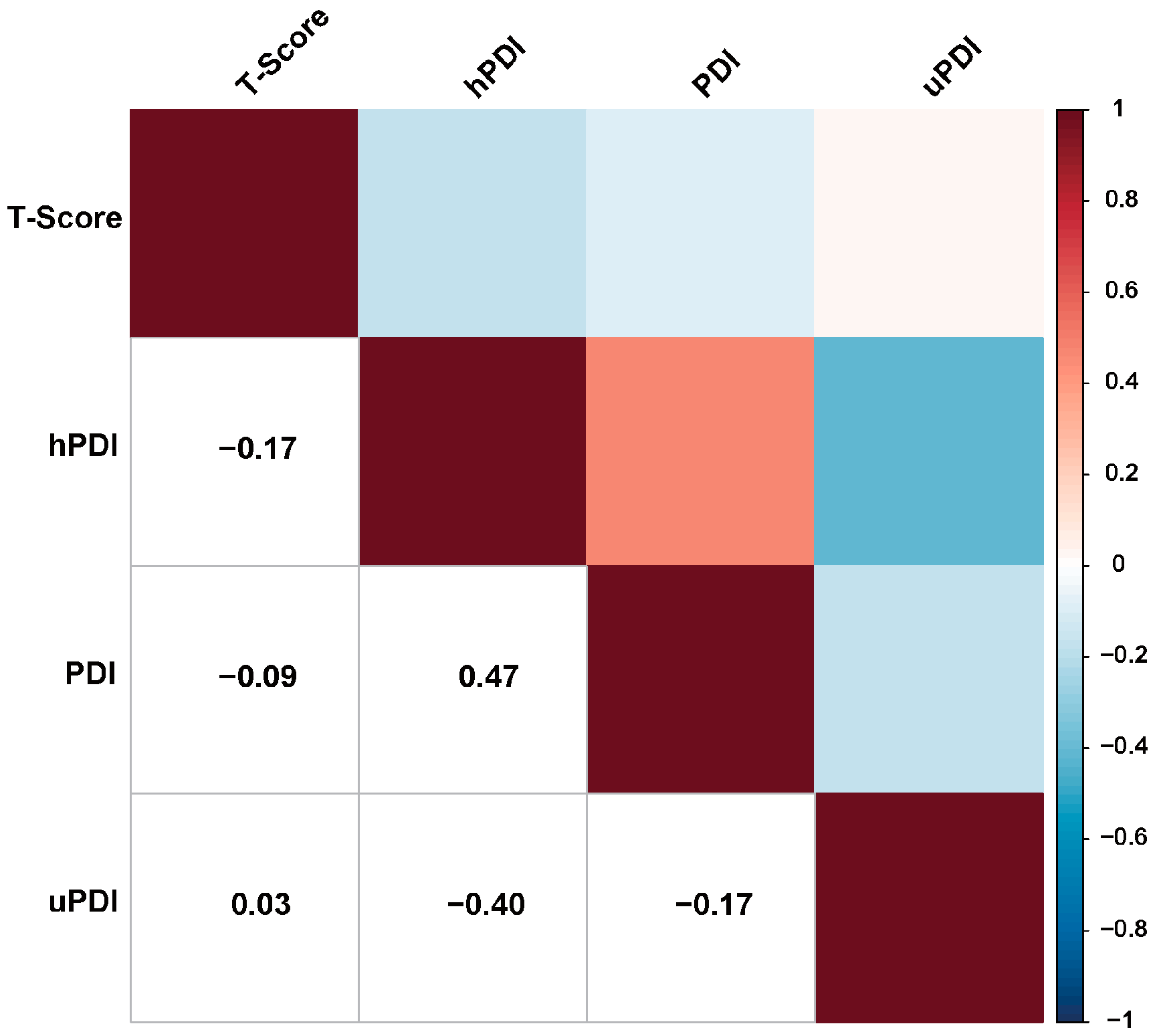
3.2. Correlation between Plant-Based Diet Indexes and BMD T-Score
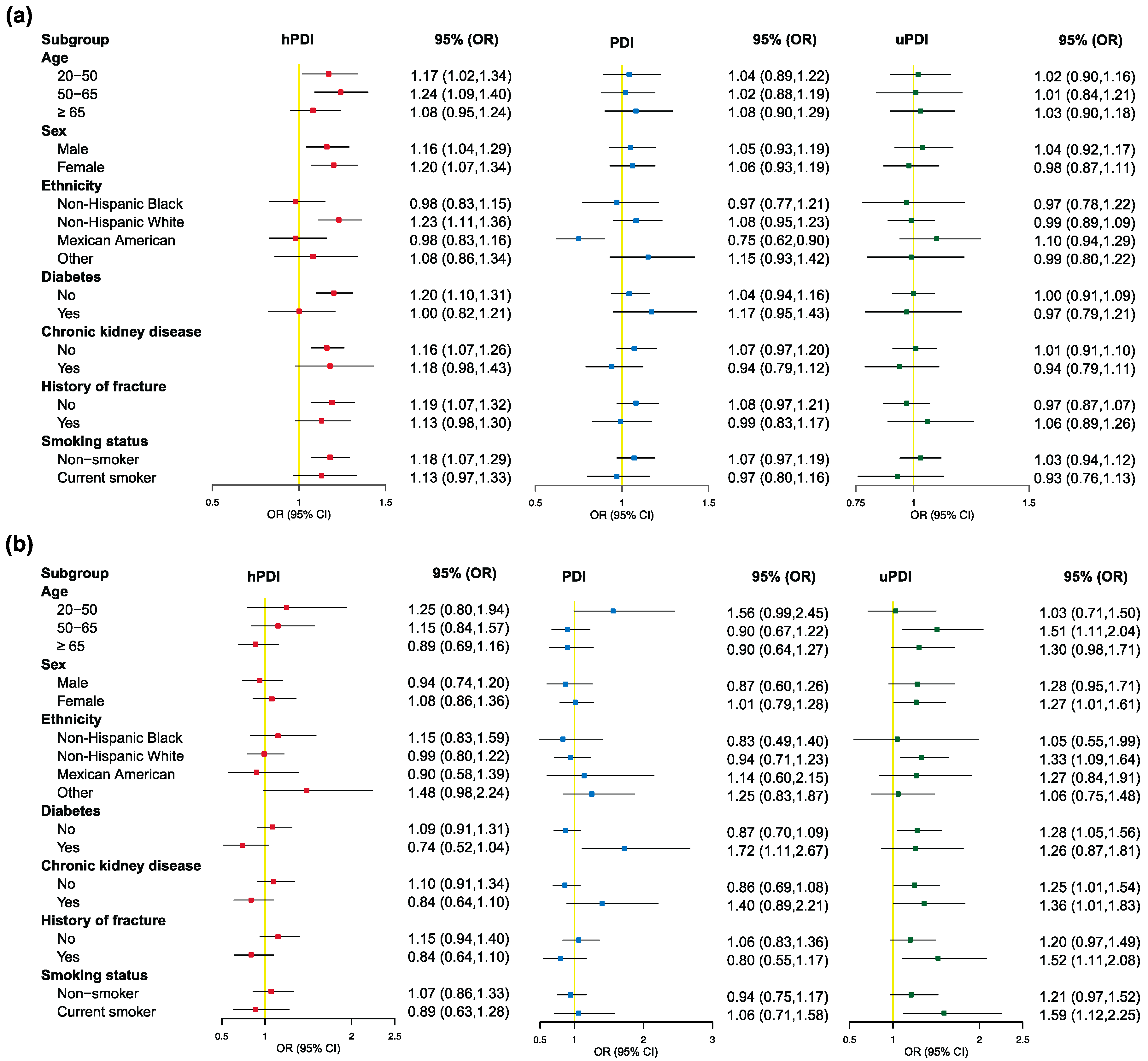
3.3. Associations between Plant-Based Diet Indexes and Different BMD Status Groups
3.4. Associations between 15 Individual Food Items and Different BMD Status Groups
3.5. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACR | albumin-to-creatinine ratio; |
| BMD | bone mineral density; |
| BMI | body mass index; |
| CI | 95% confidence interval |
| CKD | chronic kidney disease; |
| DBP | diastolic blood pressures; |
| eGFR | estimated glomerular filtration rate; |
| FPG | fasting plasma glucose; |
| HbA1c | hemoglobin A1c; |
| hPDI | healthy plant-based dietary index; |
| IGF-I | insulin-like growth factor I; |
| NCHS | National Center for Health Statistics; |
| NHANES | National Health and Nutrition Examination Survey; |
| OGTT | oral glucose tolerance test; |
| OR | odds ratio; |
| PDI | overall plant-based dietary index; |
| PIR | poverty income ratio; |
| SBP | systolic blood pressures; |
| T2DM | type 2 diabetes mellitus; |
| uPDI | unhealthy plant-based dietary index; |
| US | United States. |
References
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis. Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.-L.; Cui, A.-Y.; Hsu, C.-J.; Peng, R.; Jiang, N.; Xu, X.-H.; Ma, Y.-G.; Liu, D.; Lu, H.-D. Global, Regional Prevalence, and Risk Factors of Osteoporosis According to the World Health Organization Diagnostic Criteria: A Systematic Review and Meta-Analysis. Osteoporos. Int. 2022, 33, 2137–2153. [Google Scholar] [CrossRef] [PubMed]
- Sözen, T.; Özışık, L.; Başaran, N.Ç. An Overview and Management of Osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef]
- Tang, Y.; Peng, B.; Liu, J.; Liu, Z.; Xia, Y.; Geng, B. Systemic Immune-Inflammation Index and Bone Mineral Density in Postmenopausal Women: A Cross-Sectional Study of the National Health and Nutrition Examination Survey (NHANES) 2007–2018. Front. Immunol. 2022, 13, 975400. [Google Scholar] [CrossRef] [PubMed]
- Clynes, M.A.; Harvey, N.C.; Curtis, E.M.; Fuggle, N.R.; Dennison, E.M.; Cooper, C. The Epidemiology of Osteoporosis. Br. Med. Bull. 2020, 133, 105–117. [Google Scholar] [CrossRef]
- Liu, Z.; Weaver, J.; de Papp, A.; Li, Z.; Martin, J.; Allen, K.; Hui, S.; Imel, E.A. Disparities in Osteoporosis Treatments. Osteoporos. Int. 2016, 27, 509–519. [Google Scholar] [CrossRef]
- Hsu, E. Plant-Based Diets and Bone Health: Sorting through the Evidence. Curr. Opin. Endocrinol. Diabetes Obes. 2020, 27, 248–252. [Google Scholar] [CrossRef]
- Shahinfar, H.; Amini, M.R.; Payandeh, N.; Naghshi, S.; Sheikhhossein, F.; Djafarian, K.; Shab-Bidar, S. The Link between Plant-Based Diet Indices with Biochemical Markers of Bone Turn over, Inflammation, and Insulin in Iranian Older Adults. Food Sci. Nutr. 2021, 9, 3000–3014. [Google Scholar] [CrossRef]
- Chen, H.; Shen, J.; Xuan, J.; Zhu, A.; Ji, J.S.; Liu, X.; Cao, Y.; Zong, G.; Zeng, Y.; Wang, X.; et al. Plant-Based Dietary Patterns in Relation to Mortality among Older Adults in China. Nat. Aging 2022, 2, 224–230. [Google Scholar] [CrossRef]
