Effect of Lifelong Exposure to Dietary Plant and Marine Sources of n-3 Polyunsaturated Fatty Acids on Morphologic and Gene Expression Biomarkers of Intestinal Health in Early Life
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice, Housing, and Diets
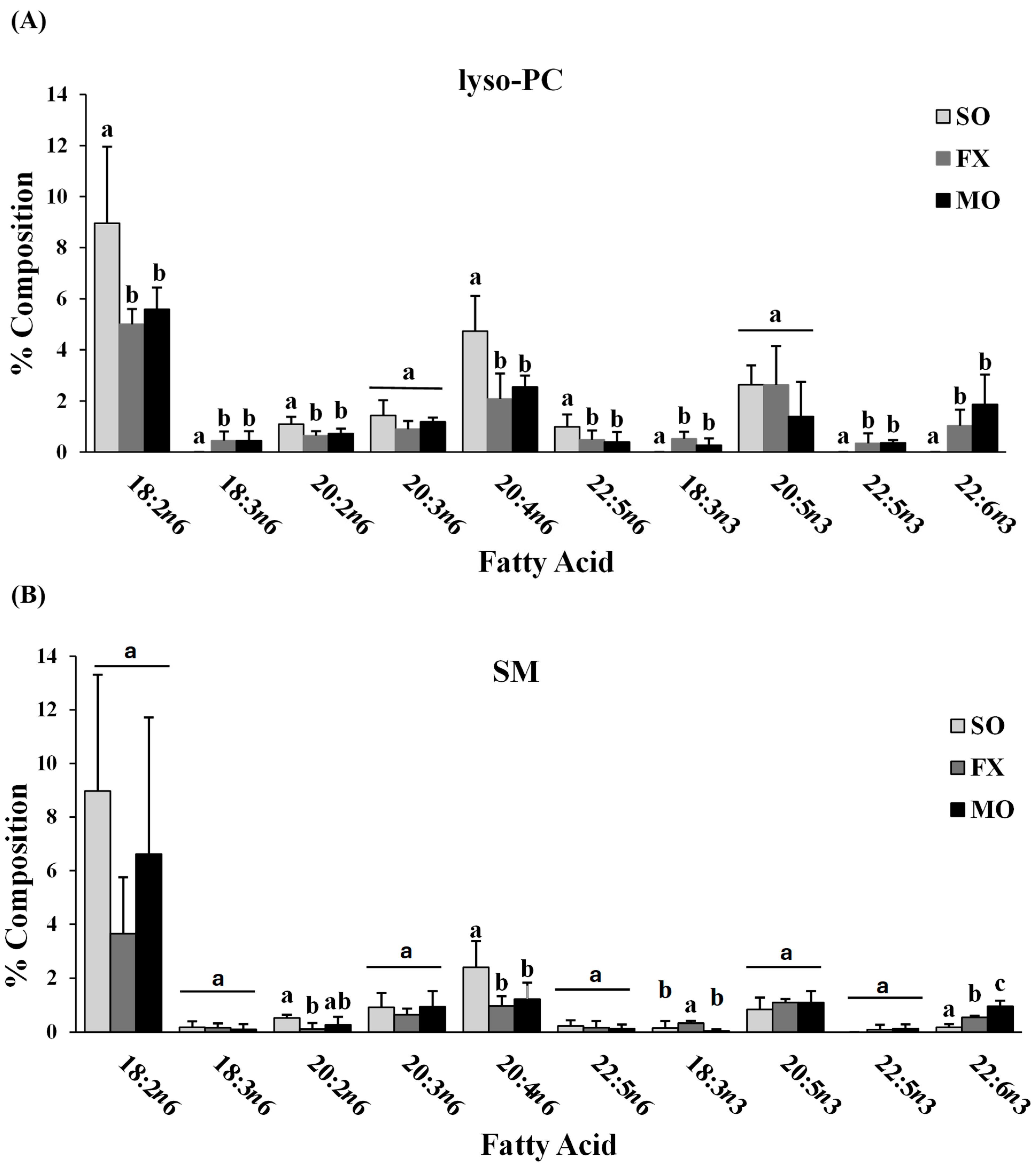
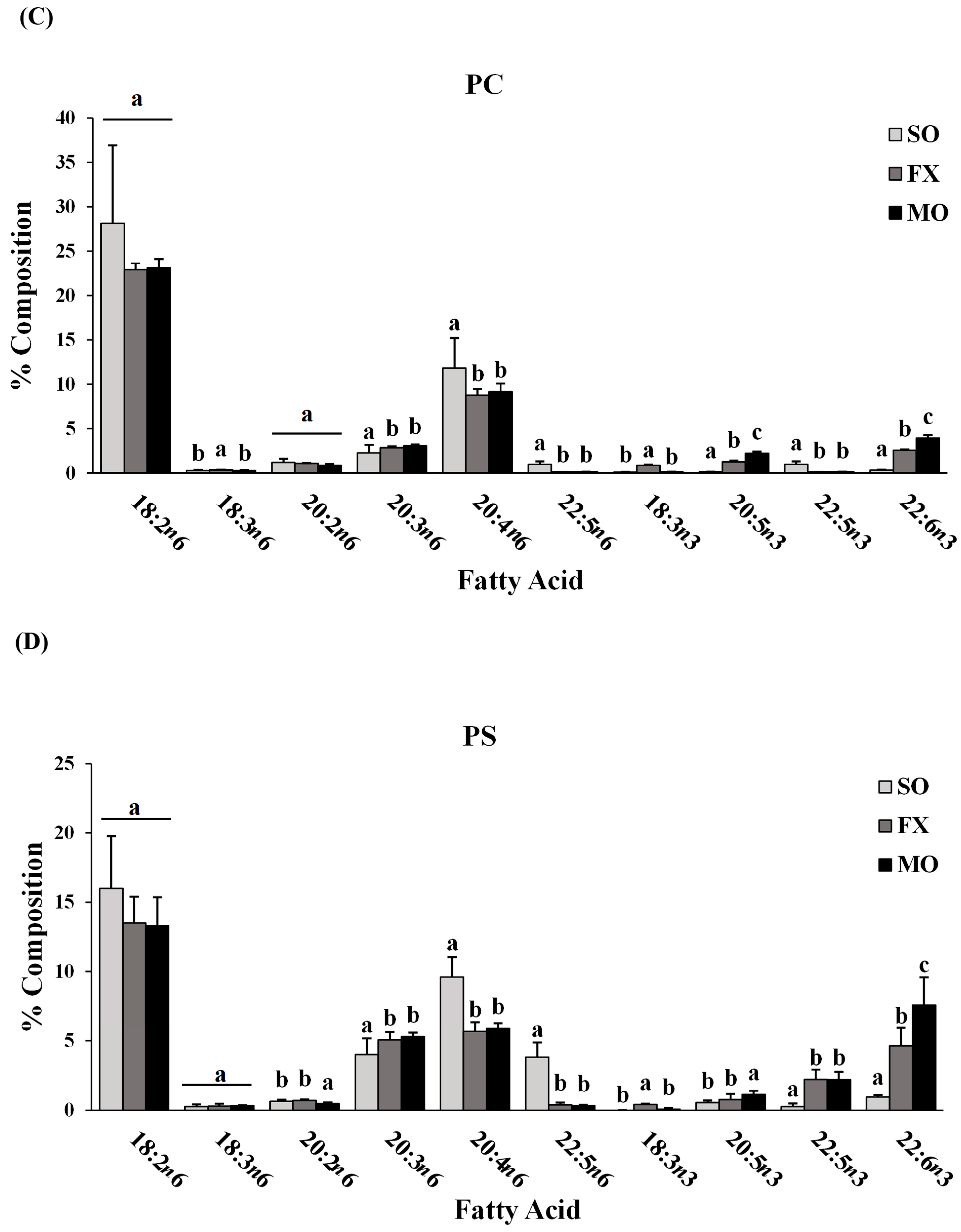
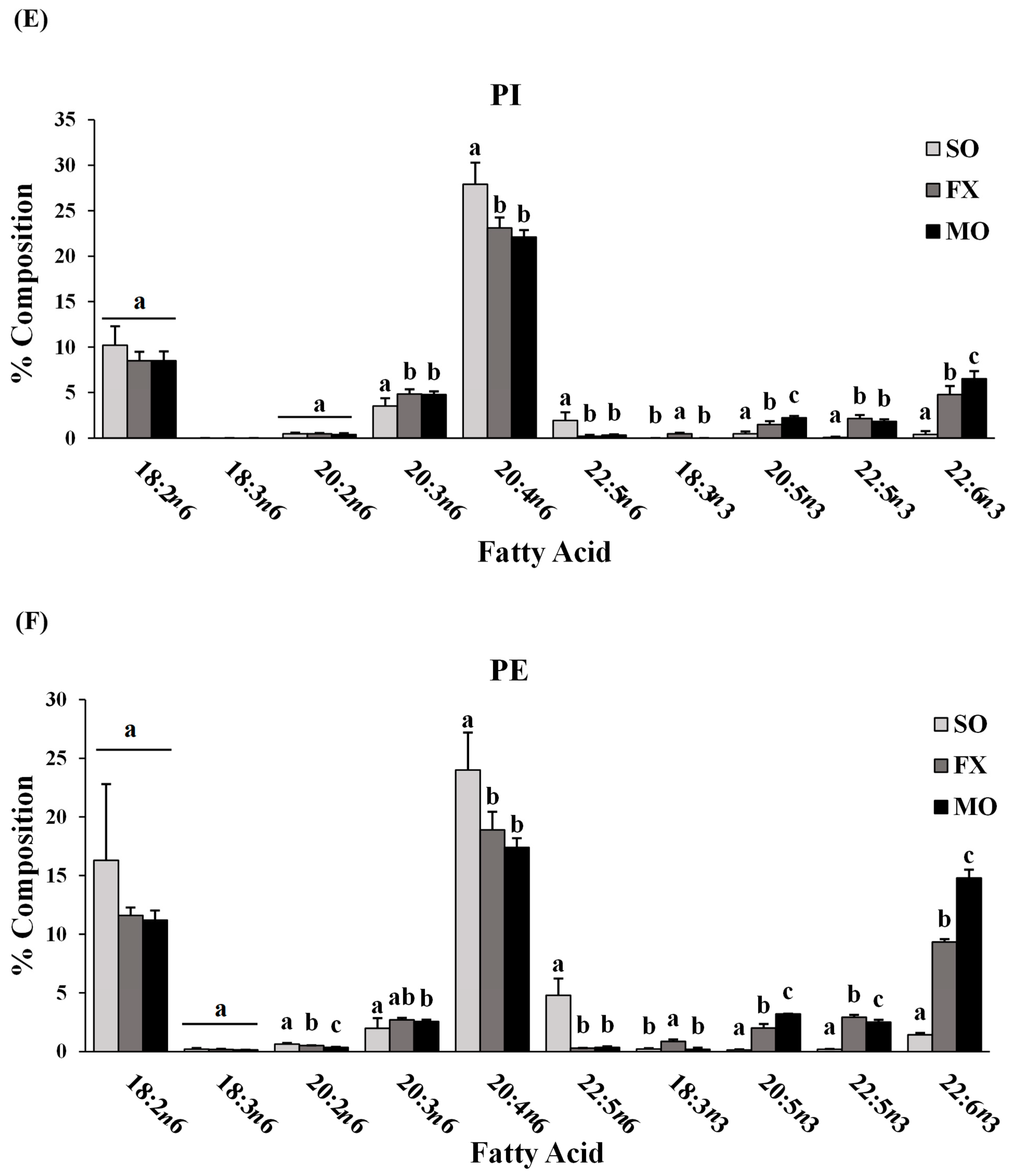
2.2. Phospholipid Fatty Acid Analysis
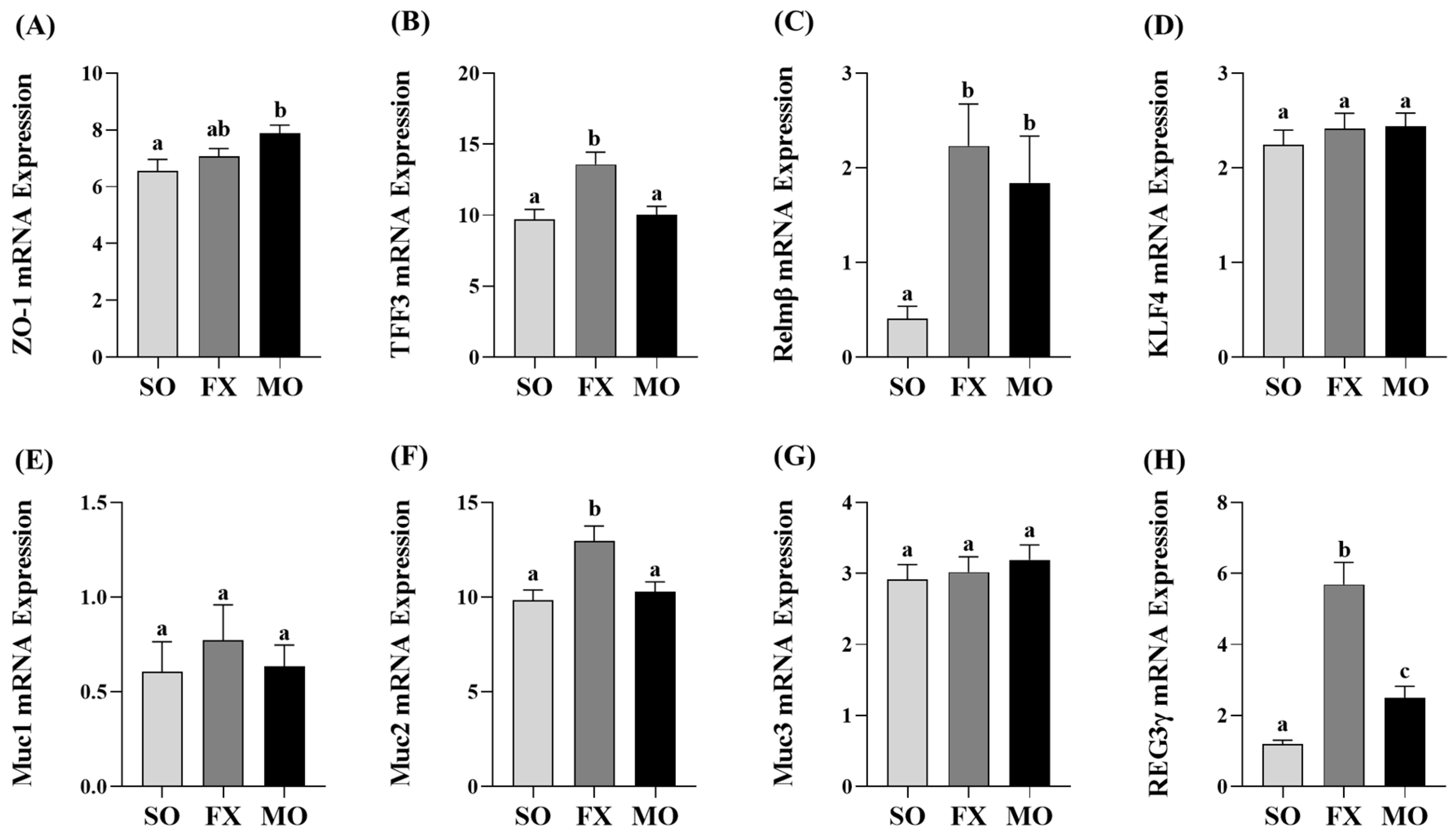
2.3. RNA Isolation and qRT-PCR
2.4. Ileum and Colon Morphology
2.5. Statistical Analysis
3. Results
3.1. Final Body Weight
3.2. Ileum Fatty Acid Analysis
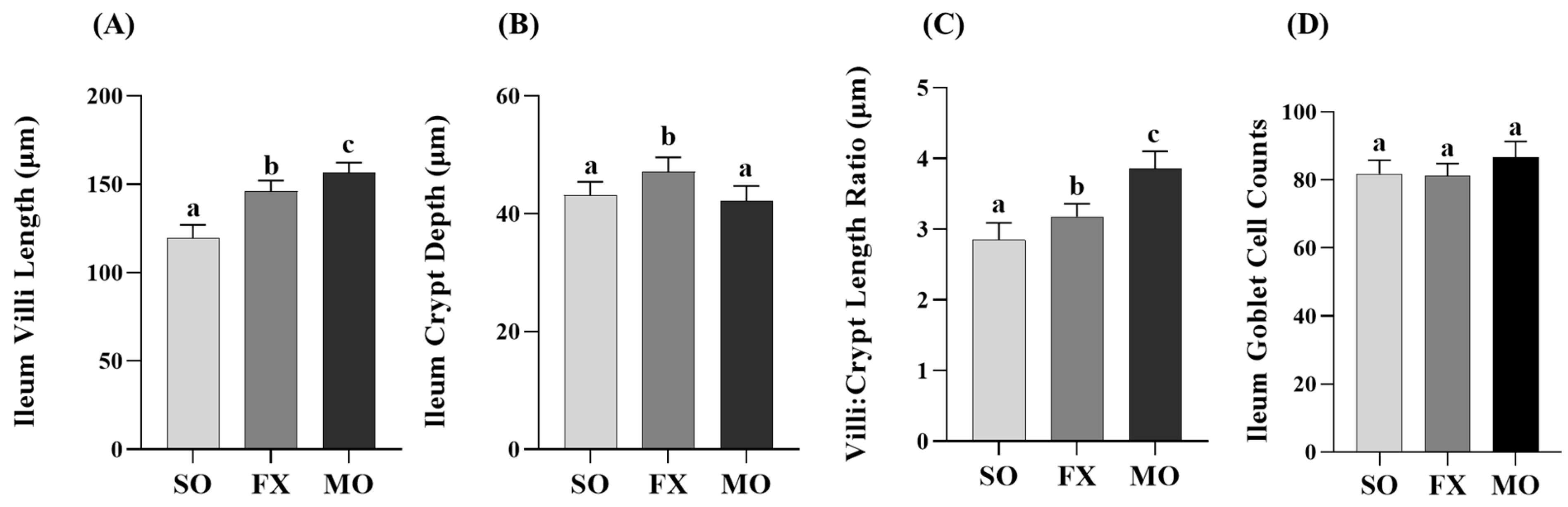
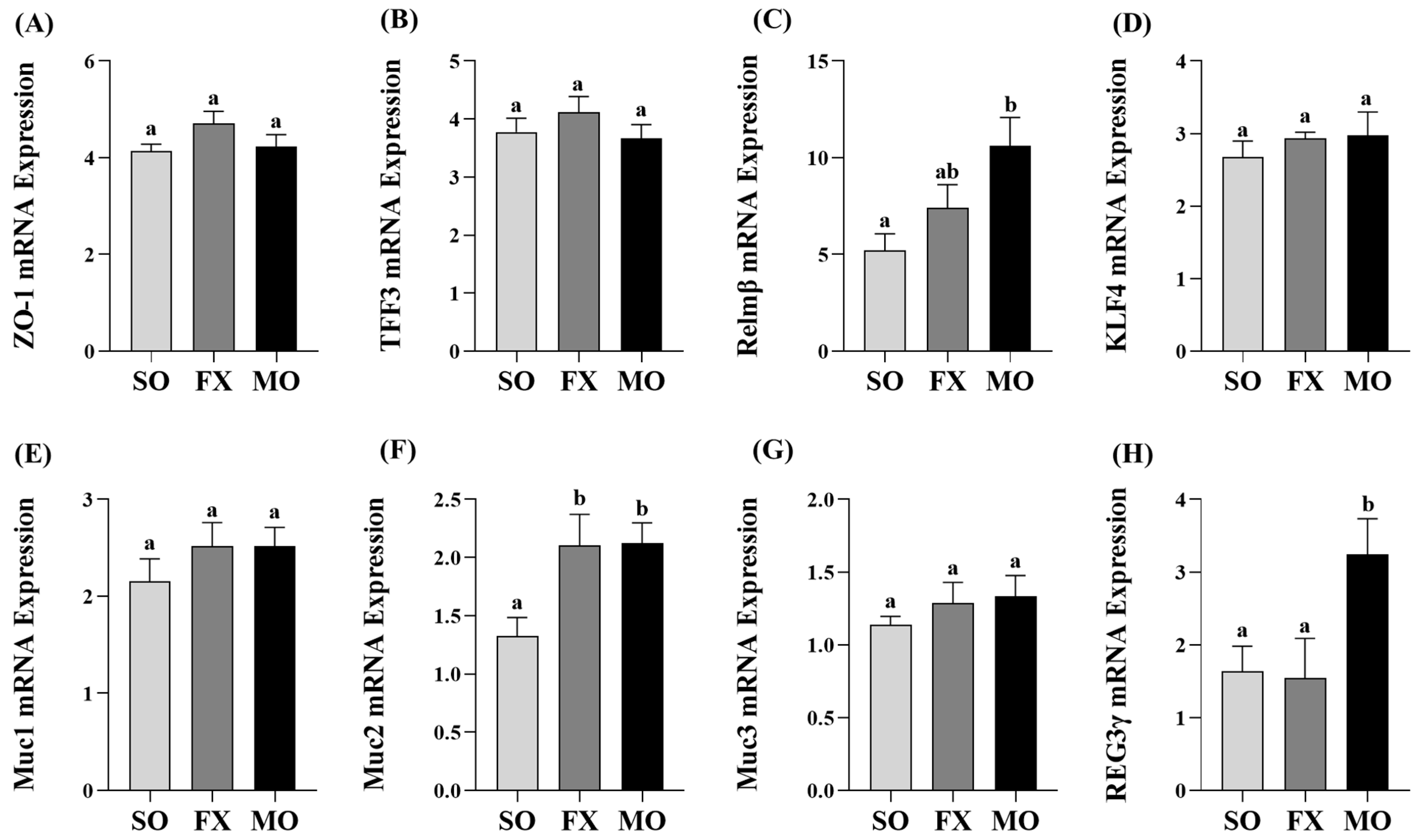
3.3. Effect of n-3 PUFA on Biomarkers of Intestinal Health in the Ileum
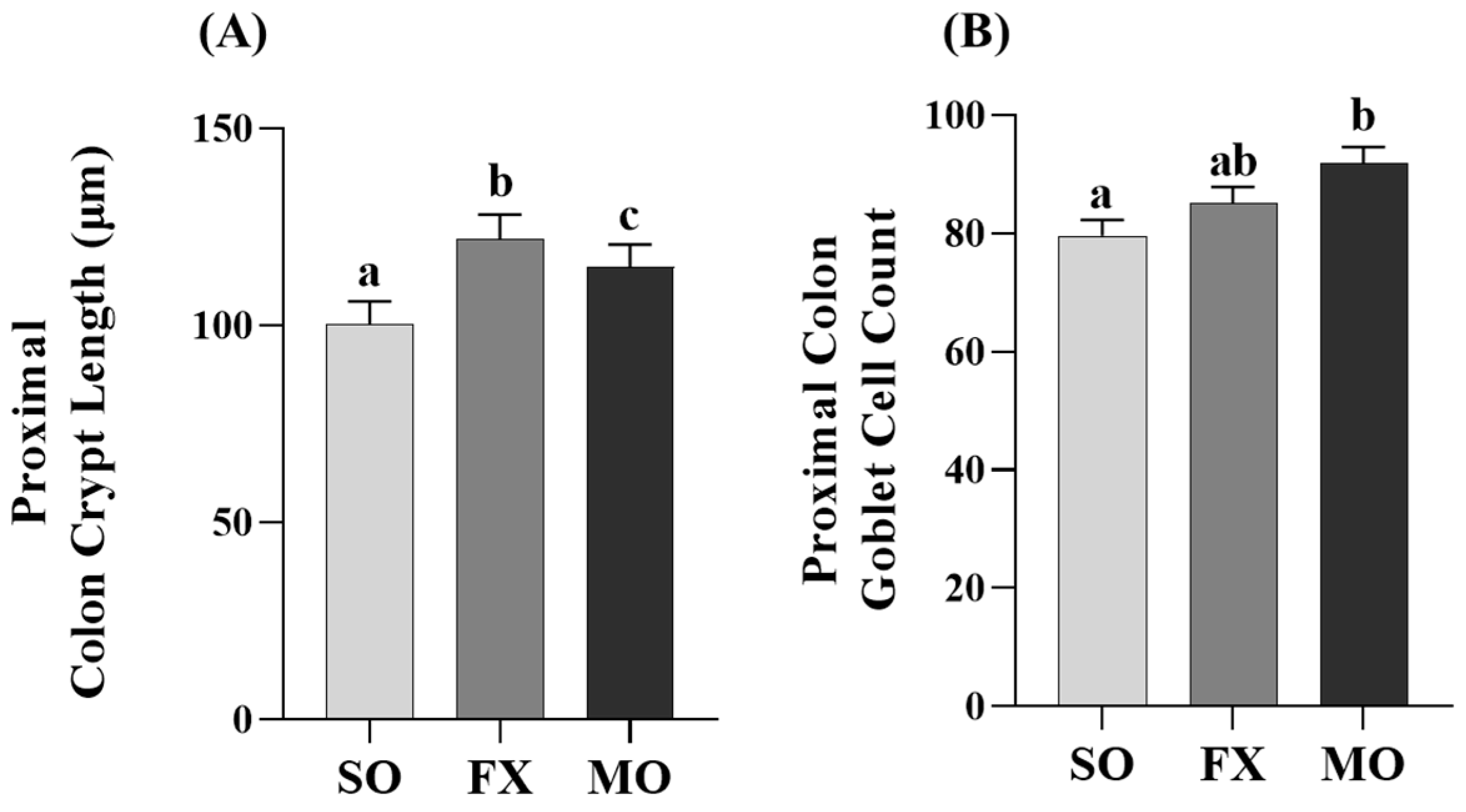
3.4. Effect of n-3 PUFA on Biomarkers of Intestinal Health in the Proximal Colon
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- König, J.; Wells, J.; Cani, P.D.; García-Ródenas, C.L.; MacDonald, T.; Mercenier, A.; Whyte, J.; Troost, F.; Brummer, R.-J. Human Intestinal Barrier Function in Health and Disease. Clin. Transl. Gastroenterol. 2016, 7, e196. [Google Scholar] [CrossRef]
- Martel, J.; Chang, S.; Ko, Y.; Hwang, T.; Young, J.D.; Ojcius, D.M. Gut barrier disruption and chronic disease. Trends Endocrinol. Metab. 2022, 33, 247–265. [Google Scholar] [CrossRef]
- Usuda, H.; Okamoto, T.; Wada, K. Leaky Gut: Effect of Dietary Fiber and Fats on Microbiome and Intestinal Barrier. Int. J. Mol. Sci. 2021, 22, 7613. [Google Scholar] [CrossRef] [PubMed]
- Aleman, R.S.; Moncada, M.; Aryana, K.J. Leaky Gut and the Ingredients That Help Treat It: A Review. Molecules 2023, 28, 619. [Google Scholar] [CrossRef] [PubMed]
- Quigley, E.M. Leaky gut—Concept or clinical entity? Curr. Opin. Gastroenterol. 2016, 32, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Knoop, K.A.; Newberry, R.D. Goblet cells: Multifaceted players in immunity at mucosal surfaces. Mucosal Immunol. 2018, 11, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.M.; Brummer, R.J.; Derrien, M.; MacDonald, T.T.; Troost, F.; Cani, P.D.; Theodorou, V.; Dekker, J.; Méheust, A.; de Vos, W.M.; et al. Homeostasis of the gut barrier and potential biomarkers. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G171–G193. [Google Scholar] [CrossRef] [PubMed]
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Fukui, H. Increased Intestinal Permeability and Decreased Barrier Function: Does It Really Influence the Risk of Inflammation? Inflamm. Intest. Dis. 2016, 1, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.; DeCoffe, D.; Molcan, E.; Gibson, D.L. Diet-induced dysbiosis of the intestinal microbiota and the effects on immunity and disease. Nutrients 2012, 4, 1095–1119. [Google Scholar] [CrossRef]
