The Effect of Alginate Encapsulated Plant-Based Carbohydrate and Protein Supplementation on Recovery and Subsequent Performance in Athletes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participant Characteristics
2.2. Experimental Design
2.3. Preliminary Test
2.4. Exhaustive Cycling Exercise (EX1)
2.5. Time-to-Exhaustion Performance Test
2.6. The Supplements
2.7. Biological Sampling and Analyses
2.8. Statistics
3. Results
3.1. Physical Exercise Bouts
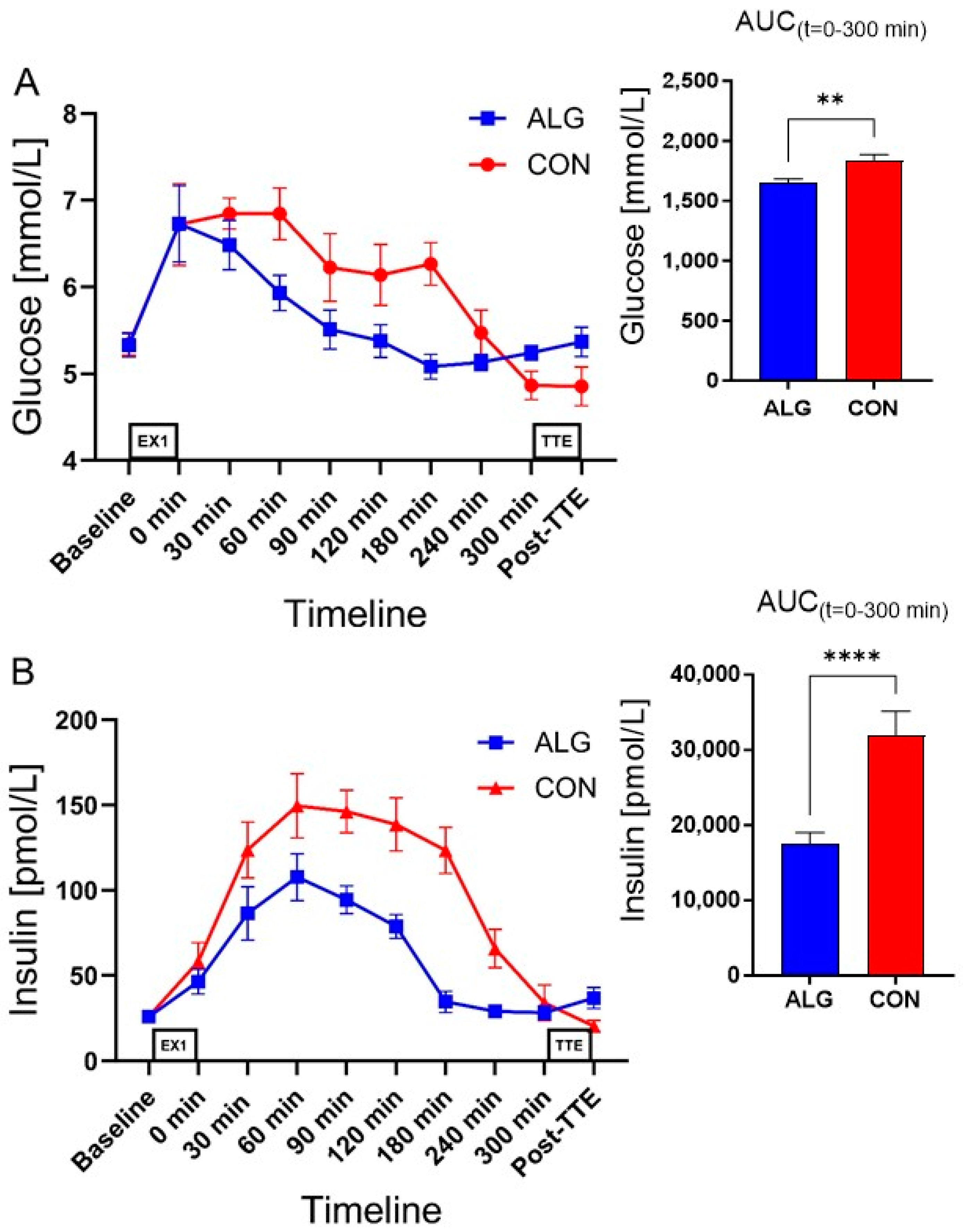
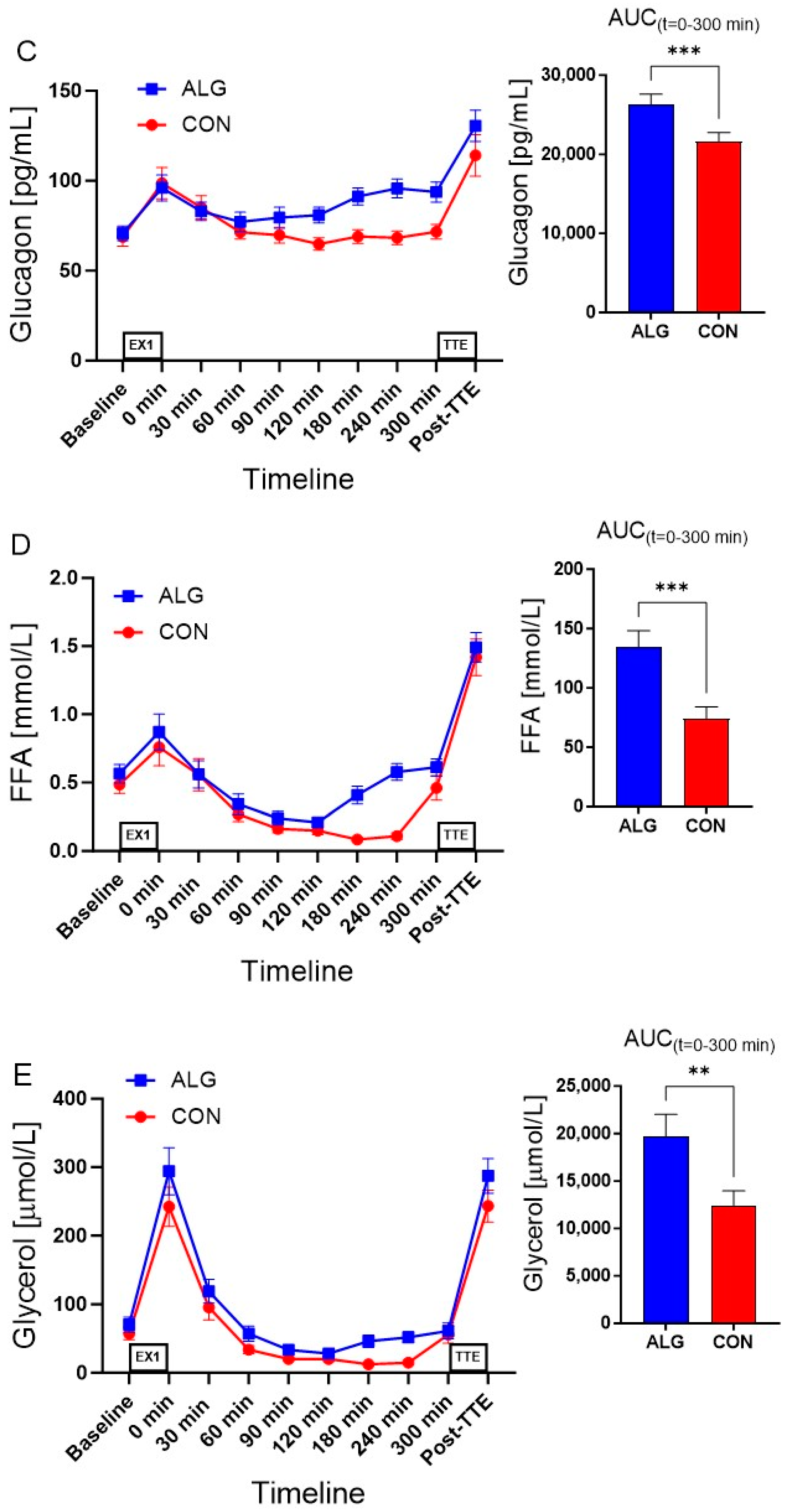
3.2. Supplements and Plasma Analyses
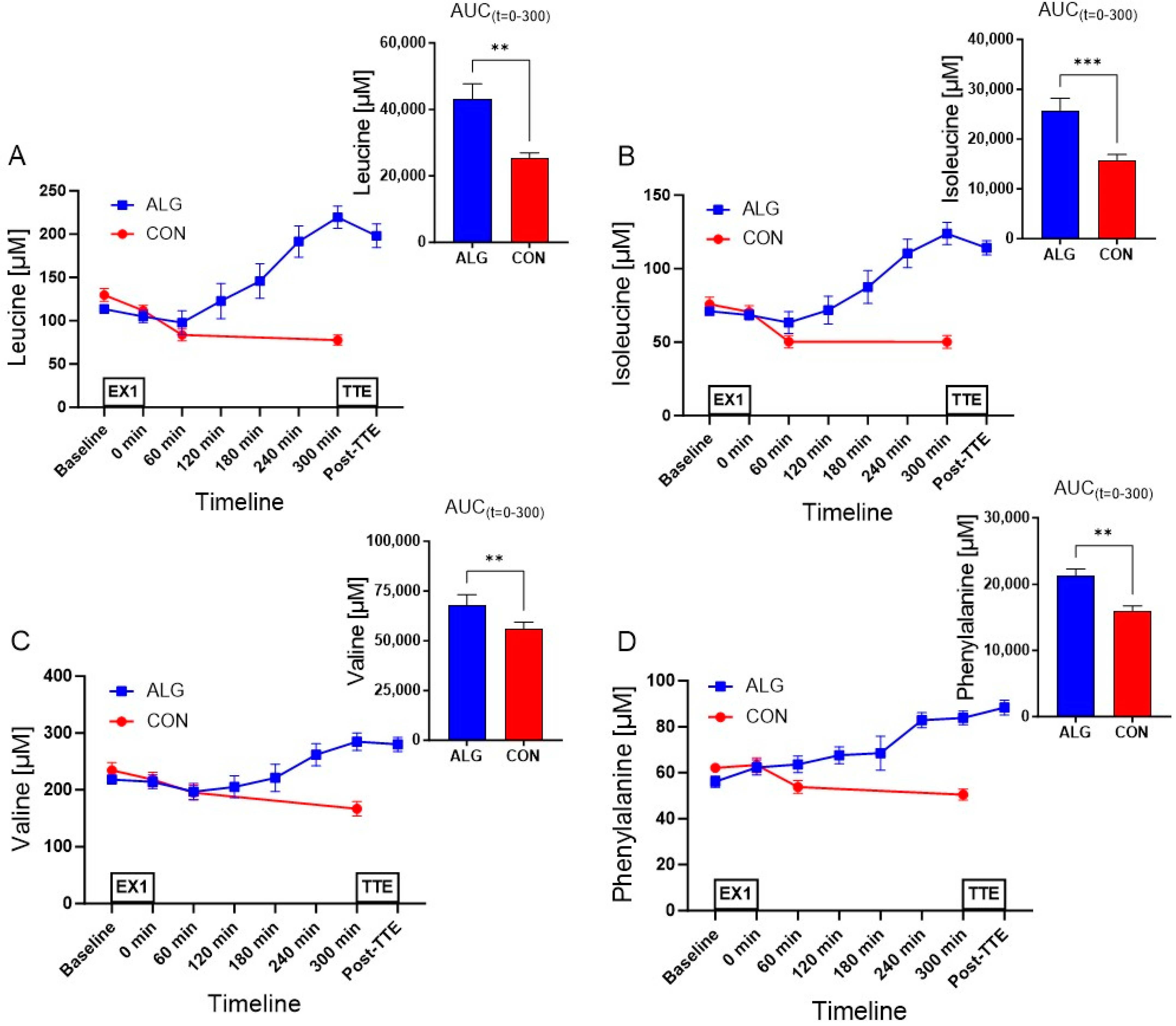
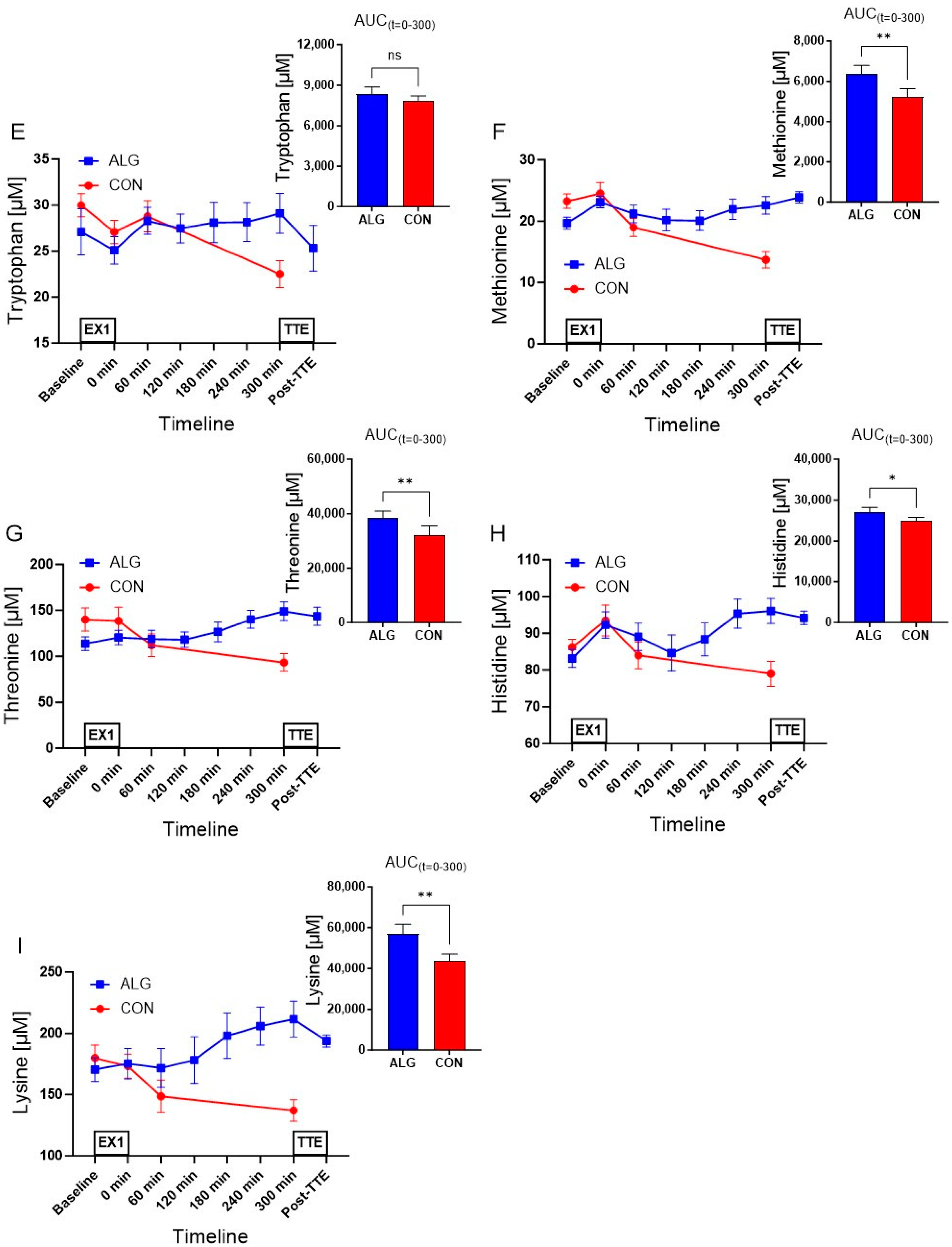
3.3. Plasma Amino Acids
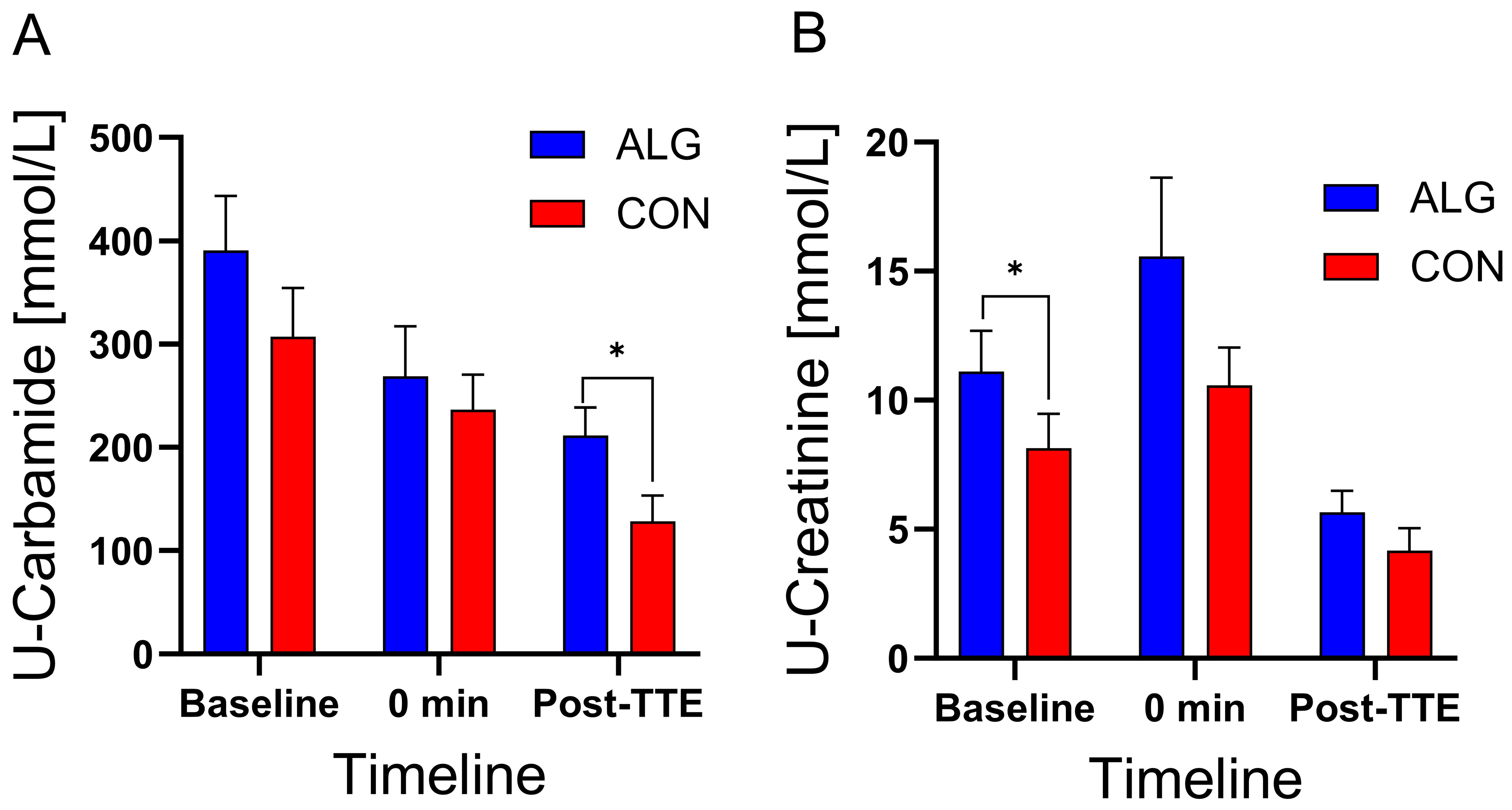
3.4. Urine and Saliva Samples
4. Discussion
5. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hansen, M.; Bangsbo, J.; Jensen, J.; Krause-Jensen, M.; Bibby, B.M.; Sollie, O.; Hall, U.A.; Madsen, K. Protein intake during training sessions has no effect on performance and recovery during a strenuous training camp for elite cyclists. J. Int. Soc. Sports Nutr. 2016, 13, 9. [Google Scholar] [CrossRef]
- Fernandez-Garcia, B.; Perez-Landaluce, J.; Rodriguez-Alonso, M.; Terrados, N. Intensity of exercise during road race pro-cycling competition. Med. Sci. Sport. Exerc. 2000, 32, 1002–1006. [Google Scholar] [CrossRef]
- Sánchez-Muñoz, C.; Zabala, M.; Muros, J.J. Nutritional intake and anthropometric changes of professional road cyclists during a 4-day competition. Scand. J. Med. Sci. Sports 2016, 26, 802–808. [Google Scholar] [CrossRef]
- Beelen, M.; Burke, L.M.; Gibala, M.J.; van Loon, L.J.C. Nutritional Strategies to Promote Postexercise Recovery. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 515–532. [Google Scholar] [CrossRef]