- Satija, A.; Bhupathiraju, S.N.; Rimm, E.B.; Spiegelman, D.; Chiuve, S.E.; Borgi, L.; Willett, W.C.; Manson, J.E.; Sun, Q.; Hu, F.B. Plant-Based Dietary Patterns and Incidence of Type 2 Diabetes in US Men and Women: Results from Three Prospective Cohort Studies. PLoS Med. 2016, 13, e1002039. [Google Scholar] [CrossRef] [Green Version]
- Loeb, S.; Fu, B.C.; Bauer, S.R.; Pernar, C.H.; Chan, J.M.; Van Blarigan, E.L.; Giovannucci, E.L.; Kenfield, S.A.; Mucci, L.A. Association of Plant-Based Diet Index with Prostate Cancer Risk. Am. J. Clin. Nutr. 2022, 115, 662–670. [Google Scholar] [CrossRef]
- Kahleova, H.; Levin, S.; Barnard, N. Cardio-Metabolic Benefits of Plant-Based Diets. Nutrients 2017, 9, 848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hargreaves, S.M.; Raposo, A.; Saraiva, A.; Zandonadi, R.P. Vegetarian Diet: An Overview through the Perspective of Quality of Life Domains. Int. J. Environ. Res. Public Health 2021, 18, 4067. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, H.; Giovannucci, E.L. Quality of Plant-Based Diets and Risk of Hypertension: A Korean Genome and Examination Study. Eur. J. Nutr. 2021, 60, 3841–3851. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Caulfield, L.E.; Garcia-Larsen, V.; Steffen, L.M.; Coresh, J.; Rebholz, C.M. Plant-Based Diets Are Associated with a Lower Risk of Incident Cardiovascular Disease, Cardiovascular Disease Mortality, and All-Cause Mortality in a General Population of Middle-Aged Adults. J. Am. Heart Assoc. 2019, 8, e012865. [Google Scholar] [CrossRef]
- Muñoz-Garach, A.; García-Fontana, B.; Muñoz-Torres, M. Nutrients and Dietary Patterns Related to Osteoporosis. Nutrients 2020, 12, 1986. [Google Scholar] [CrossRef]
- Juan, W.; Yamini, S.; Britten, P. Food Intake Patterns of Self-Identified Vegetarians Among the U.S. Population, 2007–2010. Procedia Food Sci. 2015, 4, 86–93. [Google Scholar] [CrossRef] [Green Version]
- Iguacel, I.; Miguel-Berges, M.L.; Gómez-Bruton, A.; Moreno, L.A.; Julián, C. Veganism, Vegetarianism, Bone Mineral Density, and Fracture Risk: A Systematic Review and Meta-Analysis. Nutr. Rev. 2019, 77, 1–18. [Google Scholar] [CrossRef]
- Appleby, P.N.; Crowe, F.L.; Bradbury, K.E.; Travis, R.C.; Key, T.J. Mortality in Vegetarians and Comparable Nonvegetarians in the United Kingdom. Am. J. Clin. Nutr. 2016, 103, 218–230. [Google Scholar] [CrossRef] [Green Version]
- Storz, M.A.; Rizzo, G.; Müller, A.; Lombardo, M. Bowel Health in U.S. Vegetarians: A 4-Year Data Report from the National Health and Nutrition Examination Survey (NHANES). Nutrients 2022, 14, 681. [Google Scholar] [CrossRef]
- Yoshida, Y.; Scribner, R.; Chen, L.; Broyles, S.; Phillippi, S.; Tseng, T.-S. Diet Quality and Its Relationship with Central Obesity among Mexican Americans: Findings from National Health and Nutrition Examination Survey (NHANES) 1999–2012. Public Health Nutr. 2017, 20, 1193–1202. [Google Scholar] [CrossRef] [Green Version]
- Mattei, J.; McClain, A.C.; Falcón, L.M.; Noel, S.E.; Tucker, K.L. Dietary Acculturation among Puerto Rican Adults Varies by Acculturation Construct and Dietary Measure. J. Nutr. 2018, 148, 1804–1813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fresán, U.; Errendal, S.; Craig, W.J. Influence of the Socio-Cultural Environment and External Factors in Following Plant-Based Diets. Sustainability 2020, 12, 9093. [Google Scholar] [CrossRef]
