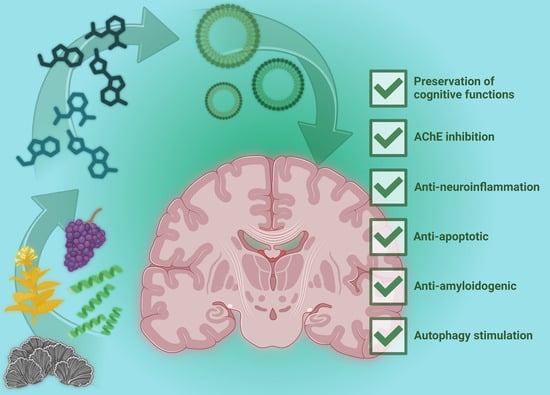
Exploiting Natural Niches with Neuroprotective Properties: A Comprehensive Review
Abstract
:1. Introduction
2. Active Natural Compounds and Targeted Bioactivities
2.1. Anti-Amyloidogenic Compounds
2.2. Inhibitors of AChE
2.3. Natural Compound with Anti-Apoptotic Effect
2.4. Autophagy Stimulation
2.5. Anti-Neuroinflammatory Compounds
2.6. Compounds Improving Cognitive Functions
3. Four Widely Explored Molecules with Neuroprotective Effects
3.1. Curcumin
3.2. Resveratrol
3.3. Ergothioneine
3.4. Phycocyanin and Spirulina Extract
4. Delivery Strategies
5. How a Better Understanding of Natural Molecules with Neuroprotective Effects Could Improve Their Utilization in the Next Decades
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a Risk Factor for Neurodegenerative Disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.M.; Cookson, M.R.; Van Den Bosch, L.; Zetterberg, H.; Holtzman, D.M.; Dewachter, I. Hallmarks of Neurodegenerative Diseases. Cell 2023, 186, 693–714. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A. Concept, Mechanism, and Applications of Phenolic Antioxidants in Foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.M.; Hossain, R.; Herrera-Bravo, J.; Islam, M.T.; Atolani, O.; Adeyemi, O.S.; Owolodun, O.A.; Kambizi, L.; Daştan, S.D.; Calina, D.; et al. Natural Antioxidants from Some Fruits, Seeds, Foods, Natural Products, and Associated Health Benefits: An Update. Food Sci. Nutr. 2023, 11, 1657–1670. [Google Scholar] [CrossRef] [PubMed]
- Sidiropoulou, G.A.; Metaxas, A.; Kourti, M. Natural Antioxidants That Act against Alzheimer’s Disease through Modulation of the NRF2 Pathway: A Focus on Their Molecular Mechanisms of Action. Front. Endocrinol. 2023, 14, 1217730. [Google Scholar] [CrossRef] [PubMed]
- Chon, S.H.; Yang, E.J.; Lee, T.; Song, K.S. β-Secretase (BACE1) Inhibitory and Neuroprotective Effects of p-Terphenyls from Polyozellus Multiplex. Food Funct. 2016, 7, 3834–3842. [Google Scholar] [CrossRef]
- Tzeng, T.T.; Chen, C.C.; Chen, C.C.; Tsay, H.J.; Lee, L.Y.; Chen, W.P.; Shen, C.C.; Shiao, Y.J. The Cyanthin Diterpenoid and Sesterterpene Constituents of Hericium erinaceus Mycelium Ameliorate Alzheimer’s Disease-Related Pathologies in APP/PS1 Transgenic Mice. Int. J. Mol. Sci. 2018, 19, 598. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.F.; Tung, S.Y.; Teng, C.C.; Shen, C.H.; Hsieh, M.C.; Huang, C.Y.; Lee, K.C.; Lee, L.Y.; Chen, W.P.; Chen, C.C.; et al. Post-Treatment with Erinacine A, a Derived Diterpenoid of H. Erinaceus, Attenuates Neurotoxicity in MPTP Model of Parkinson’s Disease. Antioxidants 2020, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Jin, H.; Sun, Q.R.; Xu, J.H.; Hu, H.T. The Neuroprotective Effects of Tanshinone IIA on β-Amyloid-Induced Toxicity in Rat Cortical Neurons. Neuropharmacology 2010, 59, 595–604. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, X.; Patal, K.; Hu, R.; Chuang, S.; Zhang, G.; Zheng, J. Tanshinones Inhibit Amyloid Aggregation by Amyloid-β Peptide, Disaggregate Amyloid Fibrils, and Protect Cultured Cells. ACS Chem. Neurosci. 2013, 4, 1004–1015. [Google Scholar] [CrossRef]
- Leri, M.; Natalello, A.; Bruzzone, E.; Stefani, M.; Bucciantini, M. Oleuropein Aglycone and Hydroxytyrosol Interfere Differently with Toxic Aβ1-42 Aggregation. Food Chem. Toxicol. 2019, 129, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Porzoor, A.; Alford, B.; Hügel, H.M.; Grando, D.; Caine, J.; Macreadie, I. Anti-Amyloidogenic Properties of Some Phenolic Compounds. Biomolecules 2015, 5, 505–527. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Huang, D.; Zhang, M.H.; Zhang, W.S.; Tang, Y.X.; Shi, Z.X.; Deng, L.; Zhou, D.H.; Lu, X.Y. Salvianolic Acid B Inhibits Aβ Generation by Modulating BACE1 Activity in SH-SY5Y-APPsw Cells. Nutrients 2016, 8, 333. [Google Scholar] [CrossRef] [PubMed]
- Rezai-Zadeh, K.; Shytle, D.; Sun, N.; Mori, T.; Hou, H.; Jeanniton, D.; Ehrhart, J.; Townsend, K.; Zeng, J.; Morgan, D.; et al. Green Tea Epigallocatechin-3-Gallate (EGCG) Modulates Amyloid Precursor Protein Cleavage and Reduces Cerebral Amyloidosis in Alzheimer Transgenic Mice. J. Neurosci. 2005, 25, 8807–8814. [Google Scholar] [CrossRef] [PubMed]
- Ehrnhoefer, D.E.; Bieschke, J.; Boeddrich, A.; Herbst, M.; Masino, L.; Lurz, R.; Engemann, S.; Pastore, A.; Wanker, E.E. EGCG Redirects Amyloidogenic Polypeptides into Unstructured, off-Pathway Oligomers. Nat. Struct. Mol. Biol. 2008, 15, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Bieschke, J.; Russ, J.; Friedrich, R.P.; Ehrnhoefer, D.E.; Wobst, H.; Neugebauer, K.; Wanker, E.E. EGCG Remodels Mature α-Synuclein and Amyloid-β Fibrils and Reduces Cellular Toxicity. Proc. Natl. Acad. Sci. USA 2010, 107, 7710–7715. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.S.; Ahn, B.R.; Choi, J.S.; Hattori, M.; Min, B.; Bae, K.H. Selective Cholinesterase Inhibition by Lanostane Triterpenes from Fruiting Bodies of Ganoderma Lucidum. Bioorganic Med. Chem. Lett. 2011, 21, 6603–6607. [Google Scholar] [CrossRef] [PubMed]
- Becher, P.G.; Beuchat, J.; Gademann, K.; Jüttner, F. Nostocarboline: Isolation and Synthesis of a New Cholinesterase Inhibitor from Nostoc 78-12A. J. Nat. Prod. 2005, 68, 1793–1795. [Google Scholar] [PubMed]
- Mahmood, N.A.; Carmichael, W.W. Anatoxin-a(s), an Anticholinesterase from the Cyanobacterium Anabaena Flos-Aquae NRC-525-17. Toxicon 1987, 25, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, J.E.; Berkov, S.; Pigni, N.B.; Theoduloz, C.; Roitman, G.; Tapia, A.; Bastida, J.; Feresin, G.E. Wild Argentinian Amaryllidaceae, a New Renewable Source of the Acetylcholinesterase Inhibitor Galanthamine and Other Alkaloids. Molecules 2012, 17, 13473–13482. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, J.; Ren, J.; Wang, W.; Xiong, W.; Zhang, Y.; Bao, L.; Liu, H. Triterpenes and Meroterpenes with Neuroprotective Effects from Ganoderma Leucocontextum. Chem. Biodivers. 2018, 15, 1700567. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Huang, Y.; Jiang, Y.; Zou, L.; Liu, X.; Liu, S.; Chen, F.; Luo, J.; Zhu, Y. Ganoderma Lucidum Triterpenoids (GLTs) Reduce Neuronal Apoptosis via Inhibition of ROCK Signal Pathway in APP/PS1 Transgenic Alzheimer’s Disease Mice. Oxid. Med. Cell. Longev. 2020, 2020, 9894037. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Huang, J.; Ma, B.; Yang, B.; Chang, D.; Liu, J. Astragaloside IV Protects Primary Cerebral Cortical Neurons from Oxygen and Glucose Deprivation/Reoxygenation by Activating the PKA/CREB Pathway. Neuroscience 2019, 404, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Guo, D.; Chen, B. Neuroprotective Effects of Astragaloside IV on Parkinson Disease Models of Mice and Primary Astrocytes. Exp. Ther. Med. 2017, 14, 5569–5575. [Google Scholar] [CrossRef]
- Zeitlin, R.; Patel, S.; Burgess, S.; Arendash, G.W.; Echeverria, V. Caffeine Induces Beneficial Changes in PKA Signaling and JNK and ERK Activities in the Striatum and Cortex of Alzheimer’s Transgenic Mice. Brain Res. 2011, 1417, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Nakaso, K.; Ito, S.; Nakashima, K. Caffeine Activates the PI3K/Akt Pathway and Prevents Apoptotic Cell Death in a Parkinson’s Disease Model of SH-SY5Y Cells. Neurosci. Lett. 2008, 432, 146–150. [Google Scholar] [CrossRef]
- Cos, P.; Li, Y.; Calomme, M.; Hu, J.P.; Cimanga, K.; Van Poel, B.; Pieters, L.; Vlietinck, A.J.; Berghe, D. Vanden Structure-Activity Relationship and Classification of Flavonoids as Inhibitors of Xanthine Oxidase and Superoxide Scavengers. J. Nat. Prod. 1998, 61, 71–76. [Google Scholar] [CrossRef]
- Watanabe, R.; Kurose, T.; Morishige, Y.; Fujimori, K. Protective Effects of Fisetin Against 6-OHDA-Induced Apoptosis by Activation of PI3K-Akt Signaling in Human Neuroblastoma SH-SY5Y Cells. Neurochem. Res. 2018, 43, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, H.; Zhou, Y.; Zhu, Y.; Fei, M. Fisetin Alleviates Oxidative Stress after Traumatic Brain Injury via the Nrf2-ARE Pathway. Neurochem. Int. 2018, 118, 304–313. [Google Scholar] [CrossRef]
