Genistein Supplementation and Bone Health in Breast Cancer in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Laboratory Animals
2.2. Histopathological and Micromorphometric Examination of Bones
2.3. Determination of Levels of Elements
2.3.1. Reagents
2.3.2. Sampling
2.3.3. Instrumental Analysis
2.4. Statistical Analysis
3. Results
3.1. Analysis of the Weight of Femurs of Rats Fed Different Diets (Standard and Supplemented with Macrogenistein, Microgenistein, or Nanogenistein)
3.2. Results of Histopathological and Micromorphometric Examination of Rat Femurs
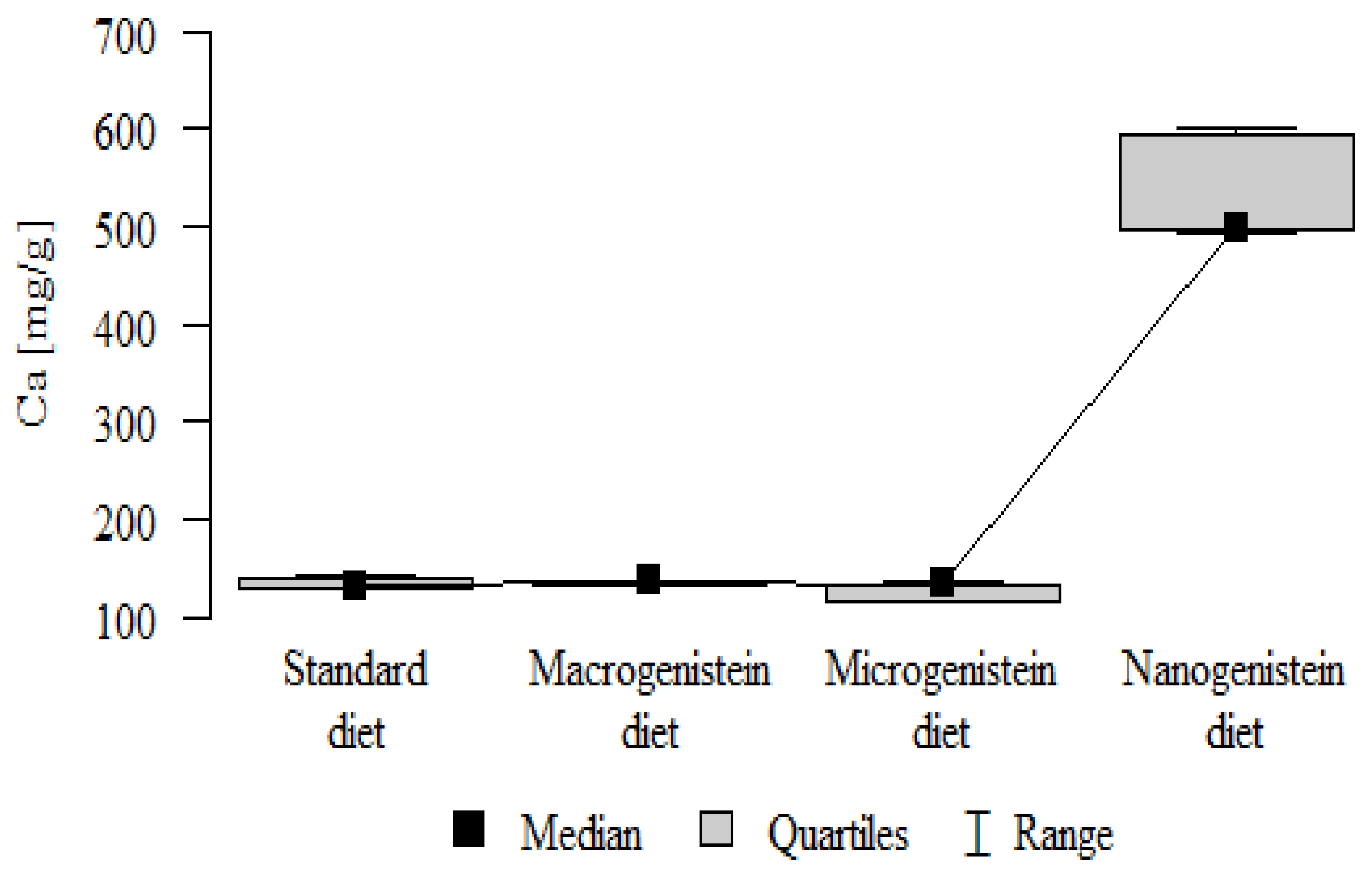
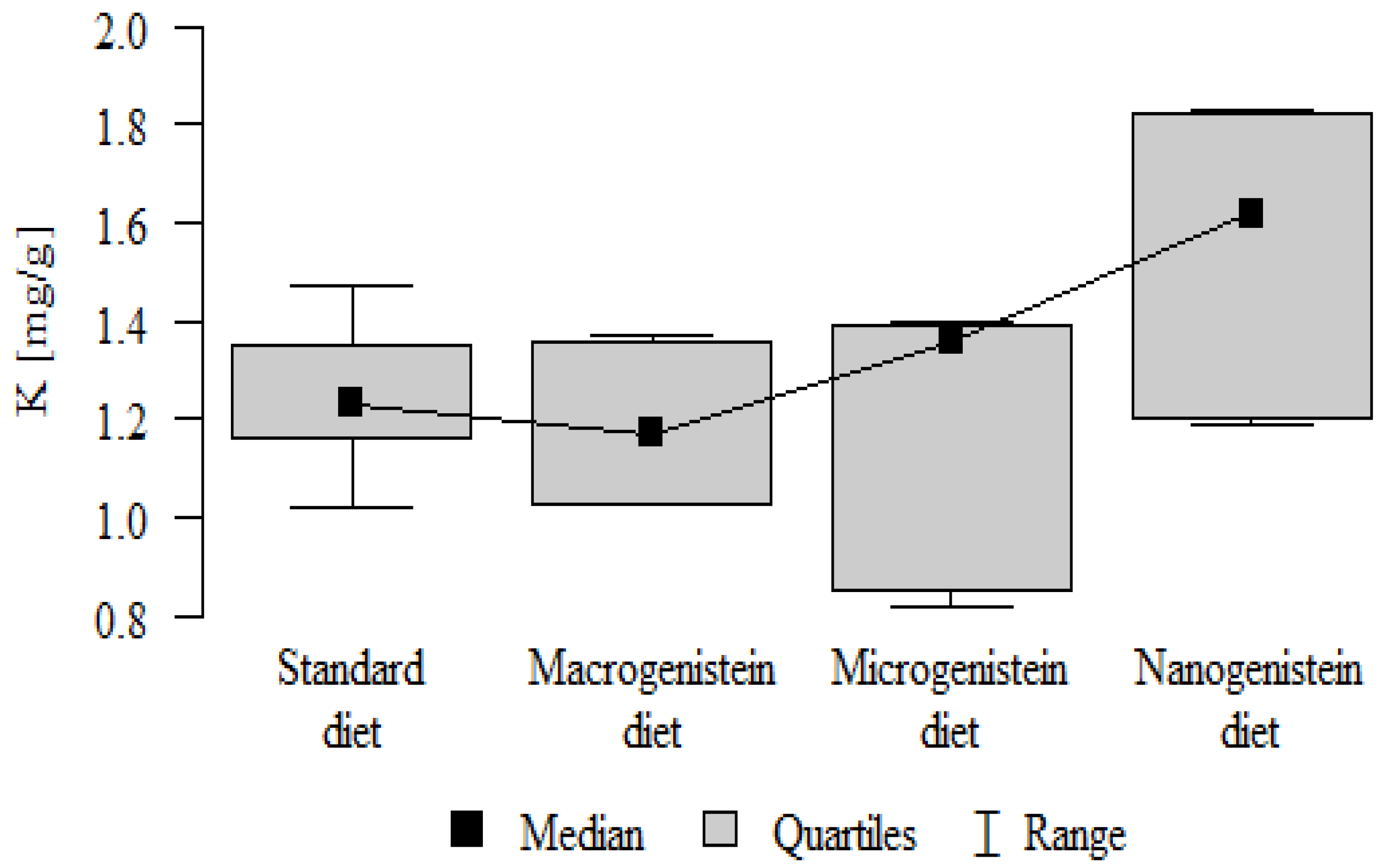
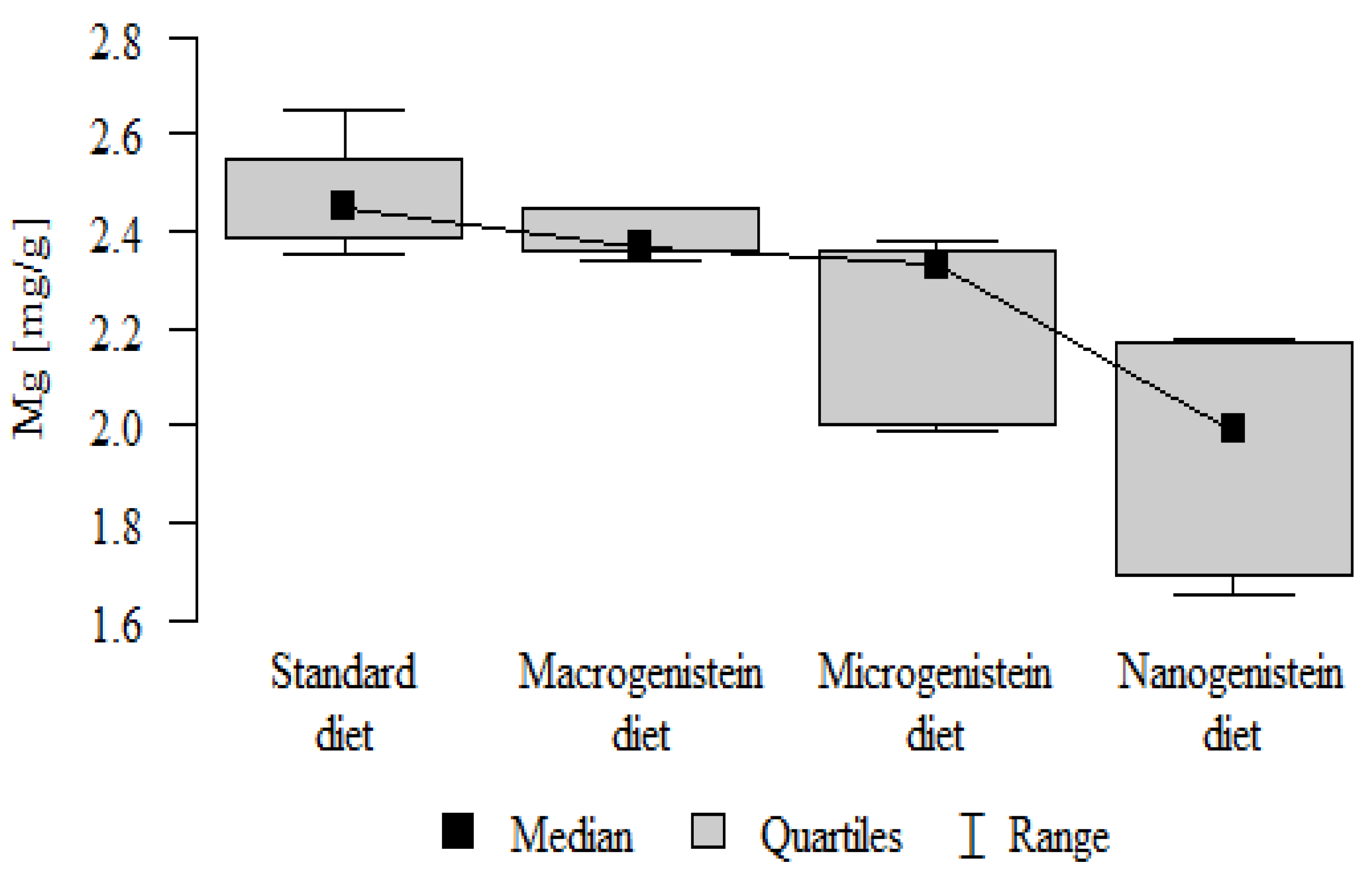
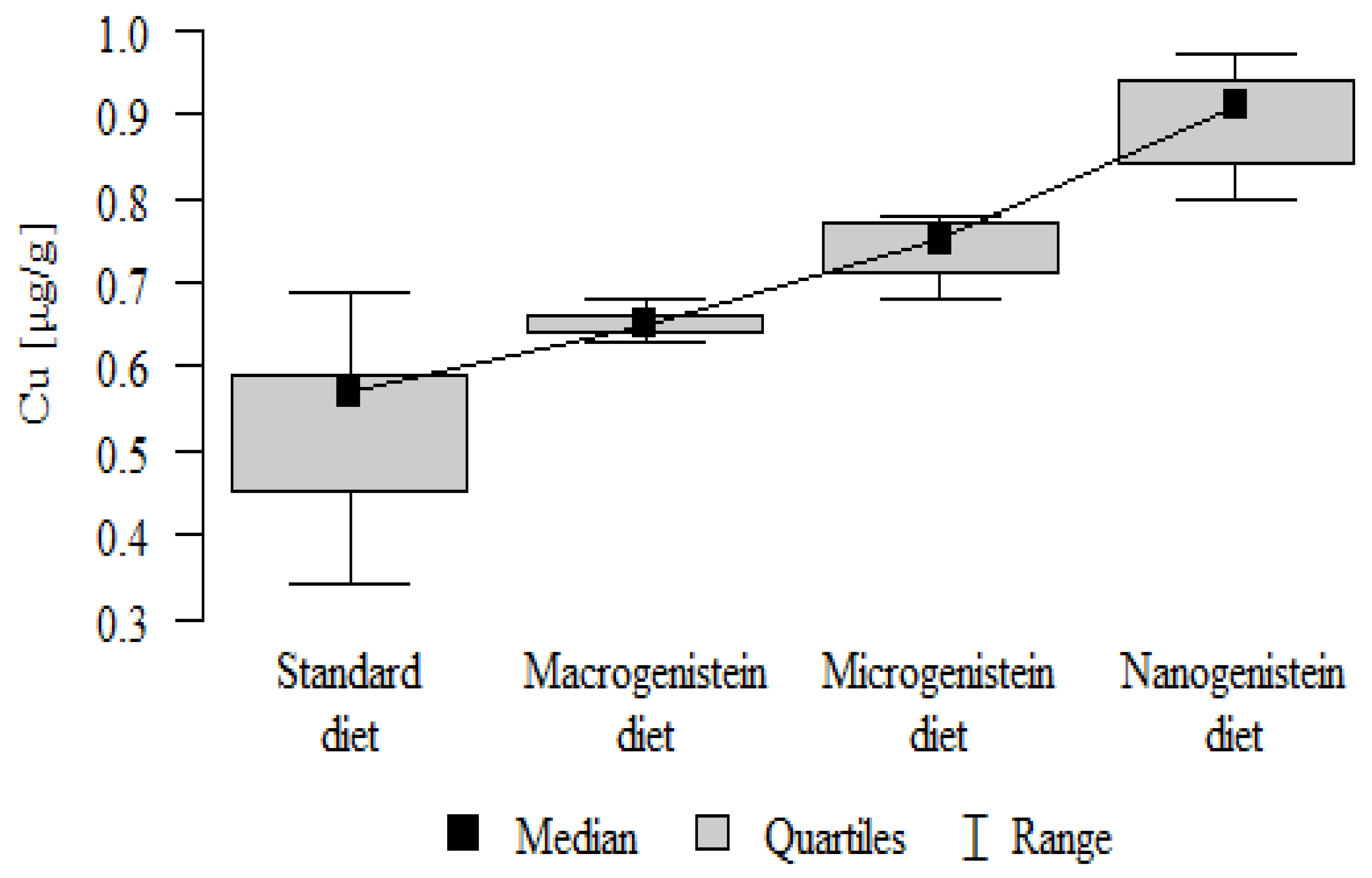
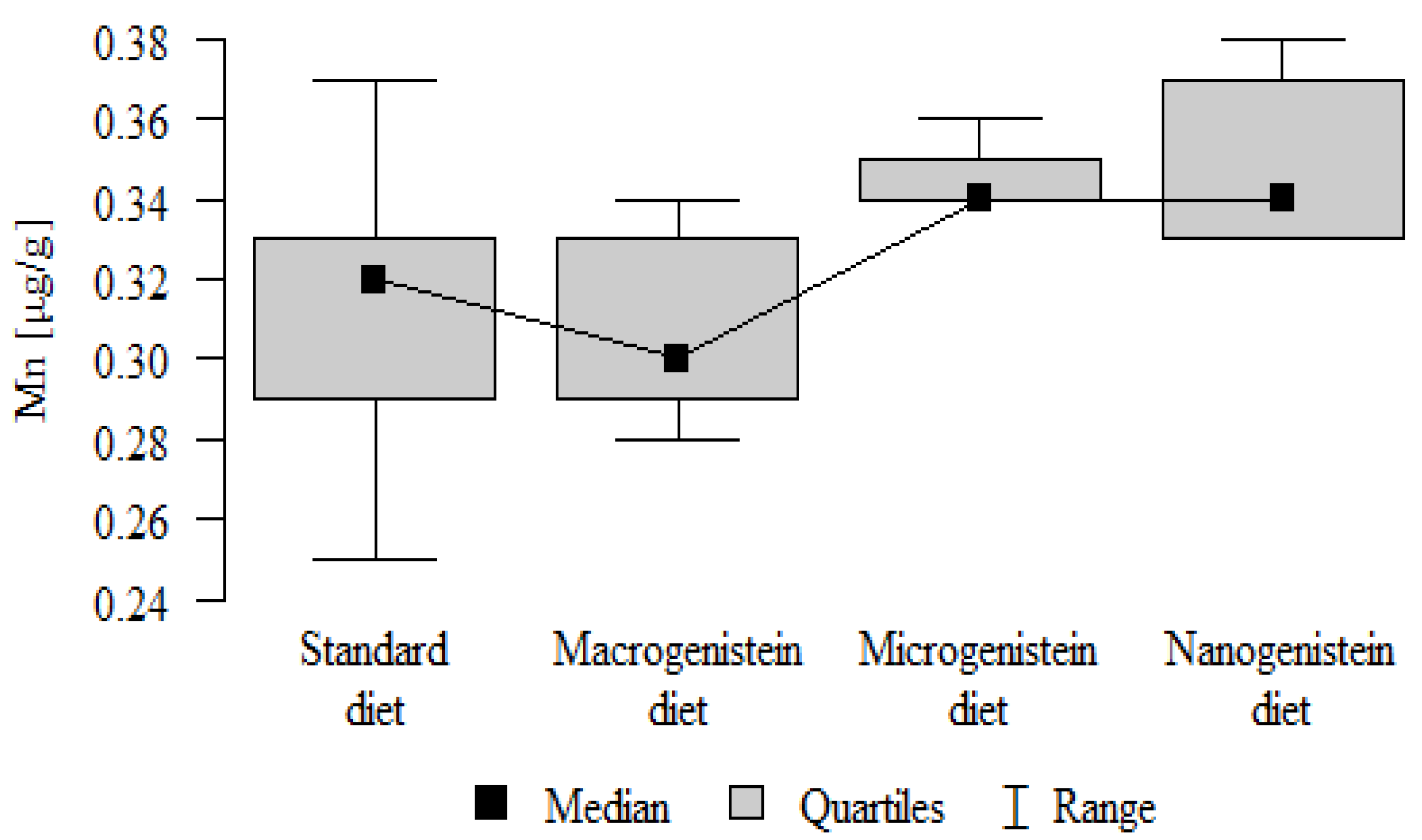