- Monk, J.M.; Liddle, D.M.; Hutchinson, A.L.; Wu, W.; Lepp, D.; Ma, D.W.L.; Robinson, L.E.; Power, K.A. Fish oil supplementation to a high-fat diet improves both intestinal health and the systemic obese phenotype. J. Nutr. Biochem. 2019, 72, 108216. [Google Scholar] [CrossRef]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070–11075. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef]
- Murphy, E.; Cotter, P.; Healy, S.; Marques, T.M.; O’sullivan, O.; Fouhy, F.; Clarke, S.; O’toole, P.; Quigley, E.M.; Stanton, C. Composition and energy harvesting capacity of the gut microbiota: Relationship to diet, obesity and time in mouse models. Gut 2010, 59, 1635–1642. [Google Scholar] [CrossRef]
- Sanmiguel, C.; Gupta, A.; Mayer, E.A. Gut microbiome and obesity: A plausible explanation for obesity. Curr. Obes. Rep. 2015, 4, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Usami, M.; Muraki, K.; Iwamoto, M.; Ohata, A.; Matsushita, E.; Miki, A. Effect of eicosapentaenoic acid (EPA) on tight junction permeability in intestinal monolayer cells. Clin. Nutr. 2001, 20, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Usami, M.; Komurasaki, T.; Hanada, A.; Kinoshita, K.; Ohata, A. Effect of gamma-linolenic acid or docosahexaenoic acid on tight junction permeability in intestinal monolayer cells and their mechanism by protein kinase C activation and/or eicosanoid formation. Nutrition 2003, 19, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Clark, D.L.; Jacobi, S.K.; Velleman, S.G. Supplementation of vitamin E and omega-3 fatty acids during the early posthatch period on intestinal morphology and gene expression differentiation in broilers. Poult. Sci. 2021, 100, 100954. [Google Scholar] [CrossRef] [PubMed]
- De Quelen, F.; Chevalier, J.; Rolli-Derkinderen, M.; Mourot, J.; Neunlist, M.; Boudry, G. n-3 polyunsaturated fatty acids in the maternal diet modify the postnatal development of nervous regulation of intestinal permeability in piglets. J. Physiol. 2011, 589, 4341–4352. [Google Scholar] [CrossRef] [PubMed]
- Pandey, U.; Aich, P. Postnatal intestinal mucosa and gut microbial composition develop hand in hand: A mouse study. Biomed. J. 2023, 46, 100519. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, M.; Paës, C.; Mussard, E.; Knudsen, C.; Cauquil, L.; Aymard, P.; Barilly, C.; Gabinaud, B.; Zemb, O.; Fourre, S.; et al. Gut microbiota derived metabolites contribute to intestinal barrier maturation at the suckling-to-weaning transition. Gut Microbes 2020, 11, 1268–1286. [Google Scholar] [CrossRef] [PubMed]
- Halpern, M.D.; Denning, P.W. The role of intestinal epithelial barrier function in the development of NEC. Tissue Barriers 2015, 3, e1000707. [Google Scholar] [CrossRef]
- Pandey, U.; Tambat, S.; Aich, P. Postnatal 14D is the Key Window for Mice Intestinal Development—An Insight from Age-Dependent Antibiotic-Mediated Gut Microbial Dysbiosis Study. Adv. Biol. 2023, 7, e2300089. [Google Scholar] [CrossRef] [PubMed]
