Differential Alterations of Expression of the Serotoninergic System Genes and Mood-Related Behavior by Consumption of Aspartame or Potassium Acesulfame in Rats
Abstract
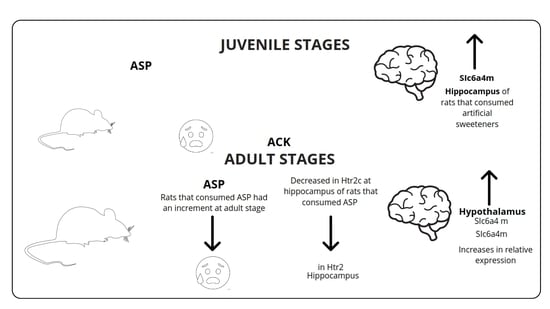
:1. Introduction
2. Material and Methods
2.1. Ethic Declaration
2.2. Experimental Design
2.3. Weight, Food, and Liquid Measurements
2.4. Behavior Tests
2.4.1. Defensive Burying Test
2.4.2. Forced Swimming Test
2.5. Relative Gene Expression Quantification
2.6. Statistical Analysis
3. Results
3.1. Weight, Food, and Liquid Consumption Measurement
3.2. Mood-Related Behavior
3.3. mRNA Expression of Serotonin System Genes (Slc6a4, Htr1a, and Htr2c)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haslam, D.W.; James, W.P. Obesity. Lancet 2005, 366, 1197–1209. [Google Scholar] [CrossRef]
- Johnson, R.J.; Segal, M.S.; Sautin, Y.; Nakagawa, T.; Feig, D.I.; Kang, D.H.; Gersch, M.S.; Benner, S.; Sánchez-Lozada, L.G. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am. J. Clin. Nutr. 2007, 86, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Nakagawa, T.; Sanchez-Lozada, L.G.; Shafiu, M.; Sundaram, S.; Le, M.; Ishimoto, T.; Sautin, Y.Y.; Lanaspa, M.A. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes 2013, 62, 3307–3315. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Amarnath, S.; Thulasimani, M.; Ramaswamy, S. Artificial sweeteners as a sugar substitute: Are they really safe? Indian J. Pharmacol. 2016, 48, 237–240. [Google Scholar] [CrossRef]
- Shao, B.R.; Zhan, C.Q.; Ha, S.H. Evaluation of the phototoxicity of five antimalarial agents and praziquantel in mice. Zhongguo Yao Li Xue Bao = Acta Pharmacol. Sin. 1986, 7, 273–275. [Google Scholar]
- Miller, P.E.; Perez, V. Low-calorie sweeteners and body weight and composition: A meta-analysis of randomized controlled trials and prospective cohort studies. Am. J. Clin. Nutr. 2014, 100, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.J.; de Banate, M.A.; Rother, K.I. Artificial sweeteners: A systematic review of metabolic effects in youth. Int. J. Pediatr. Obes. 2010, 5, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Fowler, S.P.; Williams, K.; Resendez, R.G.; Hunt, K.J.; Hazuda, H.P.; Stern, M.P. Fueling the obesity epidemic? Artificially sweetened beverage use and long-term weight gain. Obesity 2008, 16, 1894–1900. [Google Scholar] [CrossRef] [PubMed]
- Forshee, R.A.; Storey, M.L. Total beverage consumption and beverage choices among children and adolescents. Int. J. Food Sci. Nutr. 2003, 54, 297–307. [Google Scholar] [CrossRef]
- Swithers, S.E.; Davidson, T.L. A role for sweet taste: Calorie predictive relations in energy regulation by rats. Behav. Neurosci. 2008, 122, 161–173. [Google Scholar] [CrossRef]
- Pierce, W.D.; Heth, C.D.; Owczarczyk, J.C.; Russell, J.C.; Proctor, S.D. Overeating by young obesity-prone and lean rats caused by tastes associated with low energy foods. Obesity 2007, 15, 1969–1979. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q. Gain weight by “going diet?” Artificial sweeteners and the neurobiology of sugar cravings: Neuroscience 2010. Yale J. Biol. Med. 2010, 83, 101–108. [Google Scholar] [PubMed]