- Li, H.; Zeng, X.; Wang, Y.; Zhang, Z.; Zhu, Y.; Li, X.; Hu, A.; Zhao, Q.; Yang, W. A Prospective Study of Healthful and Unhealthful Plant-Based Diet and Risk of Overall and Cause-Specific Mortality. Eur. J. Nutr. 2022, 61, 387–398. [Google Scholar] [CrossRef]
- Satija, A.; Bhupathiraju, S.N.; Spiegelman, D.; Chiuve, S.E.; Manson, J.E.; Willett, W.; Rexrode, K.M.; Rimm, E.B.; Hu, F.B. Healthful and Unhealthful Plant-Based Diets and the Risk of Coronary Heart Disease in U.S. Adults. J. Am. Coll. Cardiol. 2017, 70, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Ghadiri, M.; Cheshmazar, E.; Shateri, Z.; Gerami, S.; Nouri, M.; Gargari, B.P. Healthy Plant-Based Diet Index as a Determinant of Bone Mineral Density in Osteoporotic Postmenopausal Women: A Case-Control Study. Front. Nutr. 2022, 9, 1083685. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Jiang, Y.; Yang, D.; Zhu, C.; Yuan, H.; Yuan, Z.; Suo, C.; Chen, X.; Xu, K. The Role of Metabolites under the Influence of Genes and Lifestyles in Bone Density Changes. Front. Nutr. 2022, 9, 934951. [Google Scholar] [CrossRef]
- Looker, A.C.; Wahner, H.W.; Dunn, W.L.; Calvo, M.S.; Harris, T.B.; Heyse, S.P.; Johnston, C.C.; Lindsay, R. Updated Data on Proximal Femur Bone Mineral Levels of US Adults. Osteoporos. Int. 1998, 8, 468–489. [Google Scholar] [CrossRef]
- Looker, A.C.; Borrud, L.G.; Hughes, J.P.; Fan, B.; Shepherd, J.A.; Melton, L.J. Lumbar Spine and Proximal Femur Bone Mineral Density, Bone Mineral Content, and Bone Area: United States, 2005–2008. Vital Health Stat. Ser. 11 Data Natl. Health Surv. 2012, 251, 1–132. [Google Scholar]
- Zamora, A.N.; Watkins, D.J.; Peterson, K.E.; Jansen, E.C. Association between Pesticide Exposure and Sleep Health among a Representative Sample of US Adults: Evidence from NHANES 2009–2014. BMC Public Health 2021, 21, 2199. [Google Scholar] [CrossRef]
- Han, C.; Lim, Y.-H.; Hong, Y.-C. Ten-Year Trends in Urinary Concentrations of Triclosan and Benzophenone-3 in the General U.S. Population from 2003 to 2012. Environ. Pollut. 2016, 208, 803–810. [Google Scholar] [CrossRef]
- Khalil, N.; Chen, A.; Lee, M.; Czerwinski, S.A.; Ebert, J.R.; DeWitt, J.C.; Kannan, K. Association of Perfluoroalkyl Substances, Bone Mineral Density, and Osteoporosis in the U.S. Population in NHANES 2009–2010. Environ. Health Perspect. 2016, 124, 81–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Global Recommendations on Physical Activity for Health; WHO Guidelines Approved by the Guidelines Review Committee; World Health Organization: Geneva, Switzerland, 2010; ISBN 978-92-4-159997-9.
- American Diabetes Association 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43, S14–S31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021, 100, S1–S276. [CrossRef] [PubMed]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Diedenhofen, B.; Musch, J. Cocor: A Comprehensive Solution for the Statistical Comparison of Correlations. PLoS ONE 2015, 10, e0121945. [Google Scholar] [CrossRef] [Green Version]