- Moore, D.R.; Camera, D.M.; Areta, J.L.; Hawley, J.A. Beyond muscle hypertrophy: Why dietary protein is important for endurance athletes. Appl. Physiol. Nutr. Metab. 2014, 39, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Romijn, J.A.; Coyle, E.F.; Sidossis, L.S.; Gastaldelli, A.; Horowitz, J.F.; Endert, E.; Wolfe, R.R. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am. J. Physiol. Metab. 1993, 265, E380–E391. [Google Scholar] [CrossRef] [PubMed]
- van Loon, L.J.C.; Greenhaff, P.L.; Constantin-Teodosiu, D.; Saris, W.H.M.; Wagenmakers, A.J.M. The effects of increasing exercise intensity on muscle fuel utilisation in humans. J. Physiol. 2001, 536, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Hermansen, L.; Hultman, E.; Saltin, B. Muscle glycogen during prolonged severe exercise. Acta Physiol. Scand. 1967, 71, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and Athletic Performance. J. Acad. Nutr. Diet. 2016, 116, 501–528. [Google Scholar] [CrossRef]
- Williams, M.B.; Raven, P.B.; Fogt, D.L.; Ivy, J.L. Effects of recovery beverages on glycogen restoration and endurance exercise performance. J. Strength Cond. Res. 2003, 17, 12–19. [Google Scholar]
- van Loon, L.J.C.; Kruijshoop, M.; Verhagen, H.; Saris, W.H.M.; Wagenmakers, A.J.M. Ingestion of Protein Hydrolysate and Amino Acid–Carbohydrate Mixtures Increases Postexercise Plasma Insulin Responses in Men. J. Nutr. 2000, 130, 2508–2513. [Google Scholar] [CrossRef] [PubMed]
- Zawadzki, K.M.; Yaspelkis, B.B.; Ivy, J.L. Carbohydrate-protein complex increases the rate of muscle glycogen storage after exercise. J. Appl. Physiol. 1992, 72, 1854–1859. [Google Scholar] [CrossRef]
- Berardi, J.M.; Price, T.B.; Noreen, E.E.; Lemon, P.W.R. Postexercise Muscle Glycogen Recovery Enhanced with a Carbohydrate-Protein Supplement. Med. Sci. Sports Exerc. 2006, 38, 1106–1113. [Google Scholar] [CrossRef]