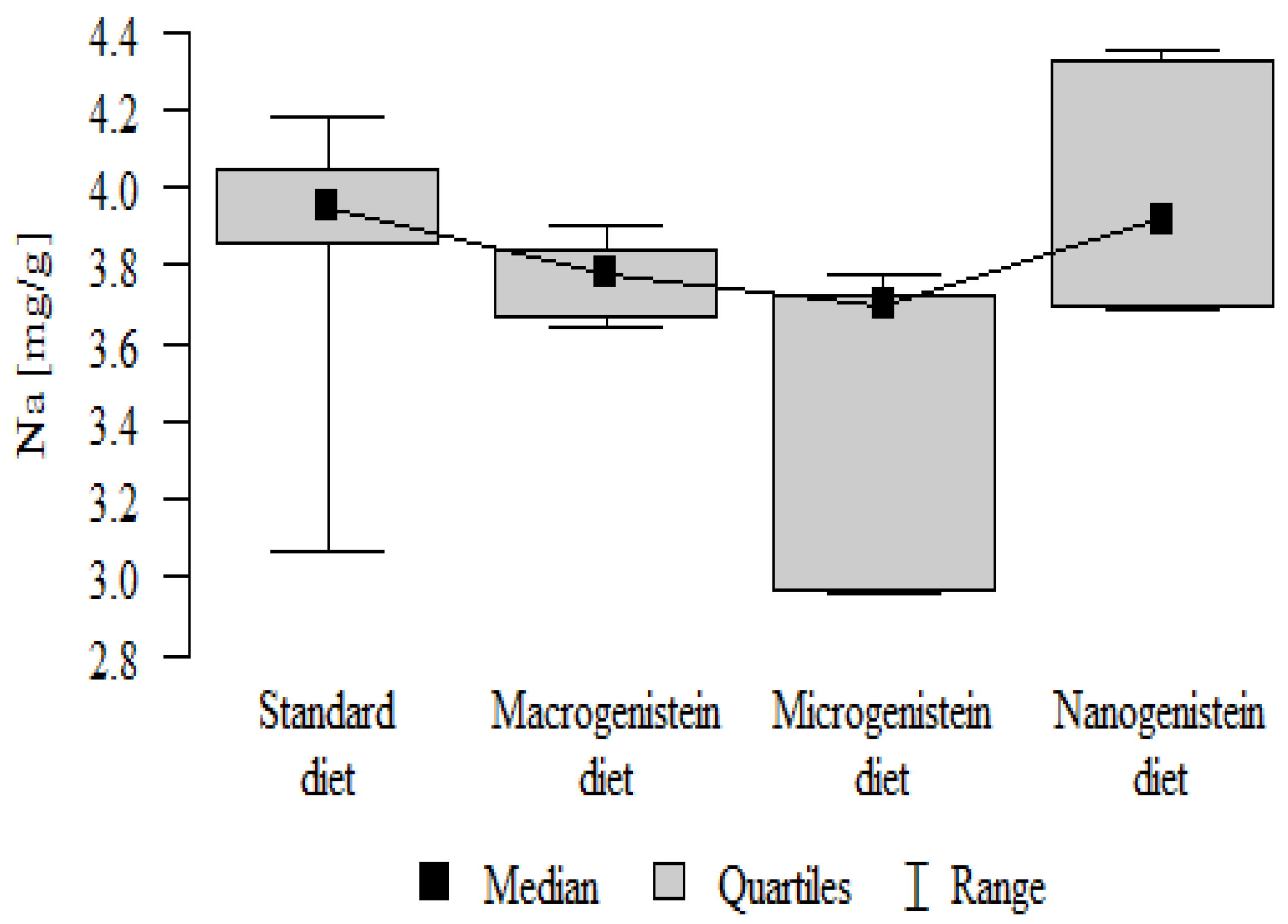
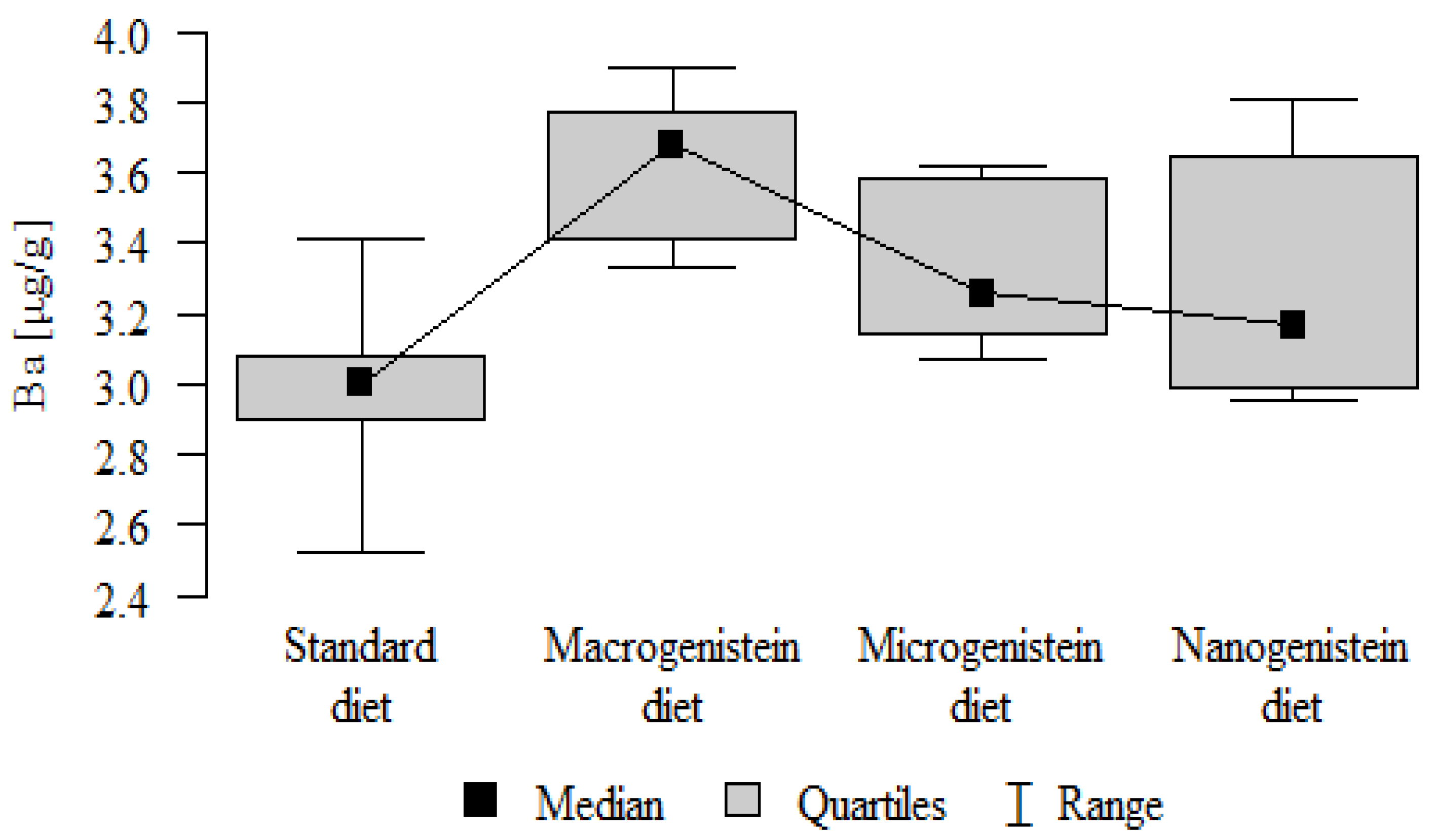
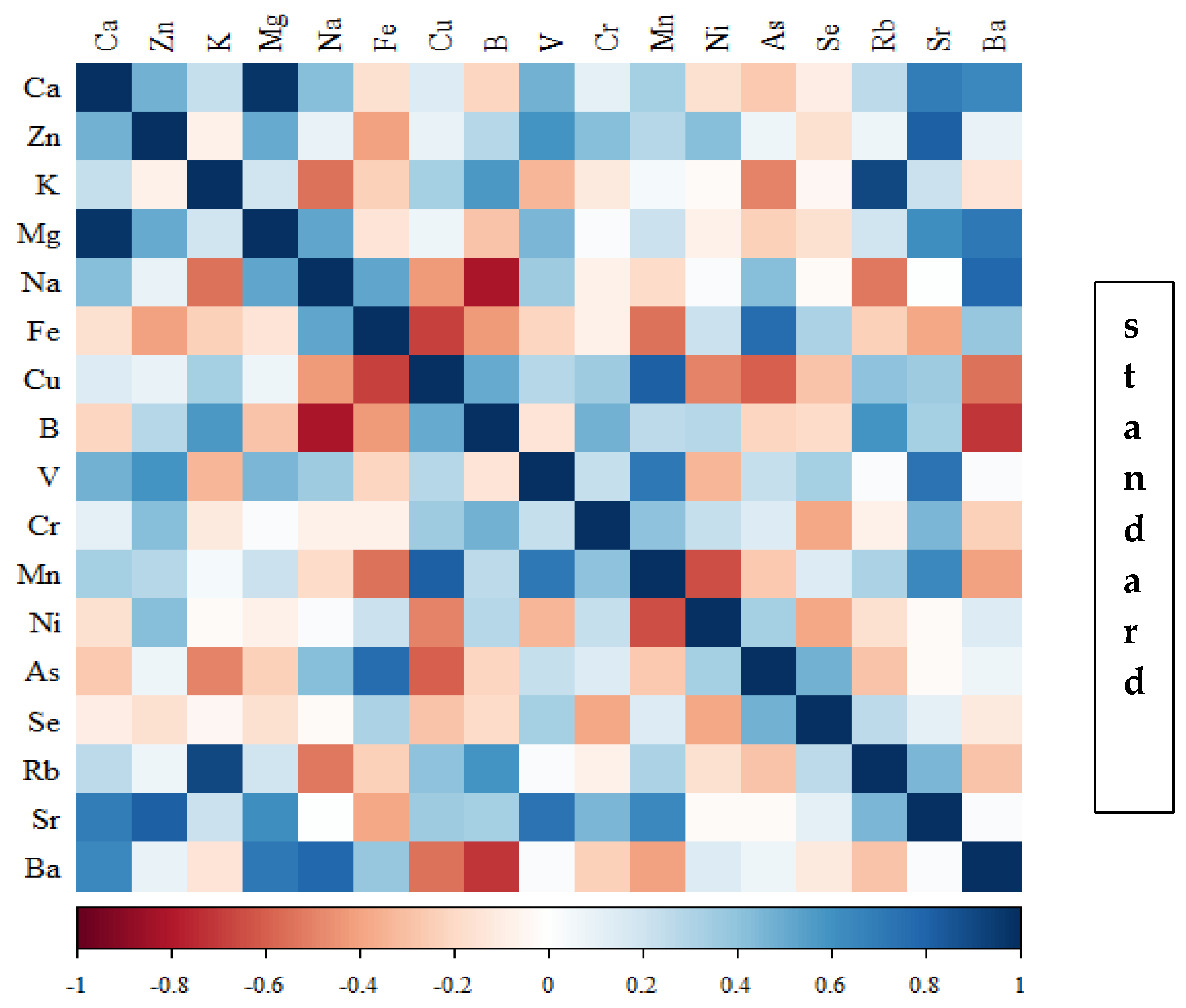
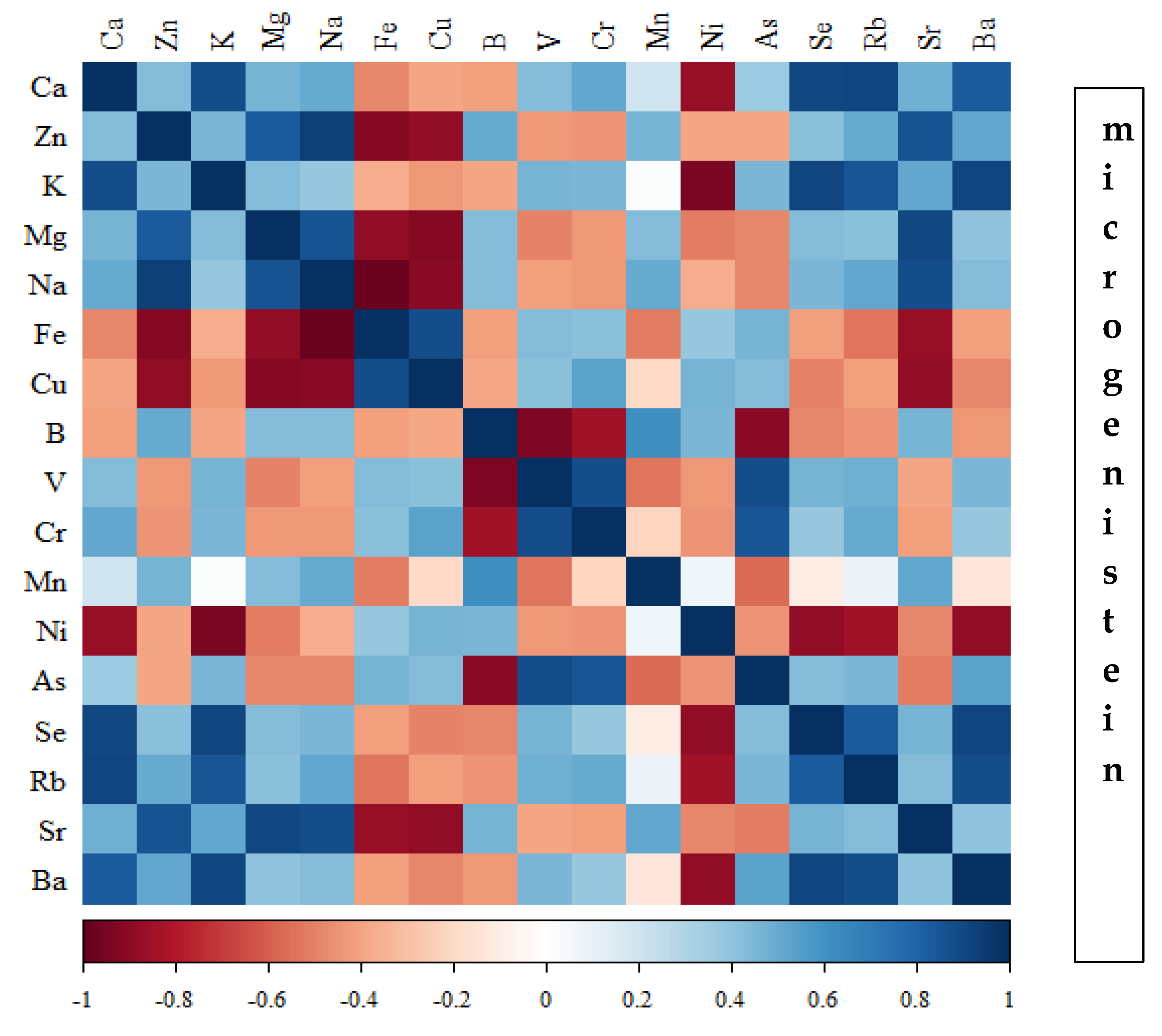
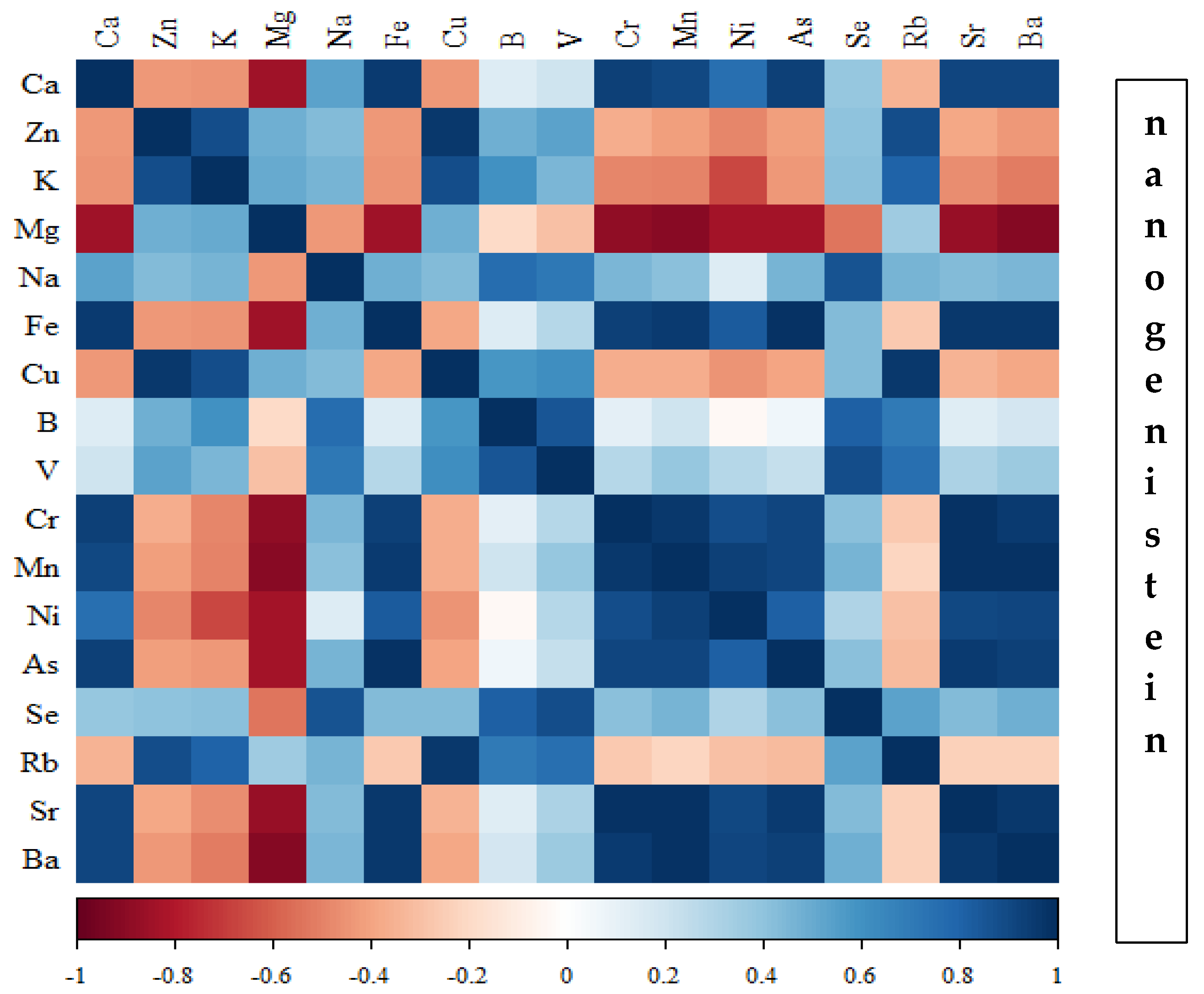
3.3. Analysis of the Content of Minerals in the Femoral Diaphysis of Rats Receiving Various Forms of Genistein: Macrogenistein, Microgenistein, Nanogenistein, or No Supplementation (Standard Diet)
- −
- −
- Content of Mg and Sr in the bone tissue was significantly higher in the rats without supplementation (standard diet) than in the groups receiving micro- and nanogenistein. The levels of these elements were significantly higher in the bones of rats supplemented with macrogenistein compared to the group supplemented with nanogenistein (Table 3; Figure 5 and Figure 6).