- Yang, W.; Tian, Z.K.; Yang, H.X.; Feng, Z.J.; Sun, J.M.; Jiang, H.; Cheng, C.; Ming, Q.L.; Liu, C.M. Fisetin Improves Lead-Induced Neuroinflammation, Apoptosis and Synaptic Dysfunction in Mice Associated with the AMPK/SIRT1 and Autophagy Pathway. Food Chem. Toxicol. 2019, 134, 110824. [Google Scholar] [CrossRef]
- Prakash, D.; Sudhandiran, G. Dietary Flavonoid Fisetin Regulates Aluminium Chloride-Induced Neuronal Apoptosis in Cortex and Hippocampus of Mice Brain. J. Nutr. Biochem. 2015, 26, 1527–1539. [Google Scholar] [CrossRef] [PubMed]
- Maitra, U.; Harding, T.; Liang, Q.; Ciesla, L. GardeninA Confers Neuroprotection against Environmental Toxin in a Drosophila Model of Parkinson’s Disease. Commun. Biol. 2021, 4, 162. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Ren, X.; Zheng, W.; Zeng, Z.; Guo, Y.; Hou, Z.; Guo, W.; Chen, X.; Li, F.; Chen, J.F. Chronic Caffeine Treatment Protects against α-Synucleinopathy by Reestablishing Autophagy Activity in the Mouse Striatum. Front. Neurosci. 2018, 12, 336493. [Google Scholar] [CrossRef]
- Qu, L.; Liang, X.C.; Gu, B.; Liu, W. Quercetin Alleviates High Glucose-Induced Schwann Cell Damage by Autophagy. Neural Regen. Res. 2014, 9, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Regitz, C.; Dußling, L.M.; Wenzel, U. Amyloid-Beta (Aβ1–42)-Induced Paralysis in Caenorhabditis Elegans Is Inhibited by the Polyphenol Quercetin through Activation of Protein Degradation Pathways. Mol. Nutr. Food Res. 2014, 58, 1931–1940. [Google Scholar] [CrossRef] [PubMed]
- Yıldız, M.O.; Çelik, H.; Caglayan, C.; Kandemir, F.M.; Gür, C.; Bayav, İ.; Genç, A.; Kandemir, Ö. Neuromodulatory Effects of Hesperidin against Sodium Fluoride-Induced Neurotoxicity in Rats: Involvement of Neuroinflammation, Endoplasmic Reticulum Stress, Apoptosis and Autophagy. Neurotoxicology 2022, 90, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Moon, J.-H.; Kim, S.-W.; Jeong, J.-K.; Nazim, U.M.D.; Lee, Y.-J.; Seol, J.-W.; Park, S.-Y.; Lee, J.-H.; Moon, J.-H.; et al. EGCG-Mediated Autophagy Flux Has a Neuroprotection Effect via a Class III Histone Deacetylase in Primary Neuron Cells. Oncotarget 2015, 6, 9701–9717. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Choi, K.J.; Cho, S.J.; Yun, S.M.; Jeon, J.P.; Koh, Y.H.; Song, J.; Johnson, G.V.W.; Jo, C. Fisetin Stimulates Autophagic Degradation of Phosphorylated Tau via the Activation of TFEB and Nrf2 Transcription Factors. Sci. Rep. 2016, 6, 24933. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cui, J.; Li, D.; Ran, S.; Huang, J.; Chen, G. Morin Exhibits a Neuroprotective Effect in MPTP-Induced Parkinson’s Disease Model via TFEB/AMPK-Mediated Mitophagy. Phytomedicine 2023, 116, 154866. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Chen, L.; Chen, Y.; Chen, X.; Dong, Y.; Zheng, S.; Zhang, L.; Li, W.; Du, J.; Li, H. A Maitake (Grifola frondosa) Polysaccharide Ameliorates Alzheimer’s Disease-like Pathology and Cognitive Impairments by Enhancing Microglial Amyloid-β Clearance. RSC Adv. 2019, 9, 37127–37135. [Google Scholar] [CrossRef]
- Lee, S.L.; Hsu, J.Y.; Chen, T.C.; Huang, C.C.; Wu, T.Y.; Chin, T.Y. Erinacine A Prevents Lipopolysaccharide-Mediated Glial Cell Activation to Protect Dopaminergic Neurons against Inflammatory Factor-Induced Cell Death In Vitro and In Vivo. Int. J. Mol. Sci. 2022, 23, 810. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Sun, J.; Miao, Z.; Chen, S.; Yang, G. Astragaloside IV Attenuates Neuroinflammation and Ameliorates Cognitive Impairment in Alzheimer’s Disease via Inhibiting NF-ΚB Signaling Pathway. Heliyon 2023, 9, e13411. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Qin, J.; Liu, M.; Luo, J.; Ding, F.; Wang, M.; Zheng, L. Astragalus Polysaccharide Attenuates Lipopolysaccharide-Induced Inflammatory Responses in Microglial Cells: Regulation of Protein Kinase B and Nuclear Factor-ΚB Signaling. Inflamm. Res. 2015, 64, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.X.; Ge, C.X.; Li, Q.; Lou, D.S.; Hu, L.F.; Sun, Y.; Xiong, M.X.; Lai, L.L.; Zhong, S.Y.; Yi, C.; et al. Fisetin Nanoparticles Protect against PM2.5 Exposure-Induced Neuroinflammation by down-Regulation of Astrocytes Activation Related NF-ΚB Signaling Pathway. J. Funct. Foods 2020, 65, 103716. [Google Scholar] [CrossRef]
- Lin, Y.L.; Lin, J.K. (−)-Epigallocatechin-3-Gallate Blocks the Induction of Nitric Oxide Synthase by Down-Regulating Lipopolysaccharide-Induced Activity of Transcription Factor Nuclear Factor-ΚB. Mol. Pharmacol. 1997, 52, 465–472. [Google Scholar] [CrossRef]
- Payne, A.; Taka, E.; Adinew, G.M.; Soliman, K.F.A. Molecular Mechanisms of the Anti-Inflammatory Effects of Epigallocatechin 3-Gallate (EGCG) in LPS-Activated BV-2 Microglia Cells. Brain Sci. 2023, 13, 632. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y.; Barro, L.; Tsai, S.T.; Feng, T.W.; Wu, X.Y.; Chao, C.W.; Yu, R.S.; Chin, T.Y.; Hsieh, M.F. Epigallocatechin-3-Gallate-Loaded Liposomes Favor Anti-Inflammation of Microglia Cells and Promote Neuroprotection. Int. J. Mol. Sci. 2021, 22, 3037. [Google Scholar] [CrossRef]
- Yadav, S.; Gupta, S.P.; Srivastava, G.; Srivastava, P.K.; Singh, M.P. Role of Secondary Mediators in Caffeine-Mediated Neuroprotection in Maneb- and Paraquat-Induced Parkinson’s Disease Phenotype in the Mouse. Neurochem. Res. 2012, 37, 875–884. [Google Scholar]
- Kang, C.H.; Jayasooriya, R.G.P.T.; Dilshara, M.G.; Choi, Y.H.; Jeong, Y.K.; Kim, N.D.; Kim, G.Y. Caffeine Suppresses Lipopolysaccharide-Stimulated BV2 Microglial Cells by Suppressing Akt-Mediated NF-ΚB Activation and ERK Phosphorylation. Food Chem. Toxicol. 2012, 50, 4270–4276. [Google Scholar] [CrossRef]
- Badshah, H.; Ikram, M.; Ali, W.; Ahmad, S.; Hahm, J.R.; Kim, M.O. Caffeine May Abrogate LPS-Induced Oxidative Stress and Neuroinflammation by Regulating Nrf2/TLR4 in Adult Mouse Brains. Biomolecules 2019, 9, 719. [Google Scholar] [CrossRef]
- Nobre, H.V.; de Andrade Cunha, G.M.; de Vasconcelos, L.M.; Magalhães, H.I.F.; Neto, R.N.O.; Maia, F.D.; de Moraes, M.O.; Leal, L.K.A.M.; de Barros Viana, G.S. Caffeine and CSC, Adenosine A2A Antagonists, Offer Neuroprotection against 6-OHDA-Induced Neurotoxicity in Rat Mesencephalic Cells. Neurochem. Int. 2010, 56, 51–58. [Google Scholar] [CrossRef]
- Chen, X.; Gawryluk, J.W.; Wagener, J.F.; Ghribi, O.; Geiger, J.D. Caffeine Blocks Disruption of Blood Brain Barrier in a Rabbit Model of Alzheimer’s Disease. J. Neuroinflamm. 2008, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Inatomi, S.; Ouchi, K.; Azumi, Y.; Tuchida, T. Improving Effects of the Mushroom Yamabushitake (Hericium erinaceus) on Mild Cognitive Impairment: A Double-Blind Placebo-Controlled Clinical Trial. Phytother. Res. 2009, 23, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Saitsu, Y.; Nishide, A.; Kikushima, K.; Shimizu, K.; Ohnuki, K. Improvement of Cognitive Functions by Oral Intake of Hericium erinaceus. Biomed. Res. 2019, 40, 125–131. [Google Scholar] [CrossRef]
- Meng, N.; Li, M.; Xu, J.; Guan, T.; Jin, M.; Teng, Z.; Zhao, L.; Fan, M.; Hao, H.; Lv, P. Astragaloside IV Improves Cognitive Impairment Caused by CCH via Improve ROS and NLRP3 Pathway by Up-Regulating the PGC1α/Nrf2 Pathway. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Arendash, G.W.; Mori, T.; Cao, C.; Mamcarz, M.; Runfeldt, M.; Dickson, A.; Rezai-Zadeh, K.; Tan, J.; Citron, B.A.; Lin, X.; et al. Caffeine Reverses Cognitive Impairment and Decreases Brain Amyloid-β Levels in Aged Alzheimer’s Disease Mice. J. Alzheimer’s Dis. 2009, 17, 661–680. [Google Scholar] [CrossRef]
- Dall’Igna, O.P.; Fett, P.; Gomes, M.W.; Souza, D.O.; Cunha, R.A.; Lara, D.R. Caffeine and Adenosine A2a Receptor Antagonists Prevent β-Amyloid (25–35)-Induced Cognitive Deficits in Mice. Exp. Neurol. 2007, 203, 241–245. [Google Scholar] [CrossRef]