- Lock, J.Y.; Carlson, T.L.; Yu, Y.; Lu, J.; Claud, E.C.; Carrier, R.L. Impact of Developmental Age, Necrotizing Enterocolitis Associated Stress, and Oral Therapeutic Intervention on Mucus Barrier Properties. Sci. Rep. 2020, 10, 6692. [Google Scholar] [CrossRef]
- Lokken-Toyli, K.L.; de Steenhuijsen Piters, W.A.A.; Zangari, T.; Martel, R.; Kuipers, K.; Shopsin, B.; Loomis, C.; Bogaert, D.; Weiser, J.N. Decreased production of epithelial-derived antimicrobial molecules at mucosal barriers during early life. Mucosal Immunol. 2021, 14, 1358–1368. [Google Scholar] [CrossRef]
- Birchenough, G.M.H.; Dalgakiran, F.; Witcomb, L.A.; Johansson, M.E.V.; McCarthy, A.J.; Hansson, G.C.; Taylor, P.W. Postnatal development of the small intestinal mucosa drives age-dependent, regio-selective susceptibility to Escherichia coli K1 infection. Sci. Rep. 2017, 7, 83. [Google Scholar] [CrossRef] [PubMed]
- Mariamenatu, A.H.; Abdu, E.M. Overconsumption of Omega-6 Polyunsaturated Fatty Acids (PUFAs) versus Deficiency of Omega-3 PUFAs in Modern-Day Diets: The Disturbing Factor for Their “Balanced Antagonistic Metabolic Functions” in the Human Body. J. Lipids 2021, 2021, 8848161. [Google Scholar] [CrossRef]
- Anderson, B.M.; MacLennan, M.B.; Hillyer, L.M.; Ma, D.W. Lifelong exposure to n-3 PUFA affects pubertal mammary gland development. Appl. Physiol. Nutr. Metab. 2014, 39, 699–706. [Google Scholar] [CrossRef]
- Hillyer, L.M.; Kang, J.X.; Ma, D.W.L. Lifelong n-3 Polyunsaturated Fatty Acid Exposure Modulates Size of Mammary Epithelial Cell Populations and Expression of Caveolae Resident Proteins in Fat-1 Mice. Nutrients 2019, 11, 2477. [Google Scholar] [CrossRef]
- Liu, J.; Abdelmagid, S.A.; Pinelli, C.J.; Monk, J.M.; Liddle, D.M.; Hillyer, L.M.; Hucik, B.; Silva, A.; Subedi, S.; Wood, G.A.; et al. Marine fish oil is more potent than plant-based n-3 polyunsaturated fatty acids in the prevention of mammary tumors. J. Nutr. Biochem. 2018, 55, 41–52. [Google Scholar] [CrossRef]
- MacLennan, M.B.; Anderson, B.M.; Ma, D.W. Differential mammary gland development in FVB and C57Bl/6 mice: Implications for breast cancer research. Nutrients 2011, 3, 929–936. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- De Boer, A.A.; Monk, J.M.; Robinson, L.E. Docosahexaenoic acid decreases pro-inflammatory mediators in an in vitro murine adipocyte macrophage co-culture model. PLoS ONE 2014, 9, e85037. [Google Scholar] [CrossRef] [PubMed]
- Monk, J.M.; Zhang, C.P.; Wu, W.; Zarepoor, L.; Lu, J.T.; Liu, R.; Pauls, K.P.; Wood, G.A.; Tsao, R.; Robinson, L.E.; et al. White and dark kidney beans reduce colonic mucosal damage and inflammation in response to dextran sodium sulfate. J. Nutr. Biochem. 2015, 26, 752–760. [Google Scholar] [CrossRef]