- −
- −
- −
- −
- −
- Content of Ba in the bones was significantly higher in the group supplemented with macrogenistein than in the groups receiving micro- and nanogenistein and the standard diet. The Ba level was significantly higher in the microgenistein group than in the group on the standard diet (Table 3; Figure 13).
- −
- There were no statistically significant differences in the content of Zn, Fe, Se, V, Cr, and Rb in the bones of the rats (Table 3).
| Diet/Supplementation | Mean | SD | Median | Min | Max | Q1 | Q3 | p | |
|---|---|---|---|---|---|---|---|---|---|
| Ca [mg/g] | Standard diet | 134.41 | 5.94 | 131.78 | 128.24 | 143.07 | 130.07 | 140.31 | p < 0.001 * |
| Macrogenistein | 134.76 | 2.71 | 136.03 | 131.06 | 137.36 | 131.40 | 136.68 | nano > macro, micro, stand | |
| Microgenistein | 127.35 | 8.54 | 132.60 | 115.73 | 134.09 | 116.18 | 133.25 | ||
| Nanogenistein | 530.73 | 50.92 | 499.66 | 492.92 | 601.43 | 495.81 | 595.52 | ||
| Zn [mg/g] | Standard diet | 0.15 | 0.01 | 0.15 | 0.13 | 0.16 | 0.14 | 0.15 | p = 0.297 |
| Macrogenistein | 0.14 | 0.01 | 0.14 | 0.13 | 0.15 | 0.13 | 0.14 | ||
| Microgenistein | 0.14 | 0.01 | 0.13 | 0.12 | 0.16 | 0.12 | 0.16 | ||
| Nanogenistein | 0.16 | 0.05 | 0.14 | 0.13 | 0.24 | 0.13 | 0.22 | ||
| K [mg/g] | Standard diet | 1.24 | 0.15 | 1.23 | 1.02 | 1.47 | 1.16 | 1.35 | p = 0.032 * |
| Macrogenistein | 1.19 | 0.15 | 1.17 | 1.03 | 1.37 | 1.03 | 1.36 | nano > micro, stand, macro | |
| Microgenistein | 1.20 | 0.27 | 1.36 | 0.82 | 1.40 | 0.85 | 1.39 | ||
| Nanogenistein | 1.55 | 0.28 | 1.62 | 1.19 | 1.83 | 1.20 | 1.82 | ||
| Mg [mg/g] | Standard diet | 2.47 | 0.11 | 2.45 | 2.35 | 2.65 | 2.39 | 2.55 | p < 0.001 * |
| Macrogenistein | 2.39 | 0.05 | 2.37 | 2.34 | 2.45 | 2.36 | 2.45 | stand > micro, nano macro > nano | |
| Microgenistein | 2.23 | 0.18 | 2.33 | 1.99 | 2.38 | 2.00 | 2.36 | ||
| Nanogenistein | 1.94 | 0.22 | 1.99 | 1.65 | 2.18 | 1.69 | 2.17 | ||
| Na [mg/g] | Standard diet | 3.87 | 0.35 | 3.95 | 3.07 | 4.18 | 3.86 | 4.05 | p = 0.005 * |
| Macrogenistein | 3.77 | 0.09 | 3.78 | 3.64 | 3.90 | 3.67 | 3.84 | stand, nano > micro | |
| Microgenistein | 3.47 | 0.38 | 3.70 | 2.96 | 3.78 | 2.97 | 3.72 | ||
| Nanogenistein | 3.99 | 0.28 | 3.92 | 3.69 | 4.35 | 3.70 | 4.33 | ||
| Fe [μg/g] | Standard diet | 66.50 | 12.93 | 63.79 | 49.10 | 94.99 | 62.30 | 67.82 | p = 0.384 |
| Macrogenistein | 61.53 | 12.87 | 62.53 | 45.92 | 76.24 | 46.55 | 75.61 | ||
| Microgenistein | 60.87 | 2.71 | 59.36 | 58.70 | 65.02 | 58.77 | 63.21 | ||
| Nanogenistein | 55.59 | 10.89 | 51.98 | 44.53 | 69.78 | 46.63 | 69.45 | ||
| Cu [μg/g] | Standard diet | 0.53 | 0.11 | 0.57 | 0.34 | 0.69 | 0.45 | 0.59 | p < 0.001 * |
| Macrogenistein | 0.65 | 0.02 | 0.65 | 0.63 | 0.68 | 0.64 | 0.66 | nano, micro > macro, stand | |
| Microgenistein | 0.74 | 0.04 | 0.75 | 0.68 | 0.78 | 0.71 | 0.77 | ||
| Nanogenistein | 0.89 | 0.06 | 0.91 | 0.80 | 0.97 | 0.84 | 0.94 | ||
| B [μg/g] | Standard diet | 0.51 | 0.12 | 0.52 | 0.31 | 0.67 | 0.45 | 0.58 | p < 0.001 * |
| Macrogenistein | 0.76 | 0.08 | 0.76 | 0.66 | 0.85 | 0.69 | 0.85 | micro, nano, macro > stand | |
| Microgenistein | 0.88 | 0.10 | 0.91 | 0.70 | 0.98 | 0.82 | 0.97 | ||
| Nanogenistein | 0.76 | 0.05 | 0.76 | 0.66 | 0.80 | 0.73 | 0.80 | ||
| V [μg/g] | Standard diet | 0.02 | 0.01 | 0.03 | 0.00 | 0.03 | 0.02 | 0.03 | p = 0.244 |
| Macrogenistein | 0.02 | 0.00 | 0.02 | 0.02 | 0.03 | 0.02 | 0.03 | ||
| Microgenistein | 0.02 | 0.00 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | ||
| Nanogenistein | 0.02 | 0.00 | 0.02 | 0.02 | 0.03 | 0.02 | 0.03 | ||
| Cr [μg/g] | Standard diet | 0.12 | 0.04 | 0.12 | 0.06 | 0.18 | 0.11 | 0.14 | p = 0.136 |
| Macrogenistein | 0.08 | 0.04 | 0.06 | 0.05 | 0.13 | 0.05 | 0.13 | ||
| Microgenistein | 0.10 | 0.03 | 0.09 | 0.07 | 0.14 | 0.07 | 0.14 | ||
| Nanogenistein | 0.11 | 0.00 | 0.11 | 0.10 | 0.11 | 0.10 | 0.11 | ||
| Mn [μg/g] | Standard diet | 0.31 | 0.04 | 0.32 | 0.25 | 0.37 | 0.29 | 0.33 | p = 0.001 * |
| Macrogenistein | 0.31 | 0.02 | 0.30 | 0.28 | 0.34 | 0.29 | 0.33 | nano, micro > stand, macro | |
| Microgenistein | 0.34 | 0.01 | 0.34 | 0.34 | 0.36 | 0.34 | 0.35 | ||
| Nanogenistein | 0.35 | 0.02 | 0.34 | 0.33 | 0.38 | 0.33 | 0.37 | ||
| Ni [μg/g] | Standard diet | 0.05 | 0.01 | 0.05 | 0.04 | 0.06 | 0.04 | 0.06 | p < 0.001 * |
| Macrogenistein | 0.09 | 0.01 | 0.09 | 0.08 | 0.10 | 0.08 | 0.09 | nano, micro, macro > stand | |
| Microgenistein | 0.12 | 0.06 | 0.09 | 0.07 | 0.21 | 0.07 | 0.20 | ||
| Nanogenistein | 0.10 | 0.01 | 0.10 | 0.08 | 0.11 | 0.09 | 0.10 | ||
| As [μg/g] | Standard diet | 0.05 | 0.01 | 0.04 | 0.04 | 0.06 | 0.04 | 0.05 | p = 0.01 * |
| Macrogenistein | 0.04 | 0.00 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | micro > macro, stand, nano | |
| Microgenistein | 0.06 | 0.00 | 0.05 | 0.05 | 0.06 | 0.05 | 0.06 | ||
| Nanogenistein | 0.04 | 0.01 | 0.04 | 0.03 | 0.06 | 0.03 | 0.06 | ||
| Se [μg/g] | Standard diet | 0.11 | 0.02 | 0.11 | 0.08 | 0.12 | 0.10 | 0.12 | p = 0.494 |
| Macrogenistein | 0.11 | 0.00 | 0.11 | 0.11 | 0.12 | 0.11 | 0.12 | ||
| Microgenistein | 0.11 | 0.01 | 0.11 | 0.09 | 0.12 | 0.10 | 0.12 | ||
| Nanogenistein | 0.10 | 0.02 | 0.10 | 0.08 | 0.12 | 0.09 | 0.11 | ||
| Rb [μg/g] | Standard diet | 1.49 | 0.16 | 1.48 | 1.25 | 1.71 | 1.41 | 1.57 | p = 0.349 |
| Macrogenistein | 1.48 | 0.20 | 1.48 | 1.24 | 1.74 | 1.30 | 1.69 | ||
| Microgenistein | 1.51 | 0.28 | 1.68 | 1.13 | 1.72 | 1.14 | 1.71 | ||
| Nanogenistein | 1.68 | 0.35 | 1.89 | 1.21 | 1.95 | 1.23 | 1.93 | ||
| Sr [μg/g] | Standard diet | 39.40 | 2.93 | 39.03 | 35.59 | 43.77 | 37.35 | 40.86 | p = 0.001 * |
| Macrogenistein | 37.85 | 1.00 | 38.36 | 36.41 | 38.79 | 36.60 | 38.55 | stand > micro, nano macro > nano | |
| Microgenistein | 36.05 | 1.79 | 35.94 | 33.84 | 38.34 | 34.15 | 38.01 | ||
| Nanogenistein | 33.03 | 3.26 | 31.49 | 30.05 | 37.42 | 30.63 | 37.08 | ||
| Ba [μg/g] | Standard diet | 2.98 | 0.25 | 3.00 | 2.52 | 3.41 | 2.90 | 3.08 | p = 0.001 * |
| Macrogenistein | 3.63 | 0.20 | 3.68 | 3.33 | 3.90 | 3.41 | 3.77 | macro > micro, nano, stand micro > stand | |
| Microgenistein | 3.32 | 0.22 | 3.26 | 3.07 | 3.62 | 3.14 | 3.58 | ||
| Nanogenistein | 3.29 | 0.34 | 3.17 | 2.95 | 3.81 | 2.99 | 3.65 |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 1, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef] [PubMed]
- Rumman, M.; Pandey, S.; Singh, B.; Gupta, M.; Ubaid, S.; Mahdi, A.A. Genistein Prevents Hypoxia-Induced Cognitive Dysfunctions by Ameliorating Oxidative Stress and Inflammation in the Hippocampus. Neurotox. Res. 2021, 39, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Hou, S. Genistein: Therapeutic and Preventive Effects, Mechanisms, and Clinical Application in Digestive Tract Tumor. Evid-Based Complement. Altern. Med. ECAM 2022, 22, 5957378. [Google Scholar] [CrossRef] [PubMed]