- Fujita, S.; Rasmussen, B.B.; Cadenas, J.G.; Grady, J.J.; Volpi, E. Effect of insulin on human skeletal muscle protein synthesis is modulated by insulin-induced changes in muscle blood flow and amino acid availability. Am. J. Physiol. Metab. 2006, 291, E745–E754. [Google Scholar] [CrossRef] [PubMed]
- Biolo, G.; Flemin, R.Y.D.; Wolfe, R.R. Physiologic hyperinsulinemia stimulates protein synthesis and enhances transport of selected amino acids in human skeletal muscle. J. Clin. Investig. 1995, 95, 811–819. [Google Scholar] [CrossRef]
- Floyd, J.C.; Fajans, S.S.; Pek, S.; Thiffault, C.A.; Knopf, R.F.; Conn, J.W. Synergistic Effect of Certain Amino Acid Pairs upon Insulin Secretion in Man. Diabetes 1970, 19, 102–108. [Google Scholar] [CrossRef]
- Liu, Z.; Jeppesen, P.B.; Gregersen, S.; Chen, X.; Hermansen, K. Dose- and Glucose-Dependent Effects of Amino Acids on Insulin Secretion from Isolated Mouse Islets and Clonal INS-1E Beta-Cells. Rev. Diabet. Stud. 2008, 5, 232–244. [Google Scholar] [CrossRef]
- van Loon, L.J.C.; Saris, W.H.M.; Verhagen, H.; Wagenmakers, A.J.M. Plasma insulin responses after ingestion of different amino acid or protein mixtures with carbohydrate. Am. J. Clin. Nutr. 2000, 72, 96–105. [Google Scholar] [CrossRef]
- Howarth, K.R.; Moreau, N.A.; Phillips, S.M.; Gibala, M.J. Coingestion of protein with carbohydrate during recovery from endurance exercise stimulates skeletal muscle protein synthesis in humans. J. Appl. Physiol. 2009, 106, 1394–1402. [Google Scholar] [CrossRef]
- Howarth, K.R.; Phillips, S.M.; MacDonald, M.J.; Richards, D.; Moreau, N.A.; Gibala, M.J. Effect of glycogen availability on human skeletal muscle protein turnover during exercise and recovery. J. Appl. Physiol. 2010, 109, 431–438. [Google Scholar] [CrossRef]