- Novick, J.S.; Stewart, J.W.; Wisniewski, S.R.; Cook, I.A.; Manev, R.; Nierenberg, A.A.; Rosenbaum, J.F.; Shores-Wilson, K.; Balasubramani, G.K.; Biggs, M.M.; et al. Clinical and demographic features of atypical depression in outpatients with major depressive disorder: Preliminary findings from STAR*D. J. Clin. Psychiatry 2005, 66, 1002–1011. [Google Scholar] [CrossRef] [PubMed]
- Simon, G.E.; Von Korff, M.; Saunders, K.; Miglioretti, D.L.; Crane, P.K.; van Belle, G.; Kessler, R.C. Association between obesity and psychiatric disorders in the US adult population. Arch. Gen. Psychiatry 2006, 63, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Luppino, F.S.; de Wit, L.M.; Bouvy, P.F.; Stijnen, T.; Cuijpers, P.; Penninx, B.W.; Zitman, F.G. Overweight, obesity, and depression: A systematic review and meta-analysis of longitudinal studies. Arch. Gen. Psychiatry 2010, 67, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.; Chuang, J.C.; Na, E.S.; Kuperman, A.; Gillman, A.G.; Mukherjee, S.; Zigman, J.M.; McClung, C.A.; Lutter, M. Differential effects of chronic social stress and fluoxetine on meal patterns in mice. Appetite 2013, 64, 81–88. [Google Scholar] [CrossRef]
- Akubuiro, A.; Bridget Zimmerman, M.; Boles Ponto, L.L.; Walsh, S.A.; Sunderland, J.; McCormick, L.; Singh, M. Hyperactive hypothalamus, motivated and non-distractible chronic overeating in ADAR2 transgenic mice. Genes Brain Behav. 2013, 12, 311–322. [Google Scholar] [CrossRef]
- Chuang, J.C.; Perello, M.; Sakata, I.; Osborne-Lawrence, S.; Savitt, J.M.; Lutter, M.; Zigman, J.M. Ghrelin mediates stress-induced food-reward behavior in mice. J. Clin. Investig. 2011, 121, 2684–2692. [Google Scholar] [CrossRef]
- Glendinning, J.I.; Elson, A.E.; Kalik, S.; Sosa, Y.; Patterson, C.M.; Myers, M.G., Jr.; Munger, S.D. Taste responsiveness to sweeteners is resistant to elevations in plasma leptin. Chem. Senses 2015, 40, 223–231. [Google Scholar] [CrossRef]
- Lindseth, G.N.; Coolahan, S.E.; Petros, T.V.; Lindseth, P.D. Neurobehavioral effects of aspartame consumption. Res. Nurs. Health 2014, 37, 185–193. [Google Scholar] [CrossRef]
- Swithers, S.E. Artificial sweeteners produce the counterintuitive effect of inducing metabolic derangements. Trends Endocrinol. Metab. TEM 2013, 24, 431–441. [Google Scholar] [CrossRef]
- Coulombe, R.A., Jr.; Sharma, R.P. Neurobiochemical alterations induced by the artificial sweetener aspartame (NutraSweet). Toxicol. Appl. Pharmacol. 1986, 83, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Wurtman, J.; Wurtman, R. The Trajectory from Mood to Obesity. Curr. Obes. Rep. 2018, 7, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Wurtman, J.J. Depression and weight gain: The serotonin connection. J. Affect. Disord. 1993, 29, 183–192. [Google Scholar] [CrossRef]
- Solis-Medina, A.; Martínez-Magaña, J.J.; Quintanar-Jurado, V.; Gallegos-Silva, I.; Juárez-Rojop, I.E.; Tovilla-Zárate, C.A.; Díaz-Zagoya, J.C.; Hernández-Díaz, Y.; González-Castro, T.B.; López-Narváez, M.L.; et al. Astrogliosis and decreased neural viability as consequences of early consumption of aspartame and acesulfame potassium in male Wistar rats. Metab. Brain Dis. 2018, 33, 2031–2038. [Google Scholar] [CrossRef] [PubMed]
- Genis-Mendoza, A.D.; Beltrán-Villalobos, I.; Nicolini-Sánchez, H. Behavioral assessment of the “schizophrenia-like” phenotype in an animal model of neonatal lesion in the ventral hippocampus (NLVH) of young and adult rats. Gac. Medica Mex. 2014, 150, 420–431. [Google Scholar]