- Pawlicka, M.A.; Filip, A. Can genistein be a potential agent against skin side effects associated with the treatment of breast cancer? Adv. Dermatol. Allergol. 2022, 39, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Liu, K.; Li, Q.; Shen, Q.; Han, F.; Ye, Q.; Zheng, C. A Mini-Review of Flavone Isomers Apigenin and Genistein in Prostate Cancer Treatment. Front. Pharmacol. 2022, 13, 851589. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, L. The protective activity of genistein against bone and cartilage diseases. Front. Pharmacol. 2022, 13, 1016981. [Google Scholar] [CrossRef]
- Hilakivi-Clarke, L.; Andrade, J.E.; Helferich, W. Is Soy Consumption Good or Bad for the Breast? J. Nutr. 2010, 140, 2326S–2334S. [Google Scholar] [CrossRef]
- Bouker, K.B.; Hilakivi-Clarke, L. Genistein: Does it prevent or promote breast cancer? Environ. Health Perspect. 2000, 108, 701–708. [Google Scholar] [CrossRef]
- Liu, R.; Yu, X.; Chen, X.; Zhong, H.; Liang, C.; Xu, X.; Xu, W.; Cheng, Y.; Wang, W.; Yu, L.; et al. Individual factors define the overall effects of dietary genistein exposure on breast cancer patients. Nutr. Res. 2019, 67, 1–16. [Google Scholar] [CrossRef]
- Pons, D.G.; Nadal-Serrano, M.; Torrens-Mas, M.; Oliver, J.; Roca, P. The Phytoestrogen Genistein Affects Breast Cancer Cells Treatment Depending on the ERα/ERβ Ratio. J. Cell Biochem. 2016, 117, 218–229. [Google Scholar] [CrossRef]
- Suen, A.A.; Kenan, A.C.; Williams, C.J. Developmental exposure to phytoestrogens found in soy: New findings and clinical implications. Biochem. Pharmacol. 2022, 195, 114848. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Imran, M.; Rauf, A.; Nadeem, M.; Gondal, T.A.; Ahmad, B.; Atif, M.; Mubarak, M.S.; Sytar, O.; et al. Genistein: An Integrative Overview of Its Mode of Action, Pharmacological Properties, and Health Benefits. Oxid. Med. Cell Longev. 2021, 19, 1–36. [Google Scholar] [CrossRef]
- Setchell, K.D. Soy isoflavones--benefits and risks from nature’s selective estrogen receptor modulators (SERMs). J. Am. Coll. Nutr. 2001, 20, 354S–362S. [Google Scholar] [CrossRef]
- Bord, S.; Ireland, D.C.; Beavan, S.R.; Compston, J.E. The effects of estrogen on osteoprotegerin, RANKL, and estrogen receptor expression in human osteoblasts. Bone 2003, 32, 136–141. [Google Scholar] [CrossRef]
- Viereck, V.; Gründker, C.; Blaschke, S.; Siggelkow, H.; Emons, G.; Hofbauer, L.C. Phytoestrogen genistein stimulates the production of osteoprotegerin by human trabecular osteoblasts. J. Cell Biochem. 2002, 84, 725–735. [Google Scholar] [CrossRef]
- Streicher, C.; Heyny, A.; Andrukhova, O.; Haigl, B.; Slavic, S.; Schüler, C.; Kollmann, K.; Kantner, I.; Sexl, V.; Kleiter, M.; et al. Estrogen Regulates Bone Turnover by Targeting RANKL Expression in Bone Lining Cells. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Chen, X.; Garner, S.C.; Quarles, L.D.; Anderson, J.B. Effects of genistein on expression of bone markers during MC3T3-E1 osteoblastic cell differentiation. J. Nutr. Biochem. 2003, 14, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Kousteni, S.; Bellido, T.; Plotkin, L.I.; O’Brien, C.A.; Bodenner, D.L.; Han, L.; Han, K.; DiGregorio, G.B.; Katzenellenbogen, J.A.; Katzenellenbogen, B.S.; et al. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: Dissociation from transcriptional activity. Cell 2001, 104, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.H.; Yamaguchi, M. Suppressive effect of genistein on rat bone osteoclasts: Involvement of protein kinase inhibition and protein tyrosine phosphatase activation. Int. J. Mol. Med. 2000, 5, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Mobeen, I.; Romero, M.A.; Yulaevna, I.M.; Attar, R.; Jabeen, S.; Fayyaz, S. Regulation of Cell Signaling Pathways by Genistein in Different Cancers: Progress, Prospects and Pitfalls. Cell Mol. Biol. 2022, 67, 318–329. [Google Scholar] [CrossRef]
- Yi, S.; Chen, S.; Xiang, J.; Tan, J.; Huang, K.; Zhang, H.; Wang, Y.; Wu, H. Genistein exerts a cell-protective effect via Nrf2/HO-1/ /PI3K signaling in Ab25-35-induced Alzheimer’s disease models in vitro. Folia Histochem. Cytobiol. 2021, 59, 49–56. [Google Scholar] [CrossRef]
- Ming, L.G.; Chen, K.M.; Xian, C.J. Functions and action mechanisms of flavonoids genistein and icariin in regulating bone remodeling. J. Cell Physiol. 2013, 228, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Quarles, L.D.; Song, L.-H.; Yu, Y.-H.; Jiao, C.; Tang, H.-B.; Jiang, C.-H.; Deng, H.-W.; Li, Y.-J.; Zhou, H.-H.; et al. Genistein stimulates the osteoblastic differentiation via NO/cGMP in bone marrow culture. J. Cell Biochem. 2005, 94, 307–316. [Google Scholar] [CrossRef]
- Ghayor, C.; Weber, F.E. Epigenetic Regulation of Bone Remodeling and Its Impacts in Osteoporosis. Int. J. Mol. Sci. 2016, 17, 1446. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-D.; Yoon, W.-J.; Kim, W.-J.; Woo, K.-M.; Baek, J.-H.; Lee, G.; Ku, Y.; van Wijnen, A.J.; Ryoo, H.-M. Epigenetic Modifications and Canonical Wingless/int-1 Class (WNT) Signaling Enable Trans-differentiation of Nonosteogenic Cells into Osteoblasts. J. Biol. Chem. 2014, 289, 20120–20128. [Google Scholar] [CrossRef] [PubMed]
- Amado, N.G.; Fonseca, B.F.; Cerqueira, D.M.; Neto, V.M.; Abreu, J.G. Flavonoids: Potential Wnt/beta-catenin signaling modulators in cancer. Life Sci. 2011, 89, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Loughlin, J.; Dowling, B.; Chapman, K.; Marcelline, L.; Mustafa, Z.; Southam, L.; Ferreira, A.; Ciesielski, C.; Carson, D.A.; Corr, M. Functional variants within the secreted frizzled-related protein 3 gene are associated with hip osteoarthritis in females. Proc. Natl. Acad. Sci. USA 2004, 101, 9757–9762. [Google Scholar] [CrossRef]
- Tarapore, R.S.; Siddiqui, I.A.; Mukhtar, H. Modulation of Wnt/β-catenin signaling pathway by bioactive food components. Carcinogenesis 2012, 33, 483–491. [Google Scholar] [CrossRef]
- Wang, W.; Sun, Y.; Guo, Y.; Cai, P.; Li, Y.; Liu, J.; Cai, G.; Kiyoshi, A.; Zhang, W. Continuous soy isoflavones exposure from weaning to maturity induces downregulation of ovarian steroidogenic factor 1 gene expression and corresponding changes in DNA methylation pattern. Toxicol. Lett. 2017, 281, 175–183. [Google Scholar] [CrossRef]
- Schimmer, B.P.; White, P.C. Minireview: Steroidogenic factor 1: Its roles in differentiation, development, and disease. Mol. Endocrinol. 2010, 24, 1322–1337. [Google Scholar] [CrossRef]
- Sato, N.; Yamakawa, N.; Masuda, M.; Sudo, K.; Hatada, I.; Muramatsu, M. Genome-wide DNA methylation analysis reveals phytoestrogen modification of promoter methylation patterns during embryonic stem cell differentiation. PLoS ONE 2011, 6, e19278. [Google Scholar] [CrossRef] [PubMed]
- Kaludjerovic, J.; Ward, W.E. Bone-specific gene expression patterns and whole bone tissue of female mice are programmed by early life exposure to soy isoflavones and folic acid. J. Nutr. Biochem. 2015, 26, 1068–1076. [Google Scholar] [CrossRef]
- Day, J.K.; Bauer, A.M.; Desbordes, C.; Zhuang, Y.; Kim, B.-E.; Newton, L.G.; Nehra, V.; Forsee, K.M.; MacDonald, R.S.; Besch-Williford, C.; et al. Genistein alters methylation patterns in mice. J. Nutr. 2002, 132, 2419S–2423S. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.C.; Zhang, Y. The Role of NPY in the Regulation of Bone Metabolism. Front. Endocrinol. 2022, 13, 833485. [Google Scholar] [CrossRef] [PubMed]