- Lemon, P.W.R.; Mullin, J.P. Effect of initial muscle glycogen levels on protein catabolism during exercise. J. Appl. Physiol. 1980, 48, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Levenhagen, D.K.; Carr, C.; Carlson, M.G.; Maron, D.J.; Borel, M.J.; Flakoll, P.J. Postexercise protein intake enhances whole-body and leg protein accretion in humans. Med. Sci. Sports Exerc. 2002, 34, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, L.L.K.; Lambert, M.N.T.; Jeppesen, P.B. The Effect of Ingesting Carbohydrate and Proteins on Athletic Performance: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2020, 12, 1483. [Google Scholar] [CrossRef]
- Rustad, P.I.; Manuela, S.; Cumming, K.K.; Jeppesen, P.B.; Kolnes, K.J.; Sollie, O.; Franch, J.; Ivy, J.L.; Daniel, H.; Jensen, J. Intake of protein plus carbohydrate during the first two hours after exhaustive cycling improves performance the following day. PLoS ONE 2016, 11, e0153229. [Google Scholar] [CrossRef]
- Ferguson-Stegall, L.; McCleave, E.L.; Ding, Z.; Doerner, P.G.; Wang, B.; Liao, Y.-H.; Kammer, L.; Liu, Y.; Hwang, J.; Dessard, B.M.; et al. Postexercise carbohydrate–protein supplementation improves subsequent exercise performance and intracellular signaling for protein synthesis. J. Strength Cond. Res. 2011, 25, 1210–1224. [Google Scholar] [CrossRef]
- Sollie, O.; Jeppesen, P.B.; Tangen, D.S.; Jernerén, F.; Nellemann, B.; Valsdottir, D.; Madsen, K.; Turner, C.; Refsum, H.; Skålhegg, B.S.; et al. Protein intake in the early recovery period after exhaustive exercise improves performance the following day. J. Appl. Physiol. 2018, 125, 1731–1742. [Google Scholar] [CrossRef] [PubMed]
- Saunders, M.J.; Luden, N.D.; Herrick, J.E. Consumption of an oral carbohydrate-protein gel improves cycling endurance and prevents postexercise muscle damage. J. Strength Cond. Res. 2007, 21, 678–684. [Google Scholar] [PubMed]