- Haneuse, S.; VanderWeele, T.J.; Arterburn, D. Using the E-Value to Assess the Potential Effect of Unmeasured Confounding in Observational Studies. JAMA 2019, 321, 602–603. [Google Scholar] [CrossRef]
- VanderWeele, T.J.; Ding, P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann. Intern. Med. 2017, 167, 268–274. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Li, Y.; Wu, S. Comparison of Human Bone Mineral Densities in Subjects on Plant-Based and Omnivorous Diets: A Systematic Review and Meta-Analysis. Arch. Osteoporos. 2021, 16, 95. [Google Scholar] [CrossRef]
- Ho-Pham, L.T.; Nguyen, N.D.; Nguyen, T.V. Effect of Vegetarian Diets on Bone Mineral Density: A Bayesian Meta-Analysis. Am. J. Clin. Nutr. 2009, 90, 943–950. [Google Scholar] [CrossRef] [Green Version]
- Merrill, R.M.; Aldana, S.G. Consequences of a Plant-Based Diet with Low Dairy Consumption on Intake of Bone-Relevant Nutrients. J. Women’s Health 2009, 18, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Functions of Osteocalcin in Bone, Pancreas, Testis, and Muscle. Int. J. Mol. Sci. 2020, 21, 7513. [Google Scholar] [CrossRef] [PubMed]
- Kwaśniewska, M.; Pikala, M.; Grygorczuk, O.; Waśkiewicz, A.; Stepaniak, U.; Pająk, A.; Kozakiewicz, K.; Nadrowski, P.; Zdrojewski, T.; Puch-Walczak, A.; et al. Dietary Antioxidants, Quality of Nutrition and Cardiovascular Characteristics among Omnivores, Flexitarians and Vegetarians in Poland-The Results of Multicenter National Representative Survey WOBASZ. Antioxidants 2023, 12, 222. [Google Scholar] [CrossRef]
- Penczynski, K.J.; Remer, T.; Menzel, J.; Abraham, K.; Weikert, C. Urinary Potential Renal Acid Load (UPRAL) among Vegans Versus Omnivores and Its Association with Bone Health in the Cross-Sectional Risks and Benefits of a Vegan Diet Study. Nutrients 2022, 14, 4468. [Google Scholar] [CrossRef] [PubMed]
- Dawson-Hughes, B. Acid-Base Balance of the Diet-Implications for Bone and Muscle. Eur. J. Clin. Nutr. 2020, 74, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Burckhardt, P. The Role of Low Acid Load in Vegetarian Diet on Bone Health: A Narrative Review. Swiss Med. Wkly. 2016, 146, w14277. [Google Scholar] [CrossRef]
- Kanis, J.A.; Cooper, C.; Rizzoli, R.; Reginster, J.-Y.; on behalf of the Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis (ESCEO) and the Committees of Scientific Advisors and National Societies of the International Osteoporosis Foundation (IOF). European Guidance for the Diagnosis and Management of Osteoporosis in Postmenopausal Women. Osteoporos. Int. 2019, 30, 3–44. [Google Scholar] [CrossRef] [Green Version]
- Zhai, T.; Chen, Q.; Xu, J.; Jia, X.; Xia, P. Prevalence and Trends in Low Bone Density, Osteopenia and Osteoporosis in U.S. Adults with Non-Alcoholic Fatty Liver Disease, 2005–2014. Front. Endocrinol. 2021, 12, 825448. [Google Scholar] [CrossRef]
- Bala, Y.; Zebaze, R.; Seeman, E. Role of Cortical Bone in Bone Fragility. Curr. Opin. Rheumatol. 2015, 27, 406–413. [Google Scholar] [CrossRef]