- Espinosa, J.; Rocha, A.; Nunes, F.; Costa, M.S.; Schein, V.; Kazlauckas, V.; Kalinine, E.; Souza, D.O.; Cunha, R.A.; Porciúncula, L.O. Caffeine Consumption Prevents Memory Impairment, Neuronal Damage, and Adenosine A 2A Receptors Upregulation in the Hippocampus of a Rat Model of Sporadic Dementia. J. Alzheimer’s Dis. 2013, 34, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, J.; Laurin, D.; Verreault, R.; Hébert, R.; Helliwell, B.; Hill, G.B.; McDowell, I. Risk Factors for Alzheimer’s Disease: A Prospective Analysis from the Canadian Study of Health and Aging. Am. J. Epidemiol. 2002, 156, 445–453. [Google Scholar] [CrossRef]
- Eskelinen, M.H.; Kivipelto, M. Caffeine as a Protective Factor in Dementia and Alzheimer’s Disease. J. Alzheimer’s Dis. 2010, 20, S167–S174. [Google Scholar] [CrossRef]
- Cho, B.H.; Choi, S.M.; Kim, J.T.; Kim, B.C. Association of Coffee Consumption and Non-Motor Symptoms in Drug-Naïve, Early-Stage Parkinson’s Disease. Park. Relat. Disord. 2018, 50, 42–47. [Google Scholar] [CrossRef]
- Guo, Y.; Zhao, Y.; Nan, Y.; Wang, X.; Chen, Y.; Wang, S. (-)-Epigallocatechin-3-Gallate Ameliorates Memory Impairment and Rescues the Abnormal Synaptic Protein Levels in the Frontal Cortex and Hippocampus in a Mouse Model of Alzheimer’s Disease. Neuroreport 2017, 28, 590–597. [Google Scholar] [CrossRef]
- de la Torre, R.; de Sola, S.; Hernandez, G.; Farré, M.; Pujol, J.; Rodriguez, J.; Espadaler, J.M.; Langohr, K.; Cuenca-Royo, A.; Principe, A.; et al. Safety and Efficacy of Cognitive Training plus Epigallocatechin-3-Gallate in Young Adults with Down’s Syndrome (TESDAD): A Double-Blind, Randomised, Placebo-Controlled, Phase 2 Trial. Lancet Neurol. 2016, 15, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Scholey, A.; Downey, L.A.; Ciorciari, J.; Pipingas, A.; Nolidin, K.; Finn, M.; Wines, M.; Catchlove, S.; Terrens, A.; Barlow, E.; et al. Acute Neurocognitive Effects of Epigallocatechin Gallate (EGCG). Appetite 2012, 58, 767–770. [Google Scholar] [CrossRef]
- Mohammadi, N.; Asle-Rousta, M.; Rahnema, M.; Amini, R. Morin Attenuates Memory Deficits in a Rat Model of Alzheimer’s Disease by Ameliorating Oxidative Stress and Neuroinflammation. Eur. J. Pharmacol. 2021, 910, 174506. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.T.; Cao, X.B.; Xiong, N.; Wang, H.C.; Huang, J.S.; Sun, S.G.; Wang, T. Morin Exerts Neuroprotective Actions in Parkinson Disease Models In Vitro and In Vivo. Acta Pharmacol. Sin. 2010, 31, 900–906. [Google Scholar] [CrossRef]
- Alharbi, M.H.; Lamport, D.J.; Dodd, G.F.; Saunders, C.; Harkness, L.; Butler, L.T.; Spencer, J.P.E. Flavonoid-Rich Orange Juice Is Associated with Acute Improvements in Cognitive Function in Healthy Middle-Aged Males. Eur. J. Nutr. 2016, 55, 2021–2029. [Google Scholar] [CrossRef] [PubMed]
- Matias, I.; Diniz, L.P.; Buosi, A.; Neves, G.; Stipursky, J.; Gomes, F.C.A. Flavonoid Hesperidin Induces Synapse Formation and Improves Memory Performance through the Astrocytic TGF-Β1. Front. Aging Neurosci. 2017, 9, 267627. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.S.; Mona, M.M.; Elsisy, R.A.; Hantash, E.M. Hesperidin Preserves Cognitive Functions and Hippocampus Histological Architecture in Albino Wistar Rats Subjected to Stress through Enhancement of Brain-Derived Neurotrophic Factor. Neurotox. Res. 2022, 40, 179–185. [Google Scholar] [CrossRef]
- Lamport, D.J.; Pal, D.; Macready, A.L.; Barbosa-Boucas, S.; Fletcher, J.M.; Williams, C.M.; Spencer, J.P.E.; Butler, L.T. The Effects of Flavanone-Rich Citrus Juice on Cognitive Function and Cerebral Blood Flow: An Acute, Randomised, Placebo-Controlled Cross-over Trial in Healthy, Young Adults. Br. J. Nutr. 2016, 116, 2160–2168. [Google Scholar] [CrossRef]
- Kean, R.J.; Lamport, D.J.; Dodd, G.F.; Freeman, J.E.; Williams, C.M.; Ellis, J.A.; Butler, L.T.; Spencer, J.P.E. Chronic Consumption of Flavanone-Rich Orange Juice Is Associated with Cognitive Benefits: An 8-Wk, Randomized, Double-Blind, Placebo-Controlled Trial in Healthy Older Adults. Am. J. Clin. Nutr. 2015, 101, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Tomata, Y.; Sugiyama, K.; Sugawara, Y.; Tsuji, I. Citrus Consumption and Incident Dementia in Elderly Japanese: The Ohsaki Cohort 2006 Study. Br. J. Nutr. 2017, 117, 1174–1180. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Kang, Z.; Zhang, J.; Qu, F.; Song, B. Neuroprotective Effects of Isoquercetin: An In Vitro and In Vivo Study. Cell J. (Yakhteh) 2021, 23, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.; Kim, H.G.; Choi, J.G.; Oh, H.; Lee, P.K.; Ha, S.K.; Kim, S.Y.; Park, Y.; Huh, Y.; Oh, M.S. 6-Shogaol, an Active Constituent of Ginger, Attenuates Neuroinflammation and Cognitive Deficits in Animal Models of Dementia. Biochem. Biophys. Res. Commun. 2014, 449, 8–13. [Google Scholar] [CrossRef]
- Na, J.Y.; Song, K.; Lee, J.W.; Kim, S.; Kwon, J. 6-Shogaol Has Anti-Amyloidogenic Activity and Ameliorates Alzheimer’s Disease via CysLT1R-Mediated Inhibition of Cathepsin B. Biochem. Biophys. Res. Commun. 2016, 477, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Hou, C.; Yang, Z.; Li, C.; Jia, L.; Liu, J.; Tang, Y.; Shi, L.; Li, Y.; Long, J.; et al. Hydroxytyrosol Mildly Improve Cognitive Function Independent of APP Processing in APP/PS1 Mice. Mol. Nutr. Food Res. 2016, 60, 2331–2342. [Google Scholar] [CrossRef] [PubMed]
- Nardiello, P.; Pantano, D.; Lapucci, A.; Stefani, M.; Casamenti, F. Diet Supplementation with Hydroxytyrosol Ameliorates Brain Pathology and Restores Cognitive Functions in a Mouse Model of Amyloid-β Deposition. J. Alzheimer’s Dis. 2018, 63, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Y.; Guo, J.; Sun, J.; Sun, Q. Salvianolic Acid B Improves Cognitive Impairment by Inhibiting Neuroinflammation and Decreasing Aβ Level in Porphyromonas gingivalis-Infected Mice. Aging 2020, 12, 10117–10128. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.; Chen, Y.; Guo, Y.; Wang, C.; Liu, N.; Gong, Q.; Fu, Y.; Fu, Y.; Cao, L.; Yao, D.; et al. Salvianolic Acid b Improves Chronic Mild Stress-Induced Depressive Behaviors in Rats: Involvement of AMPK/SIRT1 Signaling Pathway. J. Inflamm. Res. 2020, 13, 195–206. [Google Scholar] [CrossRef]
- He, Y.; Ruganzu, J.B.; Lin, C.; Ding, B.; Zheng, Q.; Wu, X.; Ma, R.; Liu, Q.; Wang, Y.; Jin, H.; et al. Tanshinone IIA Ameliorates Cognitive Deficits by Inhibiting Endoplasmic Reticulum Stress-Induced Apoptosis in APP/PS1 Transgenic Mice. Neurochem. Int. 2020, 133, 104610. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry, Nutrition, and Health-Promoting Properties of Hericium erinaceus (Lion’s Mane) Mushroom Fruiting Bodies and Mycelia and Their Bioactive Compounds. J. Agric. Food Chem. 2015, 63, 7108–7123. [Google Scholar] [CrossRef] [PubMed]
- Subedi, L.; Gaire, B.P. Tanshinone IIA: A Phytochemical as a Promising Drug Candidate for Neurodegenerative Diseases. Pharmacol. Res. 2021, 169, 105661. [Google Scholar] [CrossRef] [PubMed]
- Fitó, M.; Cladellas, M.; De La Torre, R.; Martí, J.; Alcántara, M.; Pujadas-Bastardes, M.; Marrugat, J.; Bruguera, J.; López-Sabater, M.C.; Vila, J.; et al. Antioxidant Effect of Virgin Olive Oil in Patients with Stable Coronary Heart Disease: A Randomized, Crossover, Controlled, Clinical Trial. Atherosclerosis 2005, 181, 149–158. [Google Scholar] [CrossRef] [PubMed]
- De La Cruz, J.P.; Ruiz-Moreno, M.I.; Guerrero, A.; López-Villodres, J.A.; Reyes, J.J.; Espartero, J.L.; Labajos, M.T.; González-Correa, J.A. Role of the Catechol Group in the Antioxidant and Neuroprotective Effects of Virgin Olive Oil Components in Rat Brain. J. Nutr. Biochem. 2015, 26, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Jaime, F.; Mendonça-Junior, B.; Scotti, M.T.; Muratov, E.N.; Scotti, L.; Nayarisseri, A. Editorial Natural Bioactive Products with Antioxidant Properties Useful in Neurodegenerative Diseases 2020. Oxidative Med. Cell. Longev. 2021, 2021, 6262316. [Google Scholar] [CrossRef] [PubMed]