- Dahl, M.A.; Areta, J.L.; Jeppesen, P.B.; Birk, J.B.; Johansen, E.I.; Ingemann-Hansen, T.; Hansen, M.; Skålhegg, B.S.; Ivy, J.L.; Wojtaszewski, J.F.P.; et al. Coingestion of protein and carbohydrate in the early recovery phase, compared with carbohydrate only, improves endurance performance despite similar glycogen degradation and AMPK phosphorylation. J. Appl. Physiol. 2020, 129, 297–310. [Google Scholar] [CrossRef]
- Needleman, I.; Ashley, P.; Meehan, L.; Petrie, A.; Weiler, R.; McNally, S.; Ayer, C.; Hanna, R.; Hunt, I.; Kell, S.; et al. Poor oral health including active caries in 187 UK professional male football players: Clinical dental examination performed by dentists. Br. J. Sports Med. 2016, 50, 41–44. [Google Scholar] [CrossRef]
- Selwitz, R.H.; Ismail, A.I.; Pitts, N.B. Dental caries. Lancet 2007, 369, 51–59. [Google Scholar] [CrossRef]
- Pachori, A.; Kambalimath, H.; Maran, S.; Niranjan, B.; Bhambhani, G.; Malhotra, G. Evaluation of Changes in Salivary pH after Intake of Different Eatables and Beverages in Children at Different Time Intervals. Int. J. Clin. Pediatr. Dent. 2018, 11, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Seethalakshmi, C.; Reddy, R.C.J.; Asifa, N.; Prabhu, S. Correlation of salivary pH, incidence of dental caries and periodontal status in diabetes mellitus patients: A cross-sectional study. J. Clin. Diagnostic Res. 2016, 10, ZC12–ZC14. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Sanchez, P.; Fredriksson, N.; Larsson, A.; Altskär, A.; Ström, A. High sugar content impacts microstructure, mechanics and release of calcium-alginate gels. Food Hydrocoll. 2018, 84, 26–33. [Google Scholar] [CrossRef]
