Prostatic Response to Supranutritional Selenium Supplementation: Comparison of the Target Tissue Potency of Selenomethionine vs. Selenium-Yeast on Markers of Prostatic Homeostasis
Abstract
:1. Introduction

2. Experimental Section
2.1. Study Design
2.2. Assessment of Selenium Concentration in Toenails and Prostate
2.3. Assessment of Intraprostatic Testosterone (T) and Dihydrotestosterone (DHT) Concentration by RIA
2.4. Assessment of Prostatic DNA Damage
2.5. Assessment of Proliferative Index and Apoptosis within the Prostate
2.6. Statistical Analysis
3. Results
3.1. Absence of Form-Dependent Effects of Selenium Supplementation on Systemic Selenium Status

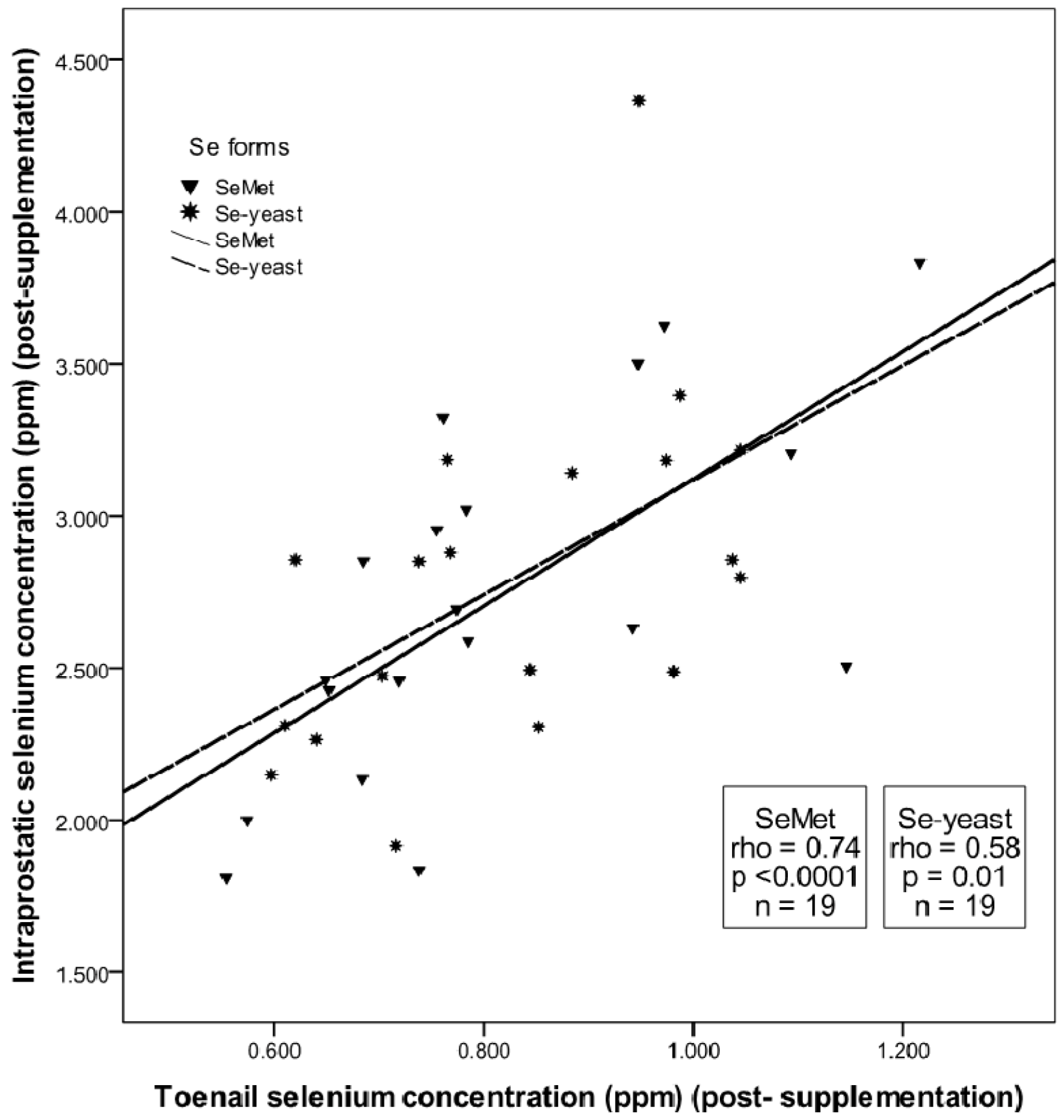
3.2. Absence of Form-Dependent Effects of Selenium Supplementation on Intraprostatic Selenium Concentration
3.3. Prostatic Response to SeMet vs. Se-yeast Supplementation: Analysis of Target Tissue Potency Using Markers of Prostatic Homeostasis
| Tertiles of Intraprostatic Selenium Concentration † | ||||
|---|---|---|---|---|
| Prostatic markers * | Lowest | Middle | Highest | |
| DHT (ng/g tissue) | SeMet (n) | 6.6 (7) | 5.7 (6) | 6.1 (6) |
| Se-yeast (n) | 5.9 (6) | 6.2 (7) | 6.3 (6) | |
| p | 0.89 | 0.48 | 0.69 | |
| T (ng/g tissue) | SeMet (n) | 2.1 (7) | 2.6 (6) | 3.2 (6) |
| Se-yeast (n) | 2.7 (6) | 2.7 (7) | 3.1 (6) | |
| p | 0.67 | 0.57 | 0.87 | |
| DHT:T | SeMet (n) | 2.7 (7) | 2.0 (6) | 2.4 (6) |
| Se-yeast (n) | 2.5 (6) | 2.9 (7) | 1.9 (6) | |
| p | 0.57 | 0.15 | 0.52 | |
| DNA damage (%) | SeMet (n) | 55 (7) | 60 (6) | 58 (6) |
| Se-yeast (n) | 52 (6) | 50 (7) | 52 (6) | |
| p | 0.31 | 0.22 | 0.16 | |
| Apoptotic index (%) | SeMet (n) | 2.0 (7) | 1.5 (6) | 2.5 (6) |
| Se-yeast (n) | 4.0 (6) | 2.0 (7) | 1.5 (6) | |
| p | 0.93 | 0.61 | 0.75 | |
| Proliferative index (%) | SeMet (n) | 1.1 (6) | 0.6 (5) | 0.4 (6) |
| Se-yeast (n) | 0.3 (5) | 0.6 (5) | 1.3 (4) | |
| p | 0.14 | 0.91 | 0.58 | |
4. Discussion
Acknowledgments
Conflict of Interest
References
- Clark, L.C.; Combs, G.F., Jr.; Turnbull, B.W.; Slate, E.H.; Chalker, D.K.; Chow, J.; Davis, L.S.; Glover, R.A.; Graham, G.F.; Gross, E.G.; et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA 1996, 276, 1957–1963. [Google Scholar]
- Lippman, S.M.; Klein, E.A.; Goodman, P.J.; Lucia, M.S.; Thompson, I.M.; Ford, L.G.; Parnes, H.L.; Minasian, L.M.; Gaziano, J.M.; Hartline, J.A.; et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2009, 301, 39–51. [Google Scholar]