- Rezai-Zadeh, K.; Arendash, G.W.; Hou, H.; Fernandez, F.; Jensen, M.; Runfeldt, M.; Shytle, R.D.; Tan, J. Green Tea Epigallocatechin-3-Gallate (EGCG) Reduces β-Amyloid Mediated Cognitive Impairment and Modulates Tau Pathology in Alzheimer Transgenic Mice. Brain Res. 2008, 1214, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.; Balaji, E.V.; Pai, S.R.K.; Kishore, A.; Pai, V.; Pemmireddy, R.; KS, C. Current Drug Targets in Alzheimer’s Associated Memory Impairment: A Comprehensive Review. CNS Neurol. Disord. Drug Targets 2022, 22, 255–275. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s Disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Yiannopoulou, K.G.; Papageorgiou, S.G. Current and Future Treatments for Alzheimer’s Disease. Ther. Adv. Neurol. Disord. 2012, 6, 19–33. [Google Scholar] [CrossRef]
- Almeida, J.R.; Freitas, M.; Cruz, S.; Leão, P.N.; Vasconcelos, V.; Cunha, I. Acetylcholinesterase in Biofouling Species: Characterization and Mode of Action of Cyanobacteria-Derived Antifouling Agents. Toxins 2015, 7, 2739–2756. [Google Scholar] [CrossRef]
- Castaneda, A.; Ferraz, R.; Vieira, M.; Cardoso, I.; Vasconcelos, V.; Martins, R. Bridging Cyanobacteria to Neurodegenerative Diseases: A New Potential Source of Bioactive Compounds against Alzheimer’s Disease. Mar. Drugs 2021, 19, 343. [Google Scholar] [CrossRef]
- Ayaz, M.; Blokland, A.; Heinrich, M.; Marcus de Andrade Paes, A.; Coelho dos Santos, T.; Mota Gomes, T.; Araújo Serra Pinto, B.; Leandro Camara, A. Naturally Occurring Acetylcholinesterase Inhibitors and Their Potential Use for Alzheimer’s Disease Therapy. Front. Pharmacol. 2018, 9, 1192. [Google Scholar] [CrossRef]
- Berkov, S.; Atanasova, M.; Georgiev, B.; Bastida, J.; Doytchinova, I. The Amaryllidaceae Alkaloids: An Untapped Source of Acetylcholinesterase Inhibitors. Phytochem. Rev. 2021, 21, 1415–1443. [Google Scholar] [CrossRef]
- Cortes, N.; Sierra, K.; Alzate, F.; Osorio, E.H.; Osorio, E. Alkaloids of Amaryllidaceae as Inhibitors of Cholinesterases (AChEs and BChEs): An Integrated Bioguided Study. Phytochem. Anal. 2018, 29, 217–227. [Google Scholar] [CrossRef]
- Nijhawan, D.; Honarpour, N.; Wang, X. Apoptosis in Neural Development and Disease. Annu. Rev. Neurosci. 2000, 23, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Heckman, M.A.; Weil, J.; de Mejia, E.G. Caffeine (1, 3, 7-Trimethylxanthine) in Foods: A Comprehensive Review on Consumption, Functionality, Safety, and Regulatory Matters. J. Food Sci. 2010, 75, R77–R87. [Google Scholar] [CrossRef]
- Khan, N.; Syed, D.N.; Ahmad, N.; Mukhtar, H. Fisetin: A Dietary Antioxidant for Health Promotion. Antioxid. Redox Signal. 2013, 19, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Li, X.; Yang, M.; Shao, Q.; Zhao, Y.; Ma, C.; Wang, P. Autophagy: An Intracellular Degradation Pathway Regulating Plant Survival and Stress Response. Front. Plant Sci. 2020, 11, 512331. [Google Scholar] [CrossRef] [PubMed]
- Parzych, K.R.; Klionsky, D.J. An Overview of Autophagy: Morphology, Mechanism, and Regulation. Antioxid. Redox Signal. 2014, 20, 460. [Google Scholar] [CrossRef] [PubMed]
- Stavoe, A.K.H.; Holzbaur, E.L.F. Autophagy in Neurons. Annu. Rev. Cell Dev. Biol. 2019, 35, 477. [Google Scholar] [CrossRef]
- Cerri, S.; Blandini, F. Role of Autophagy in Parkinson’s Disease. Curr. Med. Chem. 2019, 26, 3702–3718. [Google Scholar] [CrossRef] [PubMed]
- De La Cueva, M.; Antequera, D.; Ordoñez-Gutierrez, L.; Wandosell, F.; Camins, A.; Carro, E.; Bartolome, F. Amyloid-β Impairs Mitochondrial Dynamics and Autophagy in Alzheimer’s Disease Experimental Models. Sci. Rep. 2022, 12, 10092. [Google Scholar] [CrossRef] [PubMed]
- Pircs, K.; Petri, R.; Madsen, S.; Brattås, P.L.; Vuono, R.; Ottosson, D.R.; St-Amour, I.; Hersbach, B.A.; Matusiak-Brückner, M.; Lundh, S.H.; et al. Huntingtin Aggregation Impairs Autophagy, Leading to Argonaute-2 Accumulation and Global MicroRNA Dysregulation. Cell Rep. 2018, 24, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Valentová, K.; Vrba, J.; Bancířová, M.; Ulrichová, J.; Křen, V. Isoquercitrin: Pharmacology, Toxicology, and Metabolism. Food Chem. Toxicol. 2014, 68, 267–282. [Google Scholar] [CrossRef]
- Wu, J.Y.; Siu, K.C.; Geng, P. Bioactive Ingredients and Medicinal Values of Grifola frondosa (Maitake). Foods 2021, 10, 95. [Google Scholar] [CrossRef]
- Crowley, S.J.; Banan, G.; Amin, M.; Tanner, J.J.; Hizel, L.; Nguyen, P.; Brumback, B.; Rodriguez, K.; McFarland, N.; Bowers, D.; et al. Statistically Defined Parkinson’s Disease Executive and Memory Cognitive Phenotypes: Demographic, Behavioral, and Structural Neuroimaging Comparisons. J. Park. Dis. 2021, 11, 283. [Google Scholar] [CrossRef] [PubMed]
- Atoki, A.V.; Aja, P.M.; Shinkafi, T.S.; Ondari, E.N.; Awuchi, C.G. Hesperidin plays beneficial roles in disorders associated with the central nervous system: A review. Int. J. Food Prop. 2023, 26, 1867–1884. [Google Scholar] [CrossRef]
- Ravi, S.K.; Narasingappa, R.B.; Vincent, B. Neuro-Nutrients as Anti-Alzheimer’s Disease Agents: A Critical Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 2999–3018. [Google Scholar] [CrossRef] [PubMed]
- Leri, M.; Oropesa-Nuñez, R.; Canale, C.; Raimondi, S.; Giorgetti, S.; Bruzzone, E.; Bellotti, V.; Stefani, M.; Bucciantini, M. Oleuropein Aglycone: A Polyphenol with Different Targets against Amyloid Toxicity. Biochim. et Biophys. Acta (BBA)-Gen. Subj. 2018, 1862, 1432–1442. [Google Scholar] [CrossRef]
- He, G.; Chen, G.; Liu, W.; Ye, D.; Liu, X.; Liang, X.; Song, J. Salvianolic Acid B: A Review of Pharmacological Effects, Safety, Combination Therapy, New Dosage Forms, and Novel Drug Delivery Routes. Pharmaceutics 2023, 15, 2235. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; El Rayess, Y.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D.; et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020, 11, 550909. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- El-Saadony, M.T.; Yang, T.; Korma, S.A.; Sitohy, M.; Abd El-Mageed, T.A.; Selim, S.; Al Jaouni, S.K.; Salem, H.M.; Mahmmod, Y.; Soliman, S.M.; et al. Impacts of Turmeric and Its Principal Bioactive Curcumin on Human Health: Pharmaceutical, Medicinal, and Food Applications: A Comprehensive Review. Front. Nutr. 2023, 9, 1040259. [Google Scholar] [CrossRef]
- Benameur, T.; Giacomucci, G.; Panaro, M.A.; Ruggiero, M.; Trotta, T.; Monda, V.; Pizzolorusso, I.; Lofrumento, D.D.; Porro, C.; Messina, G. New Promising Therapeutic Avenues of Curcumin in Brain Diseases. Molecules 2022, 27, 236. [Google Scholar] [CrossRef] [PubMed]
- Sathyabhama, M.; Priya Dharshini, L.C.; Karthikeyan, A.; Kalaiselvi, S.; Min, T. The Credible Role of Curcumin in Oxidative Stress-Mediated Mitochondrial Dysfunction in Mammals. Biomolecules 2022, 12, 1405. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Beevers, C.S.; Huang, S. Targets of Curcumin. Curr. Drug Targets 2011, 12, 332–347. [Google Scholar] [CrossRef]
- Menon, V.P.; Sudheer, A.R. Antioxidant and Anti-Inflammatory Properties of Curcumin. Adv. Exp. Med. Biol. 2007, 595, 105–125. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, N.; Lin, L.; Wu, N. Curcumin Suppresses Apoptosis and Inflammation in Hypoxia/Reperfusion-Exposed Neurons via Wnt Signaling Pathway. Med. Sci. Monit. 2020, 26, e920445-1–e920445-8. [Google Scholar] [CrossRef]
- Maiti, P.; Dunbar, G.L. Use of Curcumin, a Natural Polyphenol for Targeting Molecular Pathways in Treating Age-Related Neurodegenerative Diseases. Int. J. Mol. Sci. 2018, 19, 1637. [Google Scholar] [CrossRef]
- Singh, P.K.; Kotia, V.; Ghosh, D.; Mohite, G.M.; Kumar, A.; Maji, S.K. Curcumin Modulates α-Synuclein Aggregation and Toxicity. ACS Chem. Neurosci. 2013, 4, 393–407. [Google Scholar] [CrossRef]
- Reddy, P.H.; Manczak, M.; Yin, X.; Grady, M.C.; Mitchell, A.; Tonk, S.; Sekhar Kuruva, C.; Singh Bhatti, J.; Kandimalla, R.; Vijayan, M.; et al. Protective Effects of Indian Spice Curcumin Against Amyloid Beta in Alzheimer’s Disease HHS Public Access Author Manuscript. J. Alzheimers Dis. 2018, 61, 843–866. [Google Scholar] [CrossRef]
- Hu, S.; Maiti, P.; Ma, Q.; Zuo, X.; Jones, M.R.; Cole, G.M.; Frautschy, S.A. Clinical Development of Curcumin in Neurodegenerative Disease. Expert. Rev. Neurother. 2015, 15, 629–637. [Google Scholar] [CrossRef] [PubMed]