- Power, K.A.; Lepp, D.; Zarepoor, L.; Monk, J.M.; Wu, W.; Tsao, R.; Liu, R. Dietary flaxseed modulates the colonic microenvironment in healthy C57Bl/6 male mice which may alter susceptibility to gut-associated diseases. J. Nutr. Biochem. 2016, 28, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Monk, J.M.; Lepp, D.; Zhang, C.P.; Wu, W.; Zarepoor, L.; Lu, J.T.; Pauls, K.P.; Tsao, R.; Wood, G.A.; Robinson, L.E.; et al. Diets enriched with cranberry beans alter the microbiota and mitigate colitis severity and associated inflammation. J. Nutr. Biochem. 2016, 28, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Monk, J.M.; Lepp, D.; Wu, W.; Pauls, K.P.; Robinson, L.E.; Power, K.A. Navy and black bean supplementation primes the colonic mucosal microenvironment to improve gut health. J. Nutr. Biochem. 2017, 49, 89–100. [Google Scholar] [CrossRef]
- Cano-Cebrián, M.J.; Dahlgren, D.; Kullenberg, F.; Peters, K.; Olander, T.; Sjöblom, M.; Lennernäs, H. Chemotherapeutics Combined with Luminal Irritants: Effects on Small-Intestinal Mannitol Permeability and Villus Length in Rats. Int. J. Mol. Sci. 2022, 23, 1021. [Google Scholar] [CrossRef]
- DE BOO, H.A.; HARDING, J.E. The developmental origins of adult disease (Barker) hypothesis. Aust. N. Z. J. Obstet. Gynaecol. 2006, 46, 4–14. [Google Scholar] [CrossRef]
- Monteiro, J.; Askarian, F.; Nakamura, M.T.; Moghadasian, M.H.; Ma, D.W. Oils rich in α-linolenic acid independently protect against characteristics of fatty liver disease in the Δ6-desaturase null mouse. Can. J. Physiol. Pharmacol. 2013, 91, 469–479. [Google Scholar] [CrossRef]
- Monk, J.M.; Liddle, D.M.; Cohen, D.J.; Tsang, D.H.; Hillyer, L.M.; Abdelmagid, S.A.; Nakamura, M.T.; Power, K.A.; Ma, D.W.; Robinson, L.E. The delta 6 desaturase knock out mouse reveals that immunomodulatory effects of essential n-6 and n-3 polyunsaturated fatty acids are both independent of and dependent upon conversion. J. Nutr. Biochem. 2016, 32, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, H.; Yin, P.; Fan, H.; Sun, L.; Liu, Y. Flaxseed oil ameliorates alcoholic liver disease via anti-inflammation and modulating gut microbiota in mice. Lipids Health Dis. 2017, 16, 44. [Google Scholar] [CrossRef] [PubMed]
- Angelakis, E.; Raoult, D. Gut microbiota modifications and weight gain in early life. Hum. Microbiome J. 2018, 7–8, 10–14. [Google Scholar] [CrossRef]
- Jian, C.; Carpén, N.; Helve, O.; de Vos, W.M.; Korpela, K.; Salonen, A. Early-life gut microbiota and its connection to metabolic health in children: Perspective on ecological drivers and need for quantitative approach. EBioMedicine 2021, 69, 103475. [Google Scholar] [CrossRef] [PubMed]