- Tang, M.; He, T.; Meng, Q.Y.; Broussard, J.I.; Yao, L.; Diao, Y.; Sang, X.B.; Liu, Q.P.; Liao, Y.J.; Li, Y.; et al. Immobility responses between mouse strains correlate with distinct hippocampal serotonin transporter protein expression and function. Int. J. Neuropsychopharmacol. 2014, 17, 1737–1750. [Google Scholar] [CrossRef]
- Mostalac-Preciado, C.R.; de Gortari, P.; López-Rubalcava, C. Antidepressant-like effects of mineralocorticoid but not glucocorticoid antagonists in the lateral septum: Interactions with the serotonergic system. Behav. Brain Res. 2011, 223, 88–98. [Google Scholar] [CrossRef]
- Genis-Mendoza, A.D.; Gallegos-Silva, R.I.; López-Casamichana, M.; López-Rubalcava, C.; Nicolini, H. Gene expression profiles of nucleus accumbens, prefrontal cortex and hippocampus in an animal model of schizophrenia: Proposed candidate genes. Actas Esp. Psiquiatr. 2013, 41, 154–163. [Google Scholar]
- Uebanso, T.; Ohnishi, A.; Kitayama, R.; Yoshimoto, A.; Nakahashi, M.; Shimohata, T.; Mawatari, K.; Takahashi, A. Effects of Low-Dose Non-Caloric Sweetener Consumption on Gut Microbiota in Mice. Nutrients 2017, 9, 560. [Google Scholar] [CrossRef]
- Choi, J.Y.; Park, M.N.; Kim, C.S.; Lee, Y.K.; Choi, E.Y.; Chun, W.Y.; Shin, D.M. Long-term consumption of sugar-sweetened beverage during the growth period promotes social aggression in adult mice with proinflammatory responses in the brain. Sci. Rep. 2017, 7, 45693. [Google Scholar] [CrossRef]
- Palmnäs, M.S.; Cowan, T.E.; Bomhof, M.R.; Su, J.; Reimer, R.A.; Vogel, H.J.; Hittel, D.S.; Shearer, J. Low-dose aspartame consumption differentially affects gut microbiota-host metabolic interactions in the diet-induced obese rat. PLoS ONE 2014, 9, e109841. [Google Scholar] [CrossRef]
- Boakes, R.A.; Kendig, M.D.; Martire, S.I.; Rooney, K.B. Sweetening yoghurt with glucose, but not with saccharin, promotes weight gain and increased fat pad mass in rats. Appetite 2016, 105, 114–128. [Google Scholar] [CrossRef]
- Swithers, S.E.; Baker, C.R.; Davidson, T.L. General and persistent effects of high-intensity sweeteners on body weight gain and caloric compensation in rats. Behav. Neurosci. 2009, 123, 772–780. [Google Scholar] [CrossRef]
- Davidson, T.L.; Martin, A.A.; Clark, K.; Swithers, S.E. Intake of high-intensity sweeteners alters the ability of sweet taste to signal caloric consequences: Implications for the learned control of energy and body weight regulation. Q. J. Exp. Psychol. 2011, 64, 1430–1441. [Google Scholar] [CrossRef] [PubMed]
- Davidson, T.L.; Hargrave, S.L.; Swithers, S.E.; Sample, C.H.; Fu, X.; Kinzig, K.P.; Zheng, W. Inter-relationships among diet, obesity and hippocampal-dependent cognitive function. Neuroscience 2013, 253, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Rogers, P.J.; Hogenkamp, P.S.; de Graaf, C.; Higgs, S.; Lluch, A.; Ness, A.R.; Penfold, C.; Perry, R.; Putz, P.; Yeomans, M.R.; et al. Does low-energy sweetener consumption affect energy intake and body weight? A systematic review, including meta-analyses, of the evidence from human and animal studies. Int. J. Obes. 2016, 40, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Raychaudhuri, U.; Chakraborty, R. Artificial sweeteners—A review. J. Food Sci. Technol. 2014, 51, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Wardenaar, F.C.; Schott, K.D.; Mohr, A.E. An Exploratory Study Investigating the Prevalence of Gastrointestinal Symptoms in Collegiate Division I American Football Athletes. Int. J. Environ. Res. Public Health 2023, 20, 6453. [Google Scholar] [CrossRef]