- He, H.-J.; Xiong, X.; Zhou, S.; Zhang, X.-R.; Zhao, X.; Chen, L.; Xie, C.-L. Neuroprotective Effects of Curcumin via Autophagy Induction in 6-Hydrox-Ydopamine Parkinson’s Models. Neurochem. Int. 2022, 155, 105297. [Google Scholar] [CrossRef]
- Jiang, T.-F.; Zhang, Y.-J.; Zhou, H.-Y.; Wang, H.-M.; Tian, L.-P.; Liu, J.; Ding, J.-Q.; Chen, S.-D. Curcumin Ameliorates the Neurodegenerative Pathology in A53T α-Synuclein Cell Model of Parkinson’s Disease through the Downregulation of MTOR/P70S6K Signaling and the Recovery of Macroautophagy. J. Neuroimmune Pharmacol. 2013, 8, 356–369. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, X.; Teng, Z.; Zhang, T.; Li, Y. Molecular and Cellular Pharmacology Downregulation of PI3K/Akt/MTOR Signaling Pathway in Curcumin-Induced Autophagy in APP/PS1 Double Transgenic Mice. Eur. J. Pharmacol. 2014, 740, 312–320. [Google Scholar] [CrossRef]
- Ringman, J.M.; Frautschy, S.A.; Teng, E.; Begum, A.N.; Bardens, J.; Beigi, M.; Gylys, K.H.; Badmaev, V.; Heath, D.D.; Apostolova, L.G.; et al. Oral Curcumin for Alzheimer’s Disease: Tolerability and Efficacy in a 24-Week Randomized, Double Blind, Placebo-Controlled Study. Alzheimers Res. Ther. 2012, 4, 43. [Google Scholar] [CrossRef] [PubMed]
- Baum, L.; Lam, C.W.K.; Cheung, S.K.K.; Kwok, T.; Lui, V.; Tsoh, J.; Lam, L.; Leung, V.; Hui, E.; Ng, C.; et al. Six-Month Randomized, Placebo-Controlled, Double-Blind, Pilot Clinical Trial of Curcumin in Patients with Alzheimer Disease. J. Clin. Psychopharmacol. 2008, 28, 110–113. [Google Scholar] [CrossRef]
- Cox, K.H.M.; Pipingas, A.; Scholey, A.B. Investigation of the Effects of Solid Lipid Curcumin on Cognition and Mood in a Healthy Older Population. J. Psychopharmacol. 2014, 29, 642–651. [Google Scholar] [CrossRef]
- Cox, K.H.M.; White, D.J.; Pipingas, A.; Poorun, K.; Scholey, A. Further Evidence of Benefits to Mood and Working Memory from Lipidated Curcumin in Healthy Older People: A 12-Week, Double-Blind, Placebo-Controlled, Partial Replication Study. Nutrients 2020, 12, 1678. [Google Scholar] [CrossRef]
- Disilvestro, R.A.; Joseph, E.; Zhao, S.; Bomser, J. Diverse Effects of a Low Dose Supplement of Lipidated Curcumin in Healthy Middle Aged People. Nutr. J. 2012, 11, 79. [Google Scholar] [CrossRef]
- Small, G.W.; Siddarth, P.; Li, Z.; Miller, K.J.; Ercoli, L.; Emerson, N.D.; Martinez, J.; Wong, K.P.; Liu, J.; Merrill, D.A.; et al. Memory and Brain Amyloid and Tau Effects of a Bioavailable Form of Curcumin in Non-Demented Adults: A Double-Blind, Placebo-Controlled 18-Month Trial. Am. J. Geriatr. Psychiatry 2018, 26, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Curcumin Market Size, Share & Trends Analysis Report By Application (Pharmaceutical, Food, Cosmetics), By Region (North America, Europe, Asia Pacific, CSA, MEA), and Segment Forecasts, 2020–2028. Available online: https://www.researchandmarkets.com/reports/4613416/curcumin-market-size-share-and-trends-analysis (accessed on 2 April 2024).
- Dhingra, A.K.; Rathi, V.; Chopra, B. Resveratrol. In Naturally Occurring Chemicals against Alzheimer’s Disease; Academic Press: Cambridge, MA, USA, 2021; pp. 33–47. [Google Scholar] [CrossRef]
- Bastianetto, S.; Zheng, W.H.; Quirion, R. Neuroprotective Abilities of Resveratrol and Other Red Wine Constituents against Nitric Oxide-Related Toxicity in Cultured Hippocampal Neurons. Br. J. Pharmacol. 2000, 131, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Bastianetto, S.; Ménard, C.; Quirion, R. Neuroprotective Action of Resveratrol. Biochim. et Biophys. Acta (BBA)-Mol. Basis Dis. 2015, 1852, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Sakata, Y.; Zhuang, H.; Kwansa, H.; Koehler, R.C.; Doré, S. Resveratrol Protects against Experimental Stroke: Putative Neuroprotective Role of Heme Oxygenase 1. Exp. Neurol. 2010, 224, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, B.; Milbrandt, J. Resveratrol Stimulates AMP Kinase Activity in Neurons. Proc. Natl. Acad. Sci. USA 2007, 104, 7217–7222. [Google Scholar] [CrossRef] [PubMed]
- Farkhondeh, T.; Folgado, S.L.; Pourbagher-Shahri, A.M.; Ashrafizadeh, M.; Samarghandian, S. The Therapeutic Effect of Resveratrol: Focusing on the Nrf2 Signaling Pathway. Biomed. Pharmacother. 2020, 127, 110234. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.L.; Yang, J.Y.; Dong, Y.X.; Wang, J.M.; Cui, Y.H.; Ikeshima, T.; Zhao, Y.Q.; Wu, C.F. Resveratrol Inhibits Nitric Oxide and TNF-α Production by Lipopolysaccharide-Activated Microglia. Int. Immunopharmacol. 2005, 5, 185–193. [Google Scholar] [CrossRef]
- Candelario-Jalil, E.; de Oliveira, A.C.P.; Gräf, S.; Bhatia, H.S.; Hüll, M.; Muñoz, E.; Fiebich, B.L. Resveratrol Potently Reduces Prostaglandin E2 Production and Free Radical Formation in Lipopolysaccharide-Activated Primary Rat Microglia. J. Neuroinflamm. 2007, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Liu, J.; Shi, J.-S. Anti-Inflammatory Activities of Resveratrol in the Brain: Role of Resveratrol in Microglial Activation. Eur. J. Pharmacol. 2010, 636, 1–7. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, Y.; Mueller-Steiner, S.; Chen, L.-F.; Kwon, H.; Yi, S.; Mucke, L.; Gan, L. SIRT1 Protects against Microglia-Dependent Amyloid-Toxicity through Inhibiting NF-B Signaling. J. Biol. Chem. 2005, 280, 40364–40374. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, X.P.; Yang, S.G.; Wang, Y.J.; Zhang, X.; Du, X.T.; Sun, X.X.; Zhao, M.; Huang, L.; Liu, R.T. Resveratrol Inhibits Beta-Amyloid Oligomeric Cytotoxicity but Does Not Prevent Oligomer Formation. Neurotoxicology 2009, 30, 986–995. [Google Scholar] [CrossRef]
- Kennedy, D.O.; Wightman, E.L.; Reay, J.L.; Lietz, G.; Okello, E.J.; Wilde, A.; Haskell, C.F. Effects of Resveratrol on Cerebral Blood Flow Variables and Cognitive Performance in Humans: A Double-Blind, Placebo-Controlled, Crossover Investigation. Am. J. Clin. Nutr. 2010, 91, 1590–1597. [Google Scholar] [CrossRef]
- Wightman, E.L.; Haskell-Ramsay, C.F.; Reay, J.L.; Williamson, G.; Dew, T.; Zhang, W.; Kennedy, D.O. The Effects of Chronic Trans-Resveratrol Supplementation on Aspects of Cognitive Function, Mood, Sleep, Health and Cerebral Blood Flow in Healthy, Young Humans. Br. J. Nutr. 2015, 114, 1427–1437. [Google Scholar] [CrossRef]
- Evans, H.M.; Howe, P.R.C.; Wong, R.H.X. Effects of Resveratrol on Cognitive Performance, Mood and Cerebrovascular Function in Post-Menopausal Women; a 14-Week Randomised Placebo-Controlled Intervention Trial. Nutrients 2017, 9, 27. [Google Scholar] [CrossRef]
- Veronica Witte, A.; Kerti, L.; Margulies, D.S.; Flöel, A. Effects of Resveratrol on Memory Performance, Hippocampal Functional Connectivity, and Glucose Metabolism in Healthy Older Adults. J. Neurosci. 2014, 34, 7862–7870. [Google Scholar] [CrossRef]
- Huhn, S.; Beyer, F.; Zhang, R.; Lampe, L.; Grothe, J.; Kratzsch, J.; Willenberg, A.; Breitfeld, J.; Kovacs, P.; Stumvoll, M.; et al. Effects of Resveratrol on Memory Performance, Hippocampus Connectivity and Microstructure in Older Adults—A Randomized Controlled Trial. Neuroimage 2018, 174, 177–190. [Google Scholar] [CrossRef]
- Moussa, C.; Hebron, M.; Huang, X.; Ahn, J.; Rissman, R.A.; Aisen, P.S.; Turner, R.S. Resveratrol Regulates Neuro-Inflammation and Induces Adaptive Immunity in Alzheimer’s Disease. J. Neuroinflamm. 2017, 14, 1. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Ruscica, M.; Banach, M. Resveratrol and Cognitive Decline: A Clinician Perspective. Arch. Med. Sci. 2019, 15, 936–943. [Google Scholar] [CrossRef]
- Santos, A.C.; Pereira, I.; Pereira-Silva, M.; Ferreira, L.; Caldas, M.; Collado-González, M.; Magalhães, M.; Figueiras, A.; Ribeiro, A.J.; Veiga, F. Nanotechnology-Based Formulations for Resveratrol Delivery: Effects on Resveratrol in Vivo Bioavailability and Bioactivity. Colloids Surf. B Biointerfaces 2019, 180, 127–140. [Google Scholar] [CrossRef]
- 360 Research Reports—Worldwide Market Research Report, Analysis & Consulting. Available online: https://www.360researchreports.com/purchase/21486428 (accessed on 2 April 2024).