- Chiodini, I.; Falchetti, A.; Merlotti, D.; Eller Vainicher, C.; Gennari, L. Updates in Epidemiology, Pathophysiology and Management Strategies of Glucocorticoid-Induced Osteoporosis. Expert Rev. Endocrinol. Metab. 2020, 15, 283–298. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, L.-J.; Xu, Q.; Wu, K.-H.; Deng, H.-W. Socioeconomic Status and Bone Mineral Density in Adults by Race/Ethnicity and Gender: The Louisiana Osteoporosis Study. Osteoporos. Int. 2017, 28, 1699–1709. [Google Scholar] [CrossRef] [PubMed]
- Ning, H.-T.; Du, Y.; Zhao, L.-J.; Tian, Q.; Feng, H.; Deng, H.-W. Racial and Gender Differences in the Relationship between Sarcopenia and Bone Mineral Density among Older Adults. Osteoporos. Int. 2021, 32, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.-Y.; Zhou, Z.; Gao, Y.; Chen, Y.; Li, R.; Zhou, M.; Zhu, D. Racial Differences and Factors Associated with Low Femoral Neck Bone Mineral Density: An Analysis of NHANES 2005–2014 Data. Arch. Osteoporos. 2021, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Cavalier, E.; Bergmann, P.; Bruyère, O.; Delanaye, P.; Durnez, A.; Devogelaer, J.-P.; Ferrari, S.L.; Gielen, E.; Goemaere, S.; Kaufman, J.-M.; et al. The Role of Biochemical of Bone Turnover Markers in Osteoporosis and Metabolic Bone Disease: A Consensus Paper of the Belgian Bone Club. Osteoporos. Int. 2016, 27, 2181–2195. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Overall (N = 16,085) | Normal (N = 9608) | Osteopenia (N = 5404) | Osteoporosis (N = 1073) | p-Value |
|---|---|---|---|---|---|
| Demographic characteristics | |||||
| Age, n (no. weighted %) | <0.001 | ||||
| 20–50 | 6377 (39.65) | 4775 (54.66) | 1501 (31.43) | 101 (11.09) | |
| 50–65 | 5077 (31.56) | 2889 (30.71) | 1839 (38.31) | 349 (33.60) | |
| ≥65 | 4631 (28.79) | 1944 (14.63) | 2064 (30.26) | 623 (55.31) | |
| Sex, n (no. weighted %) | <0.001 | ||||
| Female | 8238 (51.22) | 4259 (45.19) | 3152 (60.87) | 827 (80.21) | |
| Male | 7847 (48.78) | 5349 (54.81) | 2252 (39.13) | 246 (19.79) | |
| Ethnicity, n (no. weighted %) | <0.001 | ||||
| Non-Hispanic black | 3163 (19.66) | 2389 (13.34) | 687 (5.99) | 87 (4.39) | |
| Mexican American | 2614 (16.26) | 1569 (7.94) | 863 (7.02) | 182 (6.00) | |
| Non-Hispanic white | 7844 (48.77) | 4329 (68.69) | 2936 (75.54) | 579 (76.79) | |
| Other | 2464 (15.32) | 1321 (10.03) | 918 (11.45) | 225 (12.82) | |
| Education, n (no. weighted %) | <0.001 | ||||
| Less than high school | 6710 (41.76) | 3921 (32.53) | 2277 (32.02) | 512 (35.85) | |
| High school | 1074 (6.68) | 561 (5.93) | 401 (8.60) | 112 (12.57) | |
| More than high school | 8283 (51.55) | 5118 (61.54) | 2717 (59.38) | 448 (51.58) | |
| PIR, n (no. weighted %) | 0.002 | ||||
| <1 | 2665 (17.95) | 1569 (11.87) | 896 (11.36) | 200 (13.72) | |
| 1–3 | 6156 (41.47) | 3580 (33.00) | 2115 (34.69) | 461 (42.84) | |
| ≥3 | 6024 (40.58) | 3751 (55.13) | 1966 (53.95) | 307 (43.45) | |
| BMI, n (no. weighted %) | <0.001 | ||||
| <25 | 4453 (27.78) | 1936 (22.70) | 1968 (39.00) | 549 (54.02) | |
| 25–30 | 5709 (35.62) | 3414 (35.01) | 1957 (34.88) | 338 (29.80) | |
| ≥30 | 5865 (36.59) | 4227 (42.29) | 1456 (26.13) | 182 (16.18) | |
| Lifestyle characteristics | |||||
| Smoking status, n (no. weighted %) | <0.001 | ||||
| Non-smoker | 11,356 (73.12) | 6561 (71.04) | 3991 (76.12) | 804 (76.49) | |