- Dehmer, J.J.; Garrison, A.P.; Speck, K.E.; Dekaney, C.M.; Van Landeghem, L.; Sun, X.; Henning, S.J.; Helmrath, M.A. Expansion of Intestinal Epithelial Stem Cells during Murine Development. PLoS ONE 2011, 6, e27070. [Google Scholar] [CrossRef] [PubMed]
- Monk, J.M.; Hou, T.Y.; Turk, H.F.; Weeks, B.; Wu, C.; McMurray, D.N.; Chapkin, R.S. Dietary n-3 polyunsaturated fatty acids (PUFA) decrease obesity-associated Th17 cell-mediated inflammation during colitis. PLoS ONE 2012, 7, e49739. [Google Scholar] [CrossRef]
- Määttänen, P.; Lurz, E.; Botts, S.R.; Wu, R.Y.; Robinson, S.C.; Yeung, C.W.; Colas, R.; Li, B.; Johnson-Henry, K.C.; Surette, M.E.; et al. Plant- and Fish-Derived n-3 PUFAs Suppress Citrobacter Rodentium-Induced Colonic Inflammation. Mol. Nutr. Food Res. 2020, 64, e1900873. [Google Scholar] [CrossRef]
- Xu, Z.; Tang, H.; Huang, F.; Qiao, Z.; Wang, X.; Yang, C.; Deng, Q. Algal Oil Rich in n-3 PUFA Alleviates DSS-Induced Colitis via Regulation of Gut Microbiota and Restoration of Intestinal Barrier. Front. Microbiol. 2020, 11, 615404. [Google Scholar] [CrossRef]
- Monk, J.M.; Jia, Q.; Callaway, E.; Weeks, B.; Alaniz, R.C.; McMurray, D.N.; Chapkin, R.S. Th17 cell accumulation is decreased during chronic experimental colitis by (n-3) PUFA in Fat-1 mice. J. Nutr. 2012, 142, 117–124. [Google Scholar] [CrossRef]
- He, J.; Luo, X.; Xin, H.; Lai, Q.; Zhou, Y.; Bai, Y. The Effects of Fatty Acids on Inflammatory Bowel Disease: A Two-Sample Mendelian Randomization Study. Nutrients 2022, 14, 2883. [Google Scholar] [CrossRef]
- Hassan, A.; Ibrahim, A.; Mbodji, K.; Coëffier, M.; Ziegler, F.; Bounoure, F.; Chardigny, J.M.; Skiba, M.; Savoye, G.; Déchelotte, P.; et al. An α-linolenic acid-rich formula reduces oxidative stress and inflammation by regulating NF-κB in rats with TNBS-induced colitis. J. Nutr. 2010, 140, 1714–1721. [Google Scholar] [CrossRef]
- Charpentier, C.; Chan, R.; Salameh, E.; Mbodji, K.; Ueno, A.; Coëffier, M.; Guérin, C.; Ghosh, S.; Savoye, G.; Marion-Letellier, R. Dietary n-3 PUFA May Attenuate Experimental Colitis. Mediat. Inflamm. 2018, 2018, 8430614. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, W.; Deng, Q.; Huang, Q.; Wang, X.; Yang, C.; Huang, F. Flaxseed oligosaccharides alleviate DSS-induced colitis through modulation of gut microbiota and repair of the intestinal barrier in mice. Food Funct. 2020, 11, 8077–8088. [Google Scholar] [CrossRef]
- Soares, I.P.; Oliveira, B.A.C.; Baal, S.C.S.; Donatti, L.; Ingberman, M.; Beirão, B.C.B.; Appel, M.H.; Fernandes, L.C. Fish oil supplementation enhances colon recovery after experimental colitis. Prostaglandins Leukot Essent Fat. Acids 2020, 163, 102212. [Google Scholar] [CrossRef]