- Apparoo, Y.; Phan, C.W.; Kuppusamy, U.R.; Sabaratnam, V. Ergothioneine and Its Prospects as an Anti-Ageing Compound. Exp. Gerontol. 2022, 170, 111982. [Google Scholar] [CrossRef]
- Gründemann, D.; Harlfinger, S.; Golz, S.; Geerts, A.; Lazar, A.; Berkels, R.; Jung, N.; Rubbert, A.; Schömig, E. Discovery of the Ergothioneine Transporter. Proc. Natl. Acad. Sci. USA 2005, 102, 5256–5261. [Google Scholar] [CrossRef]
- Inazu, M.; Takeda, H.; Maehara, K.; Miyashita, K.; Tomoda, A.; Matsumiya, T. Functional Expression of the Organic Cation/Carnitine Transporter 2 in Rat Astrocytes. J. Neurochem. 2006, 97, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Kubo, Y.; Iwata, D.; Kato, S.; Sudo, T.; Sugiura, T.; Kagaya, T.; Wakayama, T.; Hirayama, A.; Sugimoto, M.; et al. Gene Knockout and Metabolome Analysis of Carnitine/Organic Cation Transporter OCTN1. Pharm. Res. 2010, 27, 832–840. [Google Scholar] [CrossRef]
- Kondoh, H.; Teruya, T.; Kameda, M.; Yanagida, M. Decline of Ergothioneine in Frailty and Cognition Impairment. FEBS Lett. 2022, 596, 1270–1278. [Google Scholar] [CrossRef]
- Beelman, R.B.; Kalaras, M.D.; Phillips, A.T.; Richie, J.P. Is Ergothioneine a “longevity Vitamin” Limited in the American Diet? J. Nutr. Sci. 2020, 9, e52. [Google Scholar] [CrossRef]
- Roda, E.; Priori, E.C.; Ratto, D.; De Luca, F.; Di Iorio, C.; Angelone, P.; Locatelli, C.A.; Desiderio, A.; Goppa, L.; Savino, E.; et al. Neuroprotective Metabolites of Hericium erinaceus Promote Neuro-Healthy Aging. Int. J. Mol. Sci. 2021, 22, 6379. [Google Scholar] [CrossRef]
- Roda, E.; Ratto, D.; De Luca, F.; Desiderio, A.; Ramieri, M.; Goppa, L.; Savino, E.; Bottone, M.G.; Locatelli, C.A.; Rossi, P. Searching for a Longevity Food, We Bump into Hericium erinaceus Primordium Rich in Ergothioneine: The “Longevity Vitamin” Improves Locomotor Performances during Aging. Nutrients 2022, 14, 1177. [Google Scholar] [CrossRef] [PubMed]
- Whitmore, C.A.; Haynes, J.R.; Behof, W.J.; Rosenberg, A.J.; Tantawy, M.N.; Hachey, B.C.; Wadzinski, B.E.; Spiller, B.W.; Peterson, T.E.; Paffenroth, K.C.; et al. Longitudinal Consumption of Ergothioneine Reduces Oxidative Stress and Amyloid Plaques and Restores Glucose Metabolism in the 5XFAD Mouse Model of Alzheimer’s Disease. Pharmaceuticals 2022, 15, 742. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, P.; Whitmore, C.A.; Campbell, M.; Li, C.; Tsuyuki, M.; To, E.; Haynes, J.; Pham, W.; Matsubara, J.A. Ergothioneine, a Dietary Antioxidant Improves Amyloid Beta Clearance in the Neuroretina of a Mouse Model of Alzheimer’s Disease. Front. Neurosci. 2023, 17, 1107436. [Google Scholar] [CrossRef]
- Qiu, Y.; Chen, Z.; Su, E.; Wang, L.; Sun, L.; Lei, P.; Xu, H.; Li, S. Recent Strategies for the Biosynthesis of Ergothioneine. J. Agric. Food Chem. 2021, 69, 13682–13690. [Google Scholar] [CrossRef]
- Chen, H.W.; Yang, T.S.; Chen, M.J.; Chang, Y.C.; Wang, E.I.C.; Ho, C.L.; Lai, Y.J.; Yu, C.C.; Chou, J.C.; Chao, L.K.P.; et al. Purification and Immunomodulating Activity of C-Phycocyanin from Spirulina Platensis Cultured Using Power Plant Flue Gas. Process Biochem. 2014, 49, 1337–1344. [Google Scholar] [CrossRef]
- Grover, P.; Bhatnagar, A.; Kumari, N.; Narayan Bhatt, A.; Kumar Nishad, D.; Purkayastha, J. C-Phycocyanin-a Novel Protein from Spirulina Platensis- In Vivo Toxicity, Antioxidant and Immunomodulatory Studies. Saudi J. Biol. Sci. 2021, 28, 1853–1859. [Google Scholar] [CrossRef] [PubMed]
- Abalde, J.; Betancourt, L.; Torres, E.; Cid, A.; Barwell, C. Purification and Characterization of Phycocyanin from the Marine Cyanobacterium Synechococcus Sp. IO9201. Plant Sci. 1998, 136, 109–120. [Google Scholar] [CrossRef]
- Benedetti, S.; Rinalducci, S.; Benvenuti, F.; Francogli, S.; Pagliarani, S.; Giorgi, L.; Micheloni, M.; D’Amici, G.M.; Zolla, L.; Canestrari, F. Purification and Characterization of Phycocyanin from the Blue-Green Alga Aphanizomenon Flos-Aquae. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2006, 833, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Chaiklahan, R.; Chirasuwan, N.; Loha, V.; Tia, S.; Bunnag, B. Separation and Purification of Phycocyanin from Spirulina Sp. Using a Membrane Process. Bioresour. Technol. 2011, 102, 7159–7164. [Google Scholar] [CrossRef] [PubMed]
- Vonshak, A. Spirulina Platensis (Arthrospira): Physiology, Cell-Biology and Biotechnology. J. Appl. Phycol. 1997, 9, 295–296. [Google Scholar] [CrossRef]
- Silveira, S.T.; Burkert, J.F.M.; Costa, J.A.V.; Burkert, C.A.V.; Kalil, S.J. Optimization of Phycocyanin Extraction from Spirulina Platensis Using Factorial Design. Bioresour. Technol. 2007, 98, 1629–1634. [Google Scholar] [CrossRef]
- Pez Jaeschke, D.; Rocha Teixeira, I.; Damasceno Ferreira Marczak, L.; Domeneghini Mercali, G. Phycocyanin from Spirulina: A Review of Extraction Methods and Stability. Food Res. Int. 2021, 143, 110314. [Google Scholar] [CrossRef]
- de Moraes Nogueira, A.O.; Felipe Kokuszi, L.T.; Poester Cordeiro, A.; Ziebell Salgado, H.; Costa, J.A.V.; Santos, L.O.; de Lima, V.R. Spirulina Sp. LEB 18-Extracted Phycocyanin: Effects on Liposomes’ Physicochemical Parameters and Correlation with Antiradical/Antioxidant Properties. Chem. Phys. Lipids 2021, 236, 105064. [Google Scholar] [CrossRef]
- Piniella-Matamoros, B.; Marín-Prida, J.; Pentón-Rol, G. Nutraceutical and Therapeutic Potential of Phycocyanobilin for Treating Alzheimer’s Disease. J. Biosci. 2021, 46, 42. [Google Scholar] [CrossRef]
- Trotta, T.; Porro, C.; Cianciulli, A.; Panaro, M.A. Beneficial Effects of Spirulina Consumption on Brain Health. Nutrients 2022, 14, 676. [Google Scholar] [CrossRef] [PubMed]
- Fernandes e Silva, E.; Figueira, F.d.S.; Lettnin, A.P.; Carrett-Dias, M.; Filgueira, D.d.M.V.B.; Kalil, S.; Trindade, G.S.; Votto, A.P.d.S. C-Phycocyanin: Cellular Targets, Mechanisms of Action and Multi Drug Resistance in Cancer. Pharmacol. Rep. 2018, 70, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Ziyaei, K.; Abdi, F.; Mokhtari, M.; Daneshmehr, M.A.; Ataie, Z. Phycocyanin as a Nature-Inspired Antidiabetic Agent: A Systematic Review. Phytomedicine 2023, 119, 154964. [Google Scholar] [CrossRef] [PubMed]
- Barone, G.D.; Cernava, T.; Ullmann, J.; Liu, J.; Lio, E.; Germann, A.T.; Nakielski, A.; Russo, D.A.; Chavkin, T.; Knufmann, K.; et al. Recent Developments in the Production and Utilization of Photosynthetic Microorganisms for Food Applications. Heliyon 2023, 9, e14708. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, Y.J.; Ryu, H.K.; Kim, M.H.; Chung, H.W.; Kim, W.Y. A Randomized Double-Blind, Placebo-Controlled Study to Establish the Effects of Spirulina in Elderly Koreans. Ann. Nutr. Metab. 2008, 52, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Marín-Prida, J.; Pavón-Fuentes, N.; Llópiz-Arzuaga, A.; Fernández-Massó, J.R.; Delgado-Roche, L.; Mendoza-Marí, Y.; Santana, S.P.; Cruz-Ramírez, A.; Valenzuela-Silva, C.; Nazábal-Gálvez, M.; et al. Phycocyanobilin Promotes PC12 Cell Survival and Modulates Immune and Inflammatory Genes and Oxidative Stress Markers in Acute Cerebral Hypoperfusion in Rats. Toxicol. Appl. Pharmacol. 2013, 272, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Hassinger, L.; Davis, J.; Devor, S.T.; DiSilvestro, R.A. A Randomized, Double Blind, Placebo Controlled Study of Spirulina Supplementation on Indices of Mental and Physical Fatigue in Men. Int. J. Food Sci. Nutr. 2016, 67, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Pavón-Fuentes, N.; Marín-Prida, J.; Llópiz-Arzuaga, A.; Falcón-Cama, V.; Campos-Mojena, R.; Cervantes-Llanos, M.; Piniella-Matamoros, B.; Pentón-Arias, E.; Pentón-Rol, G. Phycocyanobilin Reduces Brain Injury after Endothelin-1- Induced Focal Cerebral Ischaemia. Clin. Exp. Pharmacol. Physiol. 2020, 47, 383–392. [Google Scholar] [CrossRef]