- Pistollato, F.; Masias, M.; Agudo, P.; Giampieri, F.; Battino, M. Effects of phytochemicals on thyroid function and their possible role in thyroid disease. Ann. N. Y. Acad. Sci. 2019, 1443, 3–19. [Google Scholar] [CrossRef]
- Chin, K.Y.; Ima-Nirwana, S.; Mohamed, I.N.; Aminuddin, A.; Johari, M.H.; Ngah, W.Z.W. Thyroid-stimulating hormone is significantly associated with bone health status in men. Int. J. Med. Sci. 2013, 10, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Tuchendler, D.; Bolanowski, M. The influence of thyroid dysfunction on bone metabolism. Thyroid. Res. 2014, 7, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Tonstad, S.; Jaceldo-Siegl, K.; Messina, M.; Haddad, E.; Fraser, G.E. The association between soya consumption and serum thyroid-stimulating hormone concentrations in the Adventist Health Study-2. Public Health Nutr. 2016, 19, 1464–1470. [Google Scholar] [CrossRef]
- Janulewicz, P.A.; Carlson, J.M.; Wesselink, A.K.; A Wise, L.; E Hatch, E.; Edwards, L.M.; Peters, J.L. Urinary Isoflavones Levels in Relation to Thyroid Function in Adults from NHANES 2007–2010. ISEE Conf. Abstr. 2018, 2018, 24. [Google Scholar] [CrossRef]
- Doerge, D.R.; Sheehan, D.M. Goitrogenic and estrogenic activity of soy isoflavones. Environ. Health Perspect. 2002, 10, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Radović, B.; Mentrup, B.; Köhrle, J. Genistein and other soya isoflavones are potent ligands for transthyretin in serum and cerebrospinal fluid. Br. J. Nutr. 2006, 95, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Šošić-Jurjević, B.; Ajdžanović, V.; Filipović, B.; Severs, W.; Milošević, V. Thyroid Mediation of the Isoflavone Effects on Osteoporotic Bone: The Endocrine Interference with a Beneficial Outcome. Front. Endocrinol. 2019, 10, 486472. [Google Scholar] [CrossRef] [PubMed]
- Ariyani, W.; Iwasaki, T.; Miyazaki, W.; Yu, L.; Takeda, S.; Koibuchi, N. A Possible Novel Mechanism of Action of Genistein and Daidzein for Activating Thyroid Hormone Receptor-Mediated Transcription. Toxicol. Sci. 2018, 164, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Swart, A.C.; Johannes, I.D.; Sathyapalan, T.; Atkin, S.L. The Effect of Soy Isoflavones on Steroid Metabolism. Front. Endocrinol. 2019, 10, 229. [Google Scholar] [CrossRef] [PubMed]
- Palha, J.A.; Episkopou, V.; Maeda, S.; Shimada, K.; Gottesman, M.E.; Saraiva, M.J. Thyroid hormone metabolism in a transthyretin-null mouse strain. J. Biol. Chem. 1994, 269, 33135–33139. [Google Scholar] [CrossRef]
- Cepeda, S.B.; Sandoval, M.J.; Crescitelli, M.C.; Rauschemberger, M.B.; Massheimer, V.L. The isoflavone genistein enhances osteoblastogenesis: Signaling pathways involved. J. Physiol. Biochem. 2020, 76, 99–110. [Google Scholar] [CrossRef]
- Kim, M.; Lim, J.; Lee, J.H.; Lee, K.M.; Kim, S.; Park, K.W.; Nho, C.W.; Cho, Y.S. Understanding the functional role of genistein in the bone differentiation in mouse osteoblastic cell line MC3T3-E1 by RNA-seq analysis. Sci. Rep. 2018, 8, 3257. [Google Scholar] [CrossRef]
- Patisaul, H.B. Endocrine disruption by dietary phyto-oestrogens: Impact on dimorphic sexual systems and behaviours. Proc. Nutr. Soc. 2017, 76, 130–144. [Google Scholar] [CrossRef]
- Singh, P.; Sharma, S.; Rath, S.K. Genistein induces deleterious effects during its acute exposure in Swiss mice. BioMed Res. Int. 2014, 619617, 1–14. [Google Scholar] [CrossRef]
- Pejčić, T.; Zeković, M.; Bumbaširević, U.; Kalaba, M.; Vovk, I.; Bensa, M.; Popović, L.; Tešić, Ž. The Role of Isoflavones in the Prevention of Breast Cancer and Prostate Cancer. Antioxidants 2023, 12, 368. [Google Scholar] [CrossRef]
- Pawlicka, M.A.; Zmorzyński, S.; Popek-Marciniec, S.; Filip, A.A. The Effects of Genistein at Different Concentrations on MCF-7 Breast Cancer Cells and BJ Dermal Fibroblasts. Int. J. Mol. Sci. 2022, 23, 12360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, G.; Zeng, X.; Wu, Y.; Yang, C.; Mei, L.; Wang, Z.; Huang, L. Fabrication of genistein-loaded biodegradable TPGS-b-PCL nanoparticles for improved therapeutic effects in cervical cancer cells. Int. J. Nanomed. 2015, 10, 2461–2473. [Google Scholar]
- Yu, L.; Rios, E.; Castro, L.; Liu, J.; Yan, Y.; Dixon, D. Genistein: Dual Role in Women’s Health. Nutrients 2021, 13, 3048. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-A.; Wen, W.; Xiang, Y.-B.; Barnes, S.; Liu, D.; Cai, Q.; Zheng, W.; Shu, X.O. Assessment of Dietary Isoflavone Intake among Middle-Aged Chinese Men. J. Nutr. 2007, 137, 1011–1016. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Sobue, T.; Sasaki, S.; Kobayashi, M.; Tsugane, S.; Arai, Y.; Uehara, M.; Adlercreutz, H.; Watanabe, S.; Takahashi, T.; et al. Validity and reproducibility of a self-administered food-frequency questionnaire to assess isoflavone intake in a japanese population in comparison with dietary records and blood and urine isoflavones. J. Nutr. 2001, 131, 2741–2747. [Google Scholar] [CrossRef] [PubMed]
- Pawlowski, J.W.; Martin, B.R.; McCabe, G.P.; Ferruzzi, M.G.; Weaver, C.M. Plum and Soy Aglycon Extracts Superior at Increasing Bone Calcium Retention in Ovariectomized Sprague Dawley Rats. J. Agric. Food Chem. 2014, 62, 6108–6117. [Google Scholar] [CrossRef] [PubMed]
- Jackson, I.L.; Zodda, A.; Gurung, G.; Pavlovic, R.; Kaytor, M.D.; Kuskowski, M.A.; Vujaskovic, Z. BIO 300, a nanosuspension of genistein, mitigates pneumonitis/fibrosis following high-dose radiation exposure in the C57L/J murine model. Br. J. Pharmacol. 2017, 174, 4738–4750. [Google Scholar] [CrossRef]
- Lesniak, A.; Salvati, A.; Santos-Martinez, M.J.; Radomski, M.W.; Dawson, K.A.; Åberg, C. Nanoparticle Adhesion to the Cell Membrane and Its Effect on Nanoparticle Uptake Efficiency. J. Am. Chem. Soc. 2013, 135, 1438–1444. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular Uptake of Nanoparticles: Journey Inside the Cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- Contini, C.; Hindley, J.W.; Macdonald, T.J.; Barritt, J.D.; Ces, O.; Quirke, N. Size dependency of gold nanoparticles interacting with model membranes. Commun. Chem. 2020, 3, 130. [Google Scholar] [CrossRef] [PubMed]
- Banys, K.; Giebultowicz, J.; Sobczak, M.; Wyrebiak, R.; Bielecki, W.; Wrzesien, R.; Bobrowska-Korczak, B. Effect of Genistein Supplementation on the Progression of Neoplasms and the Level of the Modified Nucleosides in Rats with Mammary Cancer. In Vivo 2021, 35, 2059–2072. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhu, R.; Liu, C.; Ma, R.; Wang, L.; Chen, B.; Li, L.; Niu, J.; Zhao, D.; Mo, F.; et al. Evaluation of Decalcification Techniques for Rat Femurs Using HE and Immunohistochemical Staining. BioMed Res. Int. 2017, 9050754, 1–6. [Google Scholar] [CrossRef]
- Safali, S.; Aydin, B.K.; Nayman, A.; Ugurluoglu, C. Effect of curcumin on bone healing: An experimental study in a rat model of femur fracture. Injury 2019, 50, 1915–1920. [Google Scholar] [CrossRef] [PubMed]
- Sier, J.H.; Thumser, A.E.; Plant, N.J. Linking physiologically-based pharmacokinetic and genome-scale metabolic networks to understand estradiol biology. BMC Syst. Biol. 2017, 11, 141. [Google Scholar] [CrossRef]