- Jia, Q.; Ivanov, I.; Zlatev, Z.Z.; Alaniz, R.C.; Weeks, B.R.; Callaway, E.S.; Goldsby, J.S.; Davidson, L.A.; Fan, Y.Y.; Zhou, L.; et al. Dietary fish oil and curcumin combine to modulate colonic cytokinetics and gene expression in dextran sodium sulphate-treated mice. Br. J. Nutr. 2011, 106, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Zarepoor, L.; Lu, J.T.; Zhang, C.; Wu, W.; Lepp, D.; Robinson, L.; Wanasundara, J.; Cui, S.; Villeneuve, S.; Fofana, B.; et al. Dietary flaxseed intake exacerbates acute colonic mucosal injury and inflammation induced by dextran sodium sulfate. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G1042–G1055. [Google Scholar] [CrossRef] [PubMed]
- Sobolewska, A.; Bogucka, J.; Dankowiakowska, A.; Elminowska-Wenda, G.; Stadnicka, K.; Bednarczyk, M. The impact of synbiotic administration through in ovo technology on the microstructure of a broiler chicken small intestine tissue on the 1(st) and 42(nd) day of rearing. J. Anim. Sci. Biotechnol. 2017, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Zhu, X.; Gong, C.; Liu, H.; Liu, F. Protective effects and mechanisms of omega-3 polyunsaturated fatty acid on intestinal injury and macrophage polarization in peritoneal dialysis rats. Nephrology 2019, 24, 1081–1089. [Google Scholar] [CrossRef]
- Morampudi, V.; Dalwadi, U.; Bhinder, G.; Sham, H.P.; Gill, S.K.; Chan, J.; Bergstrom, K.S.; Huang, T.; Ma, C.; Jacobson, K.; et al. The goblet cell-derived mediator RELM-β drives spontaneous colitis in Muc2-deficient mice by promoting commensal microbial dysbiosis. Mucosal Immunol. 2016, 9, 1218–1233. [Google Scholar] [CrossRef] [PubMed]
- Bergstrom, K.S.; Xia, L. Mucin-type O-glycans and their roles in intestinal homeostasis. Glycobiology 2013, 23, 1026–1037. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.C. Role of mucus layers in gut infection and inflammation. Curr. Opin. Microbiol. 2012, 15, 57–62. [Google Scholar] [CrossRef]
- Vaishnava, S.; Yamamoto, M.; Severson, K.M.; Ruhn, K.A.; Yu, X.; Koren, O.; Ley, R.; Wakeland, E.K.; Hooper, L.V. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science 2011, 334, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Cash, H.L.; Whitham, C.V.; Behrendt, C.L.; Hooper, L.V. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 2006, 313, 1126–1130. [Google Scholar] [CrossRef]
- Kim, Y.S.; Ho, S.B. Intestinal goblet cells and mucins in health and disease: Recent insights and progress. Curr. Gastroenterol. Rep. 2010, 12, 319–330. [Google Scholar] [CrossRef]
- Hogan, S.P.; Seidu, L.; Blanchard, C.; Groschwitz, K.; Mishra, A.; Karow, M.L.; Ahrens, R.; Artis, D.; Murphy, A.J.; Valenzuela, D.M.; et al. Resistin-like molecule beta regulates innate colonic function: Barrier integrity and inflammation susceptibility. J. Allergy Clin. Immunol. 2006, 118, 257–268. [Google Scholar] [CrossRef]
- Krimi, R.B.; Kotelevets, L.; Dubuquoy, L.; Plaisancié, P.; Walker, F.; Lehy, T.; Desreumaux, P.; Van Seuningen, I.; Chastre, E.; Forgue-Lafitte, M.E.; et al. Resistin-like molecule beta regulates intestinal mucous secretion and curtails TNBS-induced colitis in mice. Inflamm. Bowel Dis. 2008, 14, 931–941. [Google Scholar] [CrossRef]