- Choi, W.Y.; Lee, W.K.; Kim, T.H.; Ryu, Y.K.; Park, A.; Lee, Y.J.; Heo, S.J.; Oh, C.; Chung, Y.C.; Kang, D.H. The Effects of Spirulina Maxima Extract on Memory Improvement in Those with Mild Cognitive Impairment: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2022, 14, 3714. [Google Scholar] [CrossRef]
- Thevarajah, B.; Nishshanka, G.K.S.H.; Premaratne, M.; Nimarshana, P.H.V.; Nagarajan, D.; Chang, J.S.; Ariyadasa, T.U. Large-Scale Production of Spirulina-Based Proteins and c-Phycocyanin: A Biorefinery Approach. Biochem. Eng. J. 2022, 185, 108541. [Google Scholar] [CrossRef]
- Küpeli Akkol, E.; Tatlı Çankaya, I.; Şeker Karatoprak, G.; Carpar, E.; Sobarzo-Sánchez, E.; Capasso, R. Natural Compounds as Medical Strategies in the Prevention and Treatment of Psychiatric Disorders Seen in Neurological Diseases. Front. Pharmacol. 2021, 12, 669638. [Google Scholar] [CrossRef] [PubMed]
- Ubeyitogullari, A.; Ciftci, O.N. A Novel and Green Nanoparticle Formation Approach to Forming Low-Crystallinity Curcumin Nanoparticles to Improve Curcumin’s Bioaccessibility. Sci. Rep. 2019, 9, 19112. [Google Scholar] [CrossRef]
- Tabibiazar, M.; Mohammadifar, M.A.; Roufegarinejad, L.; Ghorbani, M.; Hashemi, M.; Hamishehkar, H. Improvement in Dispersibility, Stability and Antioxidant Activity of Resveratrol Using a Colloidal Nanodispersion of BSA-Resveratrol. Food Biosci. 2019, 27, 46–53. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Cardoso, R.V.; Pereira, P.R.; Freitas, C.S.; Paschoalin, V.M.F. Trends in Drug Delivery Systems for Natural Bioactive Molecules to Treat Health Disorders: The Importance of Nano-Liposomes. Pharmaceutics 2022, 14, 2808. [Google Scholar] [CrossRef] [PubMed]
- Pateiro, M.; Gómez, B.; Munekata, P.E.S.; Barba, F.J.; Putnik, P.; Kovačević, D.B.; Lorenzo, J.M. Nanoencapsulation of Promising Bioactive Compounds to Improve Their Absorption, Stability, Functionality and the Appearance of the Final Food Products. Molecules 2021, 26, 1547. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Liao, W.; Charcosset, C. Recent Advances in Encapsulation of Curcumin in Nanoemulsions: A Review of Encapsulation Technologies, Bioaccessibility and Applications. Food Res. Int. 2020, 132, 109035. [Google Scholar] [CrossRef]
- Zheng, B.; Peng, S.; Zhang, X.; McClements, D.J. Impact of Delivery System Type on Curcumin Bioaccessibility: Comparison of Curcumin-Loaded Nanoemulsions with Commercial Curcumin Supplements. J. Agric. Food Chem. 2018, 66, 10816–10826. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Rao, Z.; Hu, J.; Wang, Q.; Sun, Y.; Lei, X.; Zhao, J.; Zeng, K.; Xu, Z.; et al. Study on the Stability and Oral Bioavailability of Curcumin Loaded (-)-Epigallocatechin-3-Gallate/Poly(N-Vinylpyrrolidone) Nanoparticles Based on Hydrogen Bonding-Driven Self-Assembly. Food Chem. 2022, 378, 132091. [Google Scholar] [CrossRef]
- Kharat, M.; Zhang, G.; McClements, D.J. Stability of Curcumin in Oil-in-Water Emulsions: Impact of Emulsifier Type and Concentration on Chemical Degradation. Food Res. Int. 2018, 111, 178–186. [Google Scholar] [CrossRef]
- Artiga-Artigas, M.; Lanjari-Pérez, Y.; Martín-Belloso, O. Curcumin-Loaded Nanoemulsions Stability as Affected by the Nature and Concentration of Surfactant. Food Chem. 2018, 266, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shin, G.H.; Lee, I.W.; Chen, X.; Park, H.J. Soluble Starch Formulated Nanocomposite Increases Water Solubility and Stability of Curcumin. Food Hydrocoll. 2016, 56, 41–49. [Google Scholar] [CrossRef]
- Ban, C.; Jo, M.; Park, Y.H.; Kim, J.H.; Han, J.Y.; Lee, K.W.; Kweon, D.H.; Choi, Y.J. Enhancing the Oral Bioavailability of Curcumin Using Solid Lipid Nanoparticles. Food Chem. 2020, 302, 125328. [Google Scholar] [CrossRef] [PubMed]
- Aditya, N.P.; Yang, H.; Kim, S.; Ko, S. Fabrication of Amorphous Curcumin Nanosuspensions Using β-Lactoglobulin to Enhance Solubility, Stability, and Bioavailability. Colloids Surf. B Biointerfaces 2015, 127, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Hadji, H.; Bouchemal, K. Advances in the Treatment of Inflammatory Bowel Disease: Focus on Polysaccharide Nanoparticulate Drug Delivery Systems. Adv. Drug Deliv. Rev. 2022, 181, 114101. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.; Zhu, F. Ultrasound Modified Polysaccharides: A Review of Structure, Physicochemical Properties, Biological Activities and Food Applications. Trends Food Sci. Technol. 2021, 107, 491–508. [Google Scholar] [CrossRef]
- Soni, R.A.; Sudhakar, K.; Rana, R.S. Spirulina—From Growth to Nutritional Product: A Review. Trends Food Sci. Technol. 2017, 69, 157–171. [Google Scholar] [CrossRef]
- Maag, P.; Dirr, S.; Karslioglu, Ö.Ö. Investigation of Bioavailability and Food-Processing Properties of Arthrospira Platensis by Enzymatic Treatment and Micro-Encapsulation by Spray Drying. Foods 2022, 11, 1922. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Jiang, M.; Fang, J.; Yang, M.F.; Zhang, S.; Yin, Y.X.; Li, D.W.; Mao, L.L.; Hou, Y.J.; Fu, X.T.; et al. Enhanced Therapeutic Potential of Nano-Curcumin Against Subarachnoid Hemorrhage-Induced Blood–Brain Barrier Disruption Through Inhibition of Inflammatory Response and Oxidative Stress. Mol. Neurobiol. 2017, 54, 1–14. [Google Scholar] [CrossRef]
- Askarizadeh, A.; Barreto, G.E.; Henney, N.C.; Majeed, M.; Sahebkar, A. Neuroprotection by Curcumin: A Review on Brain Delivery Strategies. Int. J. Pharm. 2020, 585, 119476. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Agarwal, S.; Seth, B.; Yadav, A.; Nair, S.; Bhatnagar, P.; Karmakar, M.; Kumari, M.; Chauhan, L.K.S.; Patel, D.K.; et al. Curcumin-Loaded Nanoparticles Potently Induce Adult Neurogenesis and Reverse Cognitive Deficits in Alzheimer’s Disease Model via Canonical Wnt/β-Catenin Pathway. ACS Nano 2014, 8, 76–103. [Google Scholar] [CrossRef] [PubMed]
- Loh, J.S.; Mak, W.Q.; Tan, L.K.S.; Ng, C.X.; Chan, H.H.; Yeow, S.H.; Foo, J.B.; Ong, Y.S.; How, C.W.; Khaw, K.Y. Microbiota-Gut-Brain Axis and Its Therapeutic Applications in Neurodegenerative Diseases. Signal Transduct. Target. Ther. 2024, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, F.; Clerici, M.; Ratto, D.; Occhinegro, A.; Licito, A.; Romeo, M.; Di Iorio, C.; Rossi, P. The Gut-Brain Axis in Alzheimer’s Disease and Omega-3. A Critical Overview of Clinical Trials. Nutrients 2018, 10, 1267. [Google Scholar] [CrossRef] [PubMed]