- Sutehall, S.; Galloway, S.D.R.; Bosch, A.; Pitsiladis, Y. Addition of an Alginate Hydrogel to a Carbohydrate Beverage Enhances Gastric Emptying. Med. Sci. Sports Exerc. 2020, 52, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Sutehall, S.; Muniz-Pardos, B.; Bosch, A.; Pitsiladis, Y. The Effect of Sodium Alginate and Pectin Added to a Carbohydrate Beverage on Endurance Performance, Substrate Oxidation and Blood Glucose Concentration: A Systematic Review and Meta-analysis. Sports Med.–Open 2022, 8, 82. [Google Scholar] [CrossRef]
- Baur, D.A.; Toney, H.R.; Saunders, M.J.; Baur, K.G.; Luden, N.D.; Womack, C.J. Carbohydrate hydrogel beverage provides no additional cycling performance benefit versus carbohydrate alone. Eur. J. Appl. Physiol. 2019, 119, 2599–2608. [Google Scholar] [CrossRef]
- Saunders, M.J.; Kane, M.D.; Todd, M.K. Effects of a carbohydrate-protein beverage on cycling endurance and muscle damage. Med. Sci. Sports Exerc. 2004, 36, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Berardi, J.M.; Noreen, E.E.; Lemon, P.W.R. Recovery from a cycling time trial is enhanced with carbohydrate-protein supplementation vs. isoenergetic carbohydrate supplementation. J. Int. Soc. Sports Nutr. 2008, 5, 24. [Google Scholar] [CrossRef]
- Osterberg, K.L.; Zachwieja, J.J.; Smith, J.W. Carbohydrate and carbohydrate + protein for cycling time-trial performance. J. Sports Sci. 2008, 26, 227–233. [Google Scholar] [CrossRef]
- Romano-Ely, B.C.; Todd, M.K.; Saunders, M.J.; St. Laurent, T Effect of an isocaloric carbohydrate-protein-antioxidant drink on cycling performance. Med. Sci. Sports Exerc. 2006, 38, 1608–1616. [Google Scholar] [CrossRef] [PubMed]