- McVay, L.D.; Keilbaugh, S.A.; Wong, T.M.; Kierstein, S.; Shin, M.E.; Lehrke, M.; Lefterova, M.I.; Shifflett, D.E.; Barnes, S.L.; Cominelli, F.; et al. Absence of bacterially induced RELMbeta reduces injury in the dextran sodium sulfate model of colitis. J. Clin. Investig. 2006, 116, 2914–2923. [Google Scholar] [CrossRef] [PubMed]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.T.; Zuo, L.; Odenwald, M.A.; Madha, S.; Singh, G.; Gurniak, C.B.; Abraham, C.; Turner, J.R. The Tight Junction Protein ZO-1 Is Dispensable for Barrier Function but Critical for Effective Mucosal Repair. Gastroenterology 2021, 161, 1924–1939. [Google Scholar] [CrossRef]
- Kuo, W.T.; Odenwald, M.A.; Turner, J.R.; Zuo, L. Tight junction proteins occludin and ZO-1 as regulators of epithelial proliferation and survival. Ann. N. Y. Acad. Sci. 2022, 1514, 21–33. [Google Scholar] [CrossRef]
- Aihara, E.; Engevik, K.A.; Montrose, M.H. Trefoil Factor Peptides and Gastrointestinal Function. Annu. Rev. Physiol. 2017, 79, 357–380. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.F.; Teng, X.; Guo, J.; Sun, M. Protective effect of intestinal trefoil factor on injury of intestinal epithelial tight junction induced by platelet activating factor. Inflammation 2012, 35, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Meyer zum Büschenfelde, D.; Tauber, R.; Huber, O. TFF3-peptide increases transepithelial resistance in epithelial cells by modulating claudin-1 and -2 expression. Peptides 2006, 27, 3383–3390. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lin, Z.; Lin, Q.; Bei, W.; Guo, J. Pathological and therapeutic roles of bioactive peptide trefoil factor 3 in diverse diseases: Recent progress and perspective. Cell Death Dis. 2022, 13, 62. [Google Scholar] [CrossRef]
- Lindén, S.K.; Florin, T.H.J.; McGuckin, M.A. Mucin Dynamics in Intestinal Bacterial Infection. PLoS ONE 2008, 3, e3952. [Google Scholar] [CrossRef]
- Johansson, M.E.; Sjövall, H.; Hansson, G.C. The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 352–361. [Google Scholar] [CrossRef]
- Ho, S.B.; Dvorak, L.A.; Moor, R.E.; Jacobson, A.C.; Frey, M.R.; Corredor, J.; Polk, D.B.; Shekels, L.L. Cysteine-rich domains of muc3 intestinal mucin promote cell migration, inhibit apoptosis, and accelerate wound healing. Gastroenterology 2006, 131, 1501–1517. [Google Scholar] [CrossRef]
- Luu, Y.; Junker, W.; Rachagani, S.; Das, S.; Batra, S.K.; Heinrikson, R.L.; Shekels, L.L.; Ho, S.B. Human intestinal MUC17 mucin augments intestinal cell restitution and enhances healing of experimental colitis. Int. J. Biochem. Cell Biol. 2010, 42, 996–1006. [Google Scholar] [CrossRef]