- Gwak, M.G.; Chang, S.Y. Gut-Brain Connection: Microbiome, Gut Barrier, and Environmental Sensors. Immune Netw. 2021, 21, e20. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Xu, Z.; Zhang, L.; Zhang, C.; Zhao, X.; Mao, Y.; Zhang, H.; Liang, X.; Wu, J.; Yang, Y.; et al. Gut-Derived β-Amyloid: Likely a Centerpiece of the Gut-Brain Axis Contributing to Alzheimer’s Pathogenesis. Gut Microbes 2023, 15, 2167172. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Li, Q.P.; Su, Z.R.; Ip, S.P.; Yuan, Q.J.; Xie, Y.L.; Xu, Q.Q.; Yang, W.; Huang, Y.F.; Xian, Y.F.; et al. Nano-Honokiol Ameliorates the Cognitive Deficits in TgCRND8 Mice of Alzheimer’s Disease via Inhibiting Neuropathology and Modulating Gut Microbiota. J. Adv. Res. 2022, 35, 231–243. [Google Scholar] [CrossRef] [PubMed]
- González-Fuentes, J.; Selva, J.; Moya, C.; Castro-Vázquez, L.; Lozano, M.V.; Marcos, P.; Plaza-Oliver, M.; Rodríguez-Robledo, V.; Santander-Ortega, M.J.; Villaseca-González, N.; et al. Neuroprotective Natural Molecules, from Food to Brain. Front. Neurosci. 2018, 12, 721. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.; Kim, Y.; Zhang, K.; Chau, L.; Nguyen, B.C.; Rayalam, S.; Wang, X. Antiaging Mechanism of Natural Compounds: Effects on Autophagy and Oxidative Stress. Molecules 2022, 27, 4396. [Google Scholar] [CrossRef] [PubMed]
- Puri, V.; Kanojia, N.; Sharma, A.; Huanbutta, K.; Dheer, D.; Sangnim, T. Natural Product-Based Pharmacological Studies for Neurological Disorders. Front. Pharmacol. 2022, 13, 1011740. [Google Scholar] [CrossRef]
- Bjørklund, G.; Shanaida, M.; Lysiuk, R.; Butnariu, M.; Peana, M.; Sarac, I.; Strus, O.; Smetanina, K.; Chirumbolo, S. Natural Compounds and Products from an Anti-Aging Perspective. Molecules 2022, 27, 7084. [Google Scholar] [CrossRef]
- Alghazwi, M.; Kan, Y.Q.; Zhang, W.; Gai, W.P.; Garson, M.J.; Smid, S. Neuroprotective Activities of Natural Products from Marine Macroalgae during 1999–2015. J. Appl. Phycol. 2016, 28, 3599–3616. [Google Scholar] [CrossRef]
- Mohd Sairazi, N.S.; Sirajudeen, K.N.S. Natural Products and Their Bioactive Compounds: Neuroprotective Potentials against Neurodegenerative Diseases. Evid.-Based Complement. Altern. Med. 2020, 2020, 6565396. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and Bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [PubMed]
- Jhamandas, J.H.; Wie, M.B.; Harris, K.; MacTavish, D.; Kar, S. Fucoidan Inhibits Cellular and Neurotoxic Effects of β-Amyloid (Aβ) in Rat Cholinergic Basal Forebrain Neurons. Eur. J. Neurosci. 2005, 21, 2649–2659. [Google Scholar] [CrossRef]
- Fang, J.; Li, Y.; Liu, R.; Pang, X.; Li, C.; Yang, R.; He, Y.; Lian, W.; Liu, A.L.; Du, G.H. Discovery of Multitarget-Directed Ligands against Alzheimer’s Disease through Systematic Prediction of Chemical-Protein Interactions. J. Chem. Inf. Model. 2015, 55, 149–164. [Google Scholar] [CrossRef]


| Class of Compound | Compound Name | Natural Source | Ref. |
|---|---|---|---|
| Anti-amyloidogenic | |||
| terpenes | Polyozellin, thelephoric acid, polyozellic acid | Polyozellus multiplex | [6] |
| Erinacine A | Hericium erinaceus | [7,8] | |
| Tanshinone IIA | Salvia miltiorrhiza | [9,10] | |
| phenols | Oleuropein aglycone | Extra virgin olive oil | [11] |
| Hydroxytyrosol | Extra virgin olive oil | [11] | |
| Salvianolic acid B | Salvia miltiorrhiza | [12,13] | |
| flavonoids | Epigallocatechin gallate | Camellia sinensis | [14,15,16] |
| AChE inhibitors | |||
| terpenes | Triterpenes | Ganoderma lucidum | [17] |
| Polyozellin, thelephoric acid, Polyozellic acid | Polyozellus multiplex | [6] | |
| alkaloids | Nostocarboline | Nostoc 78-12A | [18] |
| Anatoxin-a(s) | Anabaena flos-aquae strain NRC 525-17 | [19] | |
| Galanthamine | Amaryllidaceae | [20] | |
| Anti-apoptotic | |||
| terpenes | Triterpenes | Ganoderma lucidum | [21,22] |
| Erinacine A | Hericium erinaceus | [8] | |
| Astragaloside IV | Astragalus membranaceus | [23,24] | |
| alkaloids | Caffeine | Coffee and cocoa beans, tea leaves, guarana berries | [25,26] |
| flavonoids | Fisetin | Strawberry, apple, persimmon, grape, onion, cucumber | [27,28,29,30,31] |
| GardeninA | Gardenia resinifera, Tamarix dioica, Murraya paniculata | [32] | |
| Autophagy stimulation | |||
| alkaloids | Caffeine | Coffee and cocoa beans, tea leaves, guarana berries | [33] |
| flavonoids | Isoquercitrin | Apocynum venetum | [34,35] |
| Hesperidin | Citrus fruits | [36] | |
| Epigallocatechin gallate | Camellia sinensis | [37] | |
| Fisetin | Strawberry, apple, persimmon, grape, onion, cucumber | [38] | |
| Morin | Mulberry | [39] | |
| Anti-neuroinflammation | |||
| polysaccharides | β-glucans | Grifola frondosa | [40] |
| terpenes | Erinacine A | Hericium erinaceus | [41] |
| Astragaloside IV | Astragalus membranaceus | [42,43] | |
| flavonoids | Fisetin | Strawberry, apple, persimmon, grape, onion, cucumber | [44] |
| Epigallocatechin gallate | Camellia sinensis | [45,46,47] | |
| GardeninA | Gardenia resinifera, Tamarix dioica, Murraya paniculata | [32] | |
| alkaloids | Caffeine | Coffee and cocoa beans, tea leaves, guarana berries | [33,48,49,50,51,52] |
| Preservation of cognitive functions | |||
| polysaccharides | β-glucans | Grifola frondosa | [40] |
| terpenes | Erinacine A | Hericium erinaceus | [7,53,54] |
| Astragaloside IV | Astragalus membranaceus | [42,55] | |
| alkaloids | Caffeine | Coffee and cocoa beans, tea leaves, guarana berries | [56,57,58,59,60,61] |
| flavonoids | Epigallocatechin gallate | Camellia sinensis | [62,63,64] |
| GardeninA | Gardenia resinifera, Tamarix dioica, Murraya paniculata | [32] | |
| Morin | Mulberry | [65,66] | |
| Hesperidin | Oranges | [67,68,69,70,71,72] | |
| Isoquercitrin | Apocynum venetum | [73] | |
| phenols | 6-Shogaol | Ginger rhizomes (Zingiber officinale) | [74,75] |
| Hydroxytyrosol | Extra virgin olive oil | [76,77] | |
| Oleuropein aglycone | Extra virgin olive oil | [11] | |
| Salvianolic acid B | Salvia miltiorrhiza | [78,79] | |
| terpenes | Tanshinone IIA | Salvia miltiorrhiza | [80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moukham, H.; Lambiase, A.; Barone, G.D.; Tripodi, F.; Coccetti, P. Exploiting Natural Niches with Neuroprotective Properties: A Comprehensive Review. Nutrients 2024, 16, 1298. https://doi.org/10.3390/nu16091298
Moukham H, Lambiase A, Barone GD, Tripodi F, Coccetti P. Exploiting Natural Niches with Neuroprotective Properties: A Comprehensive Review. Nutrients. 2024; 16(9):1298. https://doi.org/10.3390/nu16091298
Chicago/Turabian StyleMoukham, Hind, Alessia Lambiase, Giovanni Davide Barone, Farida Tripodi, and Paola Coccetti. 2024. "Exploiting Natural Niches with Neuroprotective Properties: A Comprehensive Review" Nutrients 16, no. 9: 1298. https://doi.org/10.3390/nu16091298