- Sollie, O.; Clauss, M.; Jeppesen, P.B.; Tangen, D.S.; Johansen, E.I.; Skålhegg, B.S.; Ivy, J.L.; Jensen, J. Similar performance after intake of carbohydrate plus whey protein and carbohydrate only in the early phase after non-exhaustive cycling. Scand. J. Med. Sci. Sports 2023, 33, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- van Loon, L.J.C.; Saris, W.H.M.; Kruijshoop, M.; Wagenmakers, A.J.M. Maximizing postexercise muscle glycogen synthesis: Carbohydrate supplementation and the application of amino acid or protein hydrolysate mixtures. Am. J. Clin. Nutr. 2000, 72, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Coyle, E.F.; Coggan, A.R.; Hemmert, M.K.; Ivy, J.L. Muscle glycogen utilization during prolonged strenuous exercise when fed carbohydrate. J. Appl. Physiol. 1986, 61, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Coyle, E.F.; Hagberg, J.M.; Hurley, B.F.; Martin, W.H.; Ehsani, A.A.; Holloszy, J.O. Carbohydrate feeding during prolonged strenuous exercise can delay fatigue. J. Appl. Physiol. 1983, 55, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Jentjens, R.; Jeukendrup, A.E. Determinants of post-exercise glycogen synthesis during short-term recovery. Sports Med. 2003, 33, 117–144. [Google Scholar] [CrossRef] [PubMed]
- Calbet, J.A.L.; MacLean, D.A. Plasma Glucagon and Insulin Responses Depend on the Rate of Appearance of Amino Acids after Ingestion of Different Protein Solutions in Humans. J. Nutr. 2002, 132, 2174–2182. [Google Scholar] [CrossRef]
- Blomstrand, E.; Saltin, B. BCAA intake affects protein metabolism in muscle after but not during exercise in humans. Am. J. Physiol. Metab. 2001, 281, E365–E374. [Google Scholar] [CrossRef]
- Ian Needleman, I.; Ashley, P.; Fine, P.; Haddad, F.; Loosemore, M.; de Medici, A.; Donos, N.; Newton, T.; van Someren, K.; Moazzez, R.; et al. Oral health and elite sport performance. Br. J. Sports Med. 2015, 49, 3–6. [Google Scholar] [CrossRef]




| Variable | ALG | CON | Δ | p-Value |