| ETS | 550 (3.54) | 381 (3.65) | 149 (2.78) | 20 (1.47) | |
| Current smoker | 3624 (23.34) | 2309 (25.31) | 1096 (21.09) | 219 (22.04) | |
| Marital status, n (no. weighted %) | <0.001 | ||||
| Married/cohabiting | 10,075 (62.67) | 6171 (67.44) | 3342 (65.16) | 562 (53.29) | |
| Widowed/divorced/separated | 3886 (24.17) | 1909 (16.43) | 1533 (24.11) | 444 (40.28) | |
| Never married | 2115 (13.16) | 1521 (16.12) | 528 (10.73) | 66 (6.43) | |
| Physical exercise, n (no. weighted %) | <0.001 | ||||
| Inactive | 4249 (26.42) | 2275 (19.36) | 1552 (22.24) | 422 (33.18) | |
| Insufficient | 2895 (18.00) | 1734 (18.99) | 972 (19.75) | 189 (17.54) | |
| Moderate | 1983 (12.33) | 1171 (12.62) | 683 (14.24) | 129 (15.31) | |
| High | 6958 (43.26) | 4428 (49.03) | 2197 (43.78) | 333 (33.97) | |
| History of disease | |||||
| T2DM, n (no. weighted %) | 0.01 | ||||
| No | 12,907 (80.24) | 7668 (84.55) | 4390 (87.10) | 849 (84.33) | |
| Yes | 3178 (19.76) | 1940 (15.45) | 1014 (12.90) | 224 (15.67) | |
| Hypertension, n (no. weighted %) | <0.001 | ||||
| No | 8591 (53.41) | 5379 (60.71) | 2758 (56.57) | 454 (45.15) | |
| Yes | 7494 (46.59) | 4229 (39.29) | 2646 (43.43) | 619 (54.85) | |
| CKD, n (no. weighted %) | <0.001 | ||||
| No | 12,428 (80.17) | 7670 (86.66) | 4055 (82.60) | 703 (71.45) | |
| Yes | 3074 (19.83) | 1573 (13.34) | 1170 (17.40) | 331 (28.55) | |
| Cancer, n (no. weighted %) | <0.001 | ||||
| No | 14,228 (88.53) | 8696 (90.88) | 4646 (85.55) | 886 (81.72) | |
| Yes | 1844 (11.47) | 902 (9.12) | 755 (14.45) | 187 (18.28) | |
| History of fracture, n (no. weighted %) | <0.001 | ||||
| No | 12,359 (76.91) | 7624 (77.87) | 3981 (72.62) | 754 (66.63) | |
| Yes | 3711 (23.09) | 1975 (22.13) | 1417 (27.38) | 319 (33.37) | |
| Plant-based diet index | |||||
| hPDI, n (no. weighted %) | <0.001 | ||||
| Q1 (22–39) | 3635 (22.60) | 2561 (26.90) | 937 (16.41) | 137 (13.15) | |
| Q2 (40–43) | 3046 (18.94) | 1922 (20.19) | 972 (18.21) | 152 (13.66) | |
| Q3 (44–47) | 3395 (21.11) | 1983 (20.58) | 1159 (20.79) | 253 (22.51) | |
| Q4 (48–51) | 2874 (17.87) | 1573 (16.06) | 1052 (19.35) | 249 (23.60) | |
| Q5 (52–69) | 3135 (19.49) | 1569 (16.27) | 1284 (25.24) | 282 (27.07) | |
| PDI, n (no. weighted %) | <0.001 | ||||
| Q1 (23–39) | 3697 (22.98) | 2392 (24.39) | 1117 (18.86) | 188 (17.10) | |
| Q2 (40–42) | 3139 (19.52) | 1962 (20.45) | 996 (17.90) | 181 (16.29) | |
| Q3 (43–45) | 3403 (21.16) | 2011 (22.22) | 1125 (22.03) | 267 (23.92) | |
| Q4 (46–48) | 2824 (17.56) | 1611 (16.39) | 995 (18.40) | 218 (19.93) | |
| Q5 (49–65) | 3022 (18.79) | 1632 (16.55) | 1171 (22.81) | 219 (22.76) | |
| uPDI, n (no. weighted %) | 0.01 | ||||
| Q1 (24–42) | 3538 (22.00) | 2092 (25.25) | 1223 (26.98) | 223 (23.57) | |
| Q2 (43–46) | 3582 (22.27) | 2085 (22.08) | 1264 (23.39) | 233 (22.42) | |
| Q3 (47–49) | 3005 (18.86) | 1726 (17.91) | 1064 (19.32) | 215 (20.10) | |
| Q4 (50–53) | 3388 (21.06) | 2048 (19.65) | 1101 (18.55) | 239 (19.71) | |
| Q5 (54–68) | 2572 (15.99) | 1657 (15.12) | 752 (11.75) | 163 (14.20) | |
| Osteopenia vs. Normal | Osteoporosis vs. Normal | |||
|---|---|---|---|---|