- Yu, T.; Chen, X.; Zhang, W.; Li, J.; Xu, R.; Wang, T.C.; Ai, W.; Liu, C. Krüppel-like Factor 4 Regulates Intestinal Epithelial Cell Morphology and Polarity. PLoS ONE 2012, 7, e32492. [Google Scholar] [CrossRef]
- Katz, J.P.; Perreault, N.; Goldstein, B.G.; Lee, C.S.; Labosky, P.A.; Yang, V.W.; Kaestner, K.H. The zinc-finger transcription factor Klf4 is required for terminal differentiation of goblet cells in the colon. Development 2002, 129, 2619–2628. [Google Scholar] [CrossRef]
- Ghaleb, A.M.; McConnell, B.B.; Kaestner, K.H.; Yang, V.W. Altered intestinal epithelial homeostasis in mice with intestine-specific deletion of the Krüppel-like factor 4 gene. Dev. Biol. 2011, 349, 310–320. [Google Scholar] [CrossRef] [PubMed]

| SO (D04092701) | MO (D04092703) | FX (D04092711N) | ||||
|---|---|---|---|---|---|---|
| Macronutrient | g% | kcal% | g% | kcal% | g% | kcal% |
| Protein | 21 | 20 | 21 | 20 | 21 | 20 |
| Carbohydrate | 60 | 58 | 60 | 58 | 60 | 58 |
| Fat | 10 | 22 | 10 | 22 | 10 | 22 |
| Ingredient | g/kg | kcal | g | kcal | g | kcal |
| Casein | 200 | 800 | 200 | 800 | 200 | 800 |
| L-Cystine | 3 | 12 | 3 | 12 | 3 | 12 |
| Corn starch | 337 | 1347 | 337 | 1347 | 337 | 1347 |
| Maltodextrin 10 | 132 | 528 | 132 | 528 | 132 | 528 |
| Sucrose | 100 | 400 | 100 | 400 | 100 | 400 |
| Cellulose, BW200 | 50 | 0 | 50 | 0 | 50 | 0 |
| Safflower oil | 97 | 873 | 68 | 611 | 68 | 611 |
| Menhaden oil | 0 | 0 | 29 | 263 | 0 | 0 |
| Flax oil | 0 | 0 | 0 | 0 | 29 | 263 |
| t-Butylhydroquinone | 0.02 | 0 | 0.02 | 0 | 0.02 | 0 |
| Mineral Mix S10022 G | 35 | 0 | 35 | 0 | 35 | 0 |
| Vitamin Mix V10037 | 10 | 40 | 10 | 40 | 10 | 40 |
| Choline bitartrate | 2.5 | 0 | 2.5 | 0 | 2.5 | 0 |
| Total | 966 | 4000 | 966 | 4000 | 966 | 4000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acosta, J.E.; Burns, J.L.; Hillyer, L.M.; Van, K.; Brendel, E.B.K.; Law, C.; Ma, D.W.L.; Monk, J.M. Effect of Lifelong Exposure to Dietary Plant and Marine Sources of n-3 Polyunsaturated Fatty Acids on Morphologic and Gene Expression Biomarkers of Intestinal Health in Early Life. Nutrients 2024, 16, 719. https://doi.org/10.3390/nu16050719
Acosta JE, Burns JL, Hillyer LM, Van K, Brendel EBK, Law C, Ma DWL, Monk JM. Effect of Lifelong Exposure to Dietary Plant and Marine Sources of n-3 Polyunsaturated Fatty Acids on Morphologic and Gene Expression Biomarkers of Intestinal Health in Early Life. Nutrients. 2024; 16(5):719. https://doi.org/10.3390/nu16050719
Chicago/Turabian StyleAcosta, Julianna E., Jessie L. Burns, Lyn M. Hillyer, Kelsey Van, Elaina B. K. Brendel, Camille Law, David W. L. Ma, and Jennifer M. Monk. 2024. "Effect of Lifelong Exposure to Dietary Plant and Marine Sources of n-3 Polyunsaturated Fatty Acids on Morphologic and Gene Expression Biomarkers of Intestinal Health in Early Life" Nutrients 16, no. 5: 719. https://doi.org/10.3390/nu16050719