|---|---|---|---|---|
| BCAAs (µM) | 62,892 ± 6240 | 48,693 ± 5788 | 14,199 ± 1764 | <0.0001 |
| Leucine (µM) | 43,194 ± 4541 | 25,210 ± 1738 | 17,984 ± 3025 | 0.001 |
| Isoleucine (µM) | 25,756 ± 2398 | 15,677 ± 1214 | 10,079 ± 1534 | 0.0006 |
| Valine (µM) | 68,077 ± 5173 | 55,829 ± 3557 | 12,248 ± 3112 | 0.008 |
| Phenylalanine (µM) | 21,341 ± 974 | 16,032 ± 687 | 5309 ± 932 | 0.001 |
| Tryptophan (µM) | 8348 ± 507 | 7831 ± 376 | 517.4 ± 217 | 0.054 |
| Methionine (µM) | 6378 ± 404 | 5230 ± 399 | 1148 ± 223 | 0.002 |
| Threonine (µM) | 38,328 ± 2592 | 32,169 ± 3318 | 6158 ± 1062 | 0.0012 |
| Histidine (µM) | 27,098 ± 1105 | 24,887 ± 881 | 2211 ± 725 | 0.023 |
| Lysine (µM) | 56,840 ± 4682 | 43,940 ± 3127 | 12,899 ± 2494 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nielsen, L.L.K.; Lambert, M.N.T.; Haubek, D.; Bastani, N.E.; Skålhegg, B.S.; Overgaard, K.; Jensen, J.; Jeppesen, P.B. The Effect of Alginate Encapsulated Plant-Based Carbohydrate and Protein Supplementation on Recovery and Subsequent Performance in Athletes. Nutrients 2024, 16, 413. https://doi.org/10.3390/nu16030413
Nielsen LLK, Lambert MNT, Haubek D, Bastani NE, Skålhegg BS, Overgaard K, Jensen J, Jeppesen PB. The Effect of Alginate Encapsulated Plant-Based Carbohydrate and Protein Supplementation on Recovery and Subsequent Performance in Athletes. Nutrients. 2024; 16(3):413. https://doi.org/10.3390/nu16030413
Chicago/Turabian StyleNielsen, Lotte L. K., Max Norman Tandrup Lambert, Dorte Haubek, Nasser E. Bastani, Bjørn S. Skålhegg, Kristian Overgaard, Jørgen Jensen, and Per Bendix Jeppesen. 2024. "The Effect of Alginate Encapsulated Plant-Based Carbohydrate and Protein Supplementation on Recovery and Subsequent Performance in Athletes" Nutrients 16, no. 3: 413. https://doi.org/10.3390/nu16030413