| Model 1 OR (95% CI) | Model 2 OR (95% CI) | Model 1 OR (95% CI) | Model 2 OR (95% CI) | |
| hPDI | ||||
| Q1 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Q2 | 1.18 (1.01, 1.39) | 1.24 (1.04, 1.48) | 0.83 (0.55, 1.27) | 0.89 (0.56, 1.41) |
| Q3 | 1.19 (0.99, 1.44) | 1.21 (1.00, 1.47) | 1.06 (0.73, 1.53) | 0.95 (0.65, 1.40) |
| Q4 | 1.23 (1.03, 1.46) | 1.24 (1.02, 1.51) | 1.06 (0.74, 1.51) | 1.02 (0.71, 1.46) |
| Q5 | 1.49 (1.27, 1.75) | 1.50 (1.24, 1.81) | 1.06 (0.72, 1.55) | 1.08 (0.73, 1.60) |
| Per 10-unit increment | 1.18 (1.10, 1.26) | 1.17 (1.08, 1.27) | 1.04 (0.89, 1.22) | 1.03 (0.88, 1.21) |
| p for trend | <0.001 | <0.001 | 0.51 | 0.49 |
| PDI | ||||
| Q1 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Q2 | 1.00 (0.84, 1.20) | 0.93 (0.77,1.13) | 0.87 (0.60,1.26) | 0.80 (0.53, 1.22) |
| Q3 | 1.08 (0.90, 1.30) | 1.00 (0.82,1.21) | 1.09 (0.76,1.57) | 1.00 (0.67, 1.49) |
| Q4 | 1.20 (1.00, 1.45) | 1.14 (0.93,1.38) | 1.15 (0.80,1.65) | 1.09 (0.71, 1.69) |
| Q5 | 1.36 (1.19, 1.56) | 1.22 (1.03,1.45) | 1.16 (0.81,1.66) | 1.01 (0.68, 1.51) |
| Per 10-unit increment | 1.13 (1.05, 1.21) | 1.06 (0.96,1.16) | 1.05 (0.88,1.25) | 0.97 (0.79, 1.19) |
| p for trend | <0.001 | 0.003 | 0.21 | 0.53 |
| uPDI | ||||
| Q1 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Q2 | 1.03 (0.91, 1.17) | 1.05 (0.90, 1.21) | 1.09 (0.79, 1.49) | 1.03 (0.71, 1.50) |
| Q3 | 1.13 (0.97, 1.32) | 1.15 (0.97, 1.37) | 1.41 (1.01, 1.97) | 1.27 (0.87, 1.86) |
| Q4 | 1.07 (0.94, 1.23) | 1.05 (0.91, 1.22) | 1.48 (1.09, 2.03) | 1.39 (0.98, 1.98) |
| Q5 | 1.02 (0.86, 1.19) | 0.97 (0.79, 1.20) | 1.78 (1.32, 2.40) | 1.48 (1.04, 2.11) |
| Per 10-unit increment | 1.02 (0.96, 1.09) | 1.00 (0.92, 1.08) | 1.37 (1.16, 1.61) | 1.29 (1.08, 1.54) |
| p for trend | 0.48 | 0.89 | <0.001 | 0.01 |
| Femoral Neck | Lumbar Spine | |||
|---|---|---|---|---|
| Osteopenia vs. Normal | Osteoporosis vs. Normal | Osteopenia vs. Normal | Osteoporosis vs. Normal | |
| hPDI | ||||
| Q1 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Q2 | 1.25 (0.95, 1.65) | 1.09 (0.51, 2.33) | 0.97 (0.79, 1.20) | 0.59 (0.34, 1.03) |
| Q3 | 1.23 (0.94, 1.61) | 0.68 (0.32, 1.43) | 1.18 (0.95, 1.46) | 0.80 (0.47, 1.38) |
| Q4 | 1.20 (0.90, 1.60) | 1.23 (0.66, 2.29) | 1.18 (0.98, 1.42) | 1.01 (0.58, 1.74) |
| Q5 | 1.27 (0.98, 1.63) | 0.74 (0.37, 1.47) | 1.25 (1.03, 1.51) | 1.08 (0.67, 1.74) |
| Per 10-unit increment | 1.07 (0.96, 1.19) | 0.88 (0.68, 1.12) | 1.11 (1.02, 1.21) | 1.07 (0.88, 1.31) |
| p for trend | 0.12 | 0.53 | 0.01 | 0.21 |
| PDI | ||||
| Q1 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Q2 | 0.81 (0.61, 1.08) | 0.58 (0.27, 1.28) | 0.98 (0.77, 1.25) | 0.86 (0.55, 1.34) |
| Q3 | 0.90 (0.68, 1.19) | 0.86 (0.42, 1.75) | 0.96 (0.76, 1.22) | 1.09 (0.71, 1.67) |
| Q4 | 0.89 (0.68, 1.15) | 0.75 (0.38, 1.50) | 1.21 (0.94, 1.55) | 1.42 (0.97, 2.06) |
| Q5 | 0.99 (0.77, 1.28) | 0.69 (0.34, 1.39) | 1.33 (1.07, 1.67) | 1.19 (0.76, 1.88) |
| Per 10-unit increment | 0.94 (0.81, 1.08) | 0.78 (0.56, 1.10) | 1.15 (1.02, 1.28) | 1.13 (0.90, 1.40) |
| p for trend | 0.84 | 0.45 | 0.003 | 0.13 |
| uPDI | ||||
| Q1 | 1 (Reference) | 1 (Reference) | 1 (Reference) | 1 (Reference) |