- Hamano, T.; Fujii, N.; Ito, T.; Imai, E.; Mikami, S.; Katayama, M.; Obi, Y. Low dose estrogen replacement therapy (ERT) for postmenopausal hemodialysis (HD) patients. Clin. Calcium 2005, 1, 161–166. [Google Scholar]
- Zielniok, K.; Gajewska, M.; Motyl, T. Molecular actions of 17β-estradiol and progesterone and their relationship with cellular signaling pathways. Postepy Hig. Med. Dosw. 2014, 68, 777–792. [Google Scholar] [CrossRef] [PubMed]
- Rosner, W. The Functions of Corticosteroid-Binding Globulin and Sex Hormone-Binding Globulin: Recent Advances. Endocr. Rev. 1990, 11, 80–91. [Google Scholar] [CrossRef]
- Mousavi, Y.; Adlercreutz, H. Genistein is an effective stimulator of sex hormone-binding globulin production in hepatocarcinoma human liver cancer cells and suppresses proliferation of these cells in culture. Steroids 1993, 58, 301–304. [Google Scholar] [CrossRef]
- Van Cromphaut, S.; Rummens, K.; Stockmans, I.; Van Herck, E.; Dijcks, F.; Ederveen, A.; Carmeliet, P.; Verhaeghe, J.; Bouillon, R.; Carmeliet, G. Intestinal Calcium Transporter Genes Are Upregulated by Estrogens and the Reproductive Cycle Through Vitamin D Receptor-Independent Mechanisms. J. Bone Miner. Res. 2003, 18, 1725–1736. [Google Scholar] [CrossRef]
- Reinwald, S.; Weaver, C.M. Soy Isoflavones and Bone Health: A Double-Edged Sword? J. Nat. Prod. 2006, 69, 450–459. [Google Scholar] [CrossRef]
- Nayeem, F.; Chen, N.W.; Nagamani, M.; Anderson, K.E.; Lu, L.J.W. Daidzein and genistein have differential effects in decreasing whole body bone mineral density but had no effect on hip and spine density in premenopausal women: A 2-year randomized, double-blind, placebo-controlled study. Nutr. Res. 2019, 68, 70–81. [Google Scholar] [CrossRef]
- Christakos, S.; Prince, R. Estrogen, Vitamin D, and Calcium Transport. J. Bone Miner. Res. 2003, 18, 1737–1739. [Google Scholar] [CrossRef]
- Goltzman, D.; Mannstadt, M.; Marcocci, C. Physiology of the Calcium-Parathyroid Hormone-Vitamin D Axis. Front. Horm. Res. 2018, 50, 1–13. [Google Scholar]
- Weise, M.; De-Levi, S.; Barnes, K.M.; Gafni, R.I.; Abad, V.; Baron, J. Effects of estrogen on growth plate senescence and epiphyseal fusion. Proc. Natl. Acad. Sci. USA 2001, 98, 6871–6876. [Google Scholar] [CrossRef] [PubMed]
- Klein-Nulend, J.; van Oers, R.F.M.; Bakker, A.D.; Bacabac, R.G. Bone cell mechanosensitivity, estrogen deficiency, and osteoporosis. J. Biomech. 2015, 48, 855–865. [Google Scholar] [CrossRef]
- Gittoes, N. Osteoporosis: Pathophysiology and Clinical Management. Clin. Endocrinol. 2003, 59, 826–837. [Google Scholar] [CrossRef]
- Pantelic, J.; Ajdzanovic, V.; Medigovic, I.; Mojic, M.; Trifunovic, S.; Milosevic, V.; Filipovic, B. Genistein affects parathyroid gland and NaPi 2a cotransporter in an animal model of the andropause. J. Physiol. Pharmacol. 2013, 64, 361–368. [Google Scholar] [PubMed]
- Branca, F. Dietary phyto-oestrogens and bone health. Proc. Nutr. Soc. 2003, 62, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.M.; Martin, B.R.; Jackson, G.S.; McCabe, G.P.; Nolan, J.R.; McCabe, L.D.; Barnes, S.; Reinwald, S.; Boris, M.E.; Peacock, M. Antiresorptive effects of phytoestrogen supplements compared with estradiol or risedronate in postmenopausal women using (41)Ca methodology. J. Clin. Endocrinol. Metab. 2009, 94, 3798–3805. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, W.; Yu, B.; Huang, J.; Sun, J.; Huo, J.; Liu, C. Effects of trans-resveratrol from Polygonum cuspidatum on bone loss using the ovariectomized rat model. J. Med. Food 2005, 8, 14–19. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Gao, Y.H.; Ma, Z.J. Synergistic effect of genistein and zinc on bone components in the femoral-metaphyseal tissues of female rats. J. Bone Miner. Metab. 2000, 18, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, S.; Gustafsson, J.A. Biological role of estrogen and estrogen receptors. Crit. Rev. Biochem. Mol. Biol. 2002, 37, 1–28. [Google Scholar] [CrossRef]
- Dang, Z.C.; Lowik, C. Dose-dependent effects of phytoestrogens on bone. Trends Endocrinol. Metab. 2005, 16, 207–213. [Google Scholar] [CrossRef]
- Button, B.J.; Patel, N. Phytoestrogens for osteoporosis. Clin. Rev. Bone Miner. Metab. 2004, 2, 341–356. [Google Scholar] [CrossRef]
- Chen, W.F.; Wong, M.S. Genistein modulates the effects of parathyroid hormone in human osteoblastic SaOS-2 cells. Br. J. Nutr. 2006, 95, 1039–1047. [Google Scholar] [CrossRef]
- Filipović, B.; Šošić-Jurjević, B.; Ajdžanović, V.; Živanović, J.; Manojlović-Stojanoski, M.; Nestorović, N.; Ristić, N.; Trifunović, S.; Milošević, V. The phytoestrogen genistein prevents trabecular bone loss and affects thyroid follicular cells in a male rat model of osteoporosis. J. Anat. 2018, 233, 204–212. [Google Scholar] [CrossRef]
- Lavi-Moshayoff, V.; Wasserman, G.; Meir, T.; Silver, J.; Naveh-Many, T. PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: A bone parathyroid feedback loop. Am. J. Physiol.-Ren. Physiol. 2010, 299, 882–889. [Google Scholar] [CrossRef]
- Biber, J.; Hernando, N.; Forster, I.; Murer, H. Regulation of phosphate transport in proximal tubules. Pflug. Arch. 2009, 458, 39–52. [Google Scholar] [CrossRef]
- Miao, Q.; Li, J.-G.; Miao, S.; Hu, N.; Zhang, J.; Zhang, S.; Xie, Y.-H.; Wang, J.-B.; Wang, S.-W. The Bone-Protective Effect of Genistein in the Animal Model of Bilateral Ovariectomy: Roles of Phytoestrogens and PTH/PTHR1 Against Post-Menopausal Osteoporosis. Int. J. Mol. Sci. 2011, 13, 56–70. [Google Scholar] [CrossRef]






| Groups | Final Body Weight ± SD (g) | Femur Weight ± SD (g) | Ratio ± SD (%) |
|---|---|---|---|
| Standard | 233.3 ± 17.3 | 0.969 ± 0.110 | 0.41 ± 0.03 |
| Macrogenistein | 214.5 ± 7.1 | 0.873 ± 0.087 | 0.41 ± 0.05 |
| Microgenistein | 225.9 ± 13.9 | 0.993 ± 0.057 | 0.44 ± 0.05 |
| Nanogenistein | 221.1 ± 10.4 | 0.950 ± 0.048 | 0.43 ± 0.03 |
| Groups → | Standard X ± SD | Macrogenistein X ± SD | Microgenistein X ± SD | Nanogenistein X ± SD |
|---|---|---|---|---|
| Diameter of the femoral bone shaft (µm) | 1221 ± 69 | 1141 ± 75 * (↓6%) | 1413 ± 67 * (↑15%) | 1367 ± 55 * (↑12%) |
| The thickness of the compact layer (µm) | 282 ± 16.47 | 239 ± 42.59 * (↓15%) | 360 ± 121 * (↑28%) | 356 ± 110 * (↑26%) |
| The total thickness of the diaphysis wall. (µm) | 32.05 ± 8.53 | 41.19 ± 4.75 * (↑29%) | 25.20 ± 5.65 ** (↓21%) | 36.68 ± 13.1 * (↑2%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skrajnowska, D.; Bielecki, W.; Szterk, A.; Ofiara, K.; Bobrowska-Korczak, B. Genistein Supplementation and Bone Health in Breast Cancer in Rats. Nutrients 2024, 16, 912. https://doi.org/10.3390/nu16060912
Skrajnowska D, Bielecki W, Szterk A, Ofiara K, Bobrowska-Korczak B. Genistein Supplementation and Bone Health in Breast Cancer in Rats. Nutrients. 2024; 16(6):912. https://doi.org/10.3390/nu16060912
Chicago/Turabian StyleSkrajnowska, Dorota, Wojciech Bielecki, Arkadiusz Szterk, Karol Ofiara, and Barbara Bobrowska-Korczak. 2024. "Genistein Supplementation and Bone Health in Breast Cancer in Rats" Nutrients 16, no. 6: 912. https://doi.org/10.3390/nu16060912