- Masoro, E.J. Overview of caloric restriction and ageing. Mech. Ageing Dev. 2005, 126, 913–922. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.; Zhao, Y.; Zhang, X.; Li, B.; Cui, R. The Effects of Calorie Restriction in Depression and Potential Mechanisms. Curr. Neuropharmacol. 2015, 13, 536–542. [Google Scholar] [CrossRef]
- Rycerz, K.; Jaworska-Adamu, J.E. Effects of aspartame metabolites on astrocytes and neurons. Folia Neuropathol. 2013, 51, 10–17. [Google Scholar] [CrossRef]
- Position of the American Dietetic Association: Use of nutritive and nonnutritive sweeteners. J. Am. Diet. Assoc. 2004, 104, 255–275. [CrossRef]
- Koeppe, R.A.; Shulkin, B.L.; Rosenspire, K.C.; Shaw, L.A.; Betz, A.L.; Mangner, T.; Price, J.C.; Agranoff, B.W. Effect of aspartame-derived phenylalanine on neutral amino acid uptake in human brain: A positron emission tomography study. J. Neurochem. 1991, 56, 1526–1535. [Google Scholar] [CrossRef]
- Burgert, S.L.; Andersen, D.W.; Stegink, L.D.; Takeuchi, H.; Schedl, H.P. Metabolism of aspartame and its L-phenylalanine methyl ester decomposition product by the porcine gut. Metab. Clin. Exp. 1991, 40, 612–618. [Google Scholar] [CrossRef]
- Møller, S.E. Effect of aspartame and protein, administered in phenylalanine-equivalent doses, on plasma neutral amino acids, aspartate, insulin and glucose in man. Pharmacol. Toxicol. 1991, 68, 408–412. [Google Scholar] [CrossRef]
- Pietz, J.; Kreis, R.; Rupp, A.; Mayatepek, E.; Rating, D.; Boesch, C.; Bremer, H.J. Large neutral amino acids block phenylalanine transport into brain tissue in patients with phenylketonuria. J. Clin. Investig. 1999, 103, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Kanai, Y.; Segawa, H.; Miyamoto, K.; Uchino, H.; Takeda, E.; Endou, H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98). J. Biol. Chem. 1998, 273, 23629–23632. [Google Scholar] [CrossRef] [PubMed]
- Chillarón, J.; Roca, R.; Valencia, A.; Zorzano, A.; Palacín, M. Heteromeric amino acid transporters: Biochemistry, genetics, and physiology. Am. J. Physiology. Ren. Physiol. 2001, 281, F995–F1018. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Gray, J.A.; Roth, B.L. The expanded biology of serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.P.; Coulombe, R.A., Jr. Effects of repeated doses of aspartame on serotonin and its metabolite in various regions of the mouse brain. Food Chem. Toxicol. 1987, 25, 565–568. [Google Scholar] [CrossRef]
- Lesch, K.P.; Araragi, N.; Waider, J.; van den Hove, D.; Gutknecht, L. Targeting brain serotonin synthesis: Insights into neurodevelopmental disorders with long-term outcomes related to negative emotionality, aggression and antisocial behaviour. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 2426–2443. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.T. Bidirectional movement of horseradish peroxidase and the demonstration of reciprocal thalamocortical connections. Brain Res. 1977, 130, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Collison, K.S.; Makhoul, N.J.; Zaidi, M.Z.; Al-Rabiah, R.; Inglis, A.; Andres, B.L.; Ubungen, R.; Shoukri, M.; Al-Mohanna, F.A. Interactive effects of neonatal exposure to monosodium glutamate and aspartame on glucose homeostasis. Nutr. Metab. 