| Q2 | 1.13 (0.93, 1.38) | 0.86 (0.50, 1.48) | 1.23 (1.01, 1.50) | 1.60 (1.04, 2.45) |
| Q3 | 1.10 (0.67, 2.18) | 1.20 (0.92, 2.01) | 1.04 (0.83, 1.30) | 1.27 (0.82, 1.98) |
| Q4 | 1.26 (1.03, 1.56) | 2.17 (1.25, 3.79) | 1.09 (0.90, 1.33) | 1.92 (1.24, 2.98) |
| Q5 | 1.15 (0.89, 1.49) | 2.27 (1.03, 5.02) | 1.14 (0.93, 1.41) | 1.58 (1.04, 2.39) |
| Per 10-unit increment | 1.09 (0.97, 1.22) | 1.92 (1.47, 2.51) | 1.03 (0.93, 1.14) | 1.31 (1.09, 1.58) |
| p for trend | 0.12 | 0.01 | 0.40 | 0.007 |
| Groups | Osteopenia vs. Normal | p for Trend | Osteoporosis vs. Normal | p for Trend |
|---|---|---|---|---|
| Vegetables | 0.40 | 0.02 | ||
| Q1 | 1 (Reference) | 1 (Reference) | ||
| Q2 | 0.86 (0.72, 1.04) | 0.98 (0.74, 1.30) | ||
| Q3 | 0.99 (0.82, 1.19) | 0.83 (0.59, 1.15) | ||
| Q4 | 0.99 (0.81, 1.20) | 0.81 (0.57, 1.14) | ||
| Q5 | 1.00 (0.84, 1.20) | 0.64 (0.43, 0.95) | ||
| Nuts | 0.01 | 0.85 | ||
| Q1 | 1 (Reference) | 1 (Reference) | ||
| Q2 | 0.99 (0.85, 1.15) | 0.96 (0.69, 1.35) | ||
| Q3 | 1.02 (0.84, 1.24) | 0.86 (0.56, 1.31) | ||
| Q4 | 1.19 (0.99, 1.43) | 1.20 (0.82, 1.75) | ||
| Q5 | 1.22 (1.01, 1.46) | 0.94 (0.66, 1.35) | ||
| Refined grain | <0.001 | 0.63 | ||
| Q1 | 1 (Reference) | 1 (Reference) | ||
| Q2 | 0.88 (0.74, 1.04) | 1.17 (0.82, 1.66) | ||
| Q3 | 0.86 (0.73, 1.01) | 1.05 (0.76, 1.46) | ||
| Q4 | 0.82 (0.69, 0.98) | 0.86 (0.59, 1.25) | ||
| Q5 | 0.73 (0.61, 0.87) | 1.29 (0.82, 2.03) | ||
| Animal fat | 0.01 | 0.004 | ||
| Q1 | 1 (Reference) | 1 (Reference) | ||
| Q2 | 0.96 (0.79, 1.17) | 0.88 (0.61, 1.25) | ||
| Q3 | 0.96 (0.78, 1.17) | 0.98 (0.72, 1.34) | ||
| Q4 | 0.80 (0.64, 0.99) | 0.82 (0.58, 1.16) | ||
| Q5 | 0.81 (0.66, 0.99) | 0.57 (0.39, 0.83) | ||
| Eggs | 0.22 | 0.67 | ||
| Q1 | 1 (Reference) | 1 (Reference) | ||
| Q2 | 0.99 (0.82, 1.20) | 0.70 (0.48, 1.01) | ||
| Q3 | 0.79 (0.65, 0.96) | 0.66 (0.47, 0.93) | ||
| Q4 | 0.89 (0.77, 1.03) | 0.82 (0.58, 1.16) | ||
| Q5 | 0.88 (0.74, 1.04) | 0.74 (0.50, 1.11) | ||
| Meat | 0.10 | 0.001 | ||
| Q1 | 1 (Reference) | 1 (Reference) | ||
| Q2 | 0.89 (0.75, 1.06) | 1.05 (0.74, 1.49) | ||
| Q3 | 0.92 (0.76, 1.12) | 1.02 (0.70, 1.47) | ||
| Q4 | 0.77 (0.65, 0.92) | 0.58 (0.39, 0.85) | ||
| Q5 | 0.88 (0.73, 1.05) | 0.64 (0.44, 0.93) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Wang, J.; Wang, Y.; Xu, K.; Chen, X. The Hidden Dangers of Plant-Based Diets Affecting Bone Health: A Cross-Sectional Study with U.S. National Health and Nutrition Examination Survey (NHANES) Data from 2005–2018. Nutrients 2023, 15, 1794. https://doi.org/10.3390/nu15071794
Zheng Y, Wang J, Wang Y, Xu K, Chen X. The Hidden Dangers of Plant-Based Diets Affecting Bone Health: A Cross-Sectional Study with U.S. National Health and Nutrition Examination Survey (NHANES) Data from 2005–2018. Nutrients. 2023; 15(7):1794. https://doi.org/10.3390/nu15071794
Chicago/Turabian StyleZheng, Yi, Jiacheng Wang, Yawen Wang, Kelin Xu, and Xingdong Chen. 2023. "The Hidden Dangers of Plant-Based Diets Affecting Bone Health: A Cross-Sectional Study with U.S. National Health and Nutrition Examination Survey (NHANES) Data from 2005–2018" Nutrients 15, no. 7: 1794. https://doi.org/10.3390/nu15071794