- El-Bayoumy, K. The negative results of the SELECT study do not necessarily discredit the selenium-cancer prevention hypothesis. Nutr. Cancer 2009, 61, 285–286. [Google Scholar] [CrossRef]
- Hatfield, D.L.; Gladyshev, V.N. The outcome of Selenium and Vitamin E Cancer Prevention Trial (SELECT) reveals the need for better understanding of selenium biology. Mol. Interv. 2009, 9, 18–21. [Google Scholar] [CrossRef]
- Ledesma, M.C.; Jung-Hynes, B.; Schmit, T.L.; Kumar, R.; Mukhtar, H.; Ahmad, N. Selenium and vitamin E for prostate cancer: Post-SELECT (Selenium and Vitamin E Cancer Prevention Trial) status. Mol. Med. 2011, 17, 134–143. [Google Scholar]
- Kotrebai, M.; Birringer, M.; Tyson, J.F.; Block, E.; Uden, P.C. Identification of the principal selenium compounds in selenium-enriched natural sample extracts by ion-pair liquid chromatography with inductively coupled plasma- and electrospray ionization-mass spectrometer detection. Anal. Commun. 1999, 36, 249–252. [Google Scholar] [CrossRef]
- Larsen, E.H.; Hansen, M.; Paulin, H.; Moesgaard, S.; Reid, M.; Rayman, M. Speciation and bioavailability of selenium in yeast-based intervention agents used in cancer chemoprevention studies. J. AOAC Int. 2004, 7, 225–232. [Google Scholar]
- Waters, D.J.; Shen, S.; Glickman, L.T.; Cooley, D.M.; Bostwick, D.G.; Qian, J.; Combs, G.F., Jr.; Morris, J.S. Prostate cancer risk and DNA damage: Translational significance of selenium supplementation in a canine model. Carcinogenesis 2005, 26, 1256–1562. [Google Scholar]
- Hurst, R.; Hooper, L.; Norat, T.; Lau, R.; Aune, D.; Greenwood, D.C.; Vieira, R.; Collings, R.; Harvey, L.J.; Sterne, J.A.C.; et al. Selenium and prostate cancer: Systematic review and meta-analysis. Am. J. Clin. Nutr. 2012, 96, 111–122. [Google Scholar] [CrossRef]
- Hoque, A.; Albanes, D.; Lippman, S.M.; Spitz, M.R.; Taylor, P.R.; Klein, E.A.; Thompson, I.M.; Goodman, P.; Stanfrod, J.L.; Crowley, J.J.; et al. Molecular epidemiologic studies within the Selenium and Vitamin E Cancer Prevention Trial (SELECT). Cancer Causes Control 2001, 12, 627–633. [Google Scholar] [CrossRef]
- Sinha, R.; El-Bayoumy, K. Apoptosis is a critical cellular event in cancer chemoprevention and chemotherapy by selenium compounds. Curr. Cancer Drug Targets 2004, 4, 13–28. [Google Scholar] [CrossRef]
- Lockett, K.L.; Hall, M.C.; Clark, P.E.; Chuang, S.-C.; Robinson, B.; Lin, H.-Y.; Su, L.J.; Hu, J.J. DNA damage levels in prostate cancer cases and controls. Carcinogenesis 2006, 27, 1187–1193. [Google Scholar] [CrossRef]