2012, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Salam, O.M.; Salem, N.A.; El-Shamarka, M.E.; Hussein, J.S.; Ahmed, N.A.; El-Nagar, M.E. Studies on the effects of aspartame on memory and oxidative stress in brain of mice. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 2092–2101. [Google Scholar] [PubMed]
- Song, N.N.; Jia, Y.F.; Zhang, L.; Zhang, Q.; Huang, Y.; Liu, X.Z.; Hu, L.; Lan, W.; Chen, L.; Lesch, K.P.; et al. Reducing central serotonin in adulthood promotes hippocampal neurogenesis. Sci. Rep. 2016, 6, 20338. [Google Scholar] [CrossRef] [PubMed]
- Voigt, J.P.; Fink, H. Serotonin controlling feeding and satiety. Behav. Brain Res. 2015, 277, 14–31. [Google Scholar] [CrossRef]
- Onaolapo, A.Y.; Onaolapo, O.J.; Nwoha, P.U. Aspartame and the hippocampus: Revealing a bi-directional, dose/time-dependent behavioural and morphological shift in mice. Neurobiol. Learn. Mem. 2017, 139, 76–88. [Google Scholar] [CrossRef]
- Timper, K.; Brüning, J.C. Hypothalamic circuits regulating appetite and energy homeostasis: Pathways to obesity. Dis. Models Mech. 2017, 10, 679–689. [Google Scholar] [CrossRef]
- Schellekens, H.; Clarke, G.; Jeffery, I.B.; Dinan, T.G.; Cryan, J.F. Dynamic 5-HT2C receptor editing in a mouse model of obesity. PLoS ONE 2012, 7, e32266. [Google Scholar] [CrossRef]
- Dourish, C.T.; Hutson, P.H.; Curzon, G. Low doses of the putative serotonin agonist 8-hydroxy-2-(di-n-propylamino) tetralin (8-OH-DPAT) elicit feeding in the rat. Psychopharmacology 1985, 86, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Moreau, J.L.; Griebel, G.; Jenck, F.; Martin, J.R.; Widmer, U.; Haefely, W.E. Behavioral profile of the 5HT1A receptor antagonist (S)-UH-301 in rodents and monkeys. Brain Res. Bull. 1992, 29, 901–904. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Magaña, J.J.; Genis-Mendoza, A.D.; Gallegos-Silva, I.; López-Narváez, M.L.; Juárez-Rojop, I.E.; Diaz-Zagoya, J.C.; Tovilla-Zárate, C.A.; González-Castro, T.B.; Nicolini, H.; Solis-Medina, A. Differential Alterations of Expression of the Serotoninergic System Genes and Mood-Related Behavior by Consumption of Aspartame or Potassium Acesulfame in Rats. Nutrients 2024, 16, 490. https://doi.org/10.3390/nu16040490
Martínez-Magaña JJ, Genis-Mendoza AD, Gallegos-Silva I, López-Narváez ML, Juárez-Rojop IE, Diaz-Zagoya JC, Tovilla-Zárate CA, González-Castro TB, Nicolini H, Solis-Medina A. Differential Alterations of Expression of the Serotoninergic System Genes and Mood-Related Behavior by Consumption of Aspartame or Potassium Acesulfame in Rats. Nutrients. 2024; 16(4):490. https://doi.org/10.3390/nu16040490
Chicago/Turabian StyleMartínez-Magaña, José Jaime, Alma Delia Genis-Mendoza, Ileana Gallegos-Silva, María Lilia López-Narváez, Isela Esther Juárez-Rojop, Juan C. Diaz-Zagoya, Carlos Alfonso Tovilla-Zárate, Thelma Beatriz González-Castro, Humberto Nicolini, and Anayelly Solis-Medina. 2024. "Differential Alterations of Expression of the Serotoninergic System Genes and Mood-Related Behavior by Consumption of Aspartame or Potassium Acesulfame in Rats" Nutrients 16, no. 4: 490. https://doi.org/10.3390/nu16040490