- Jiang, W.; Jiang, C.; Pei, H.; Wang, L.; Zhang, J.; Hu, H.; Lu, J. In vivo molecular mediators of cancer growth suppression and apoptosis by selenium in mammary and prostate models: Lack of involvement of gadd genes. Mol. Cancer Ther. 2009, 8, 682–691. [Google Scholar] [CrossRef]
- Kaaks, R.; Stattin, P. Obesity, endogenous hormone metabolism, and prostate cancer risk: A conundrum of “highs” and “lows”. Cancer Prev. Res. 2010, 3, 259–262. [Google Scholar] [CrossRef]
- Waters, D.J.; Shen, S.; Cooley, D.M.; Bostwick, D.G.; Qian, J.; Combs, G.F., Jr.; Glickman, L.T.; Oteham, C.; Schlittler, D.; Morris, J.S. Effects of dietary selenium supplementation on DNA damage and apoptosis in canine prostate. J. Natl. Cancer Inst. 2003, 95, 237–241. [Google Scholar] [CrossRef]
- Chiang, E.C.; Shen, S.; Kengeri, S.S.; Xu, H.; Combs, G.F.; Morris, J.S.; Bostwick, D.G.; Waters, D.J. Defining the optimal selenium dose for prostate cancer risk reduction: Insights from the U-shaped relationship between selenium status, DNA damage, and apoptosis. Dose Response 2009, 8, 285–300. [Google Scholar]
- Patronek, G.J.; Waters, D.J.; Glickman, L.T. Comparative longevity of pet dogs and humans: Implications for gerontology research. J. Gerontol. Biol. Sci. Med. Sci. 1997, 52, B171–B178. [Google Scholar]
- McKown, D.M.; Morris, J.S. Rapid measurement of selenium in biological samples using instrumental neutron activation analysis. J. Radioanal. Chem. 1978, 43, 411–420. [Google Scholar] [CrossRef]
- Tice, R.R.; Andrews, P.W.; Hirai, O.; Singh, N.P. The Single Cell Gel (SCG) Assay: An Electrophoretic Technique for the Detection of DNA Damage in Individual Cells. In Biological Reactive Intermediates IV. Molecular and Cellular Effects and Their Impact on Human Health; Whitmer, C.R., Snyder, R.R., Jollow, D.J., Kalf, G.F., Kocsis, J.J., Sipes, I.G., Eds.; Plenum Press: New York, NY, USA, 1991; pp. 157–164. [Google Scholar]
- Collins, A.R.; Ma, A.G.; Duthie, S.J. The kinetics of repair of oxidative DNA damage (strand breaks and oxidised pyrimidines) in human cells. Mutat. Res. 1995, 336, 69–77. [Google Scholar] [CrossRef]
- Waters, D.J.; Hayden, D.W.; Bell, F.W.; Klausner, J.S.; Qian, J.; Bostwick, D.G. Prostatic intraepithelial neoplasia in dogs with spontaneous prostate cancer. Prostate 1997, 30, 92–97. [Google Scholar] [CrossRef]
- Gavrieli, Y.; Sherman, Y.; Ben-Sasson, S.A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol. 1992, 19, 493–501. [Google Scholar]
- Sabichi, A.L.; Lee, J.J.; Taylor, R.J.; Thompson, I.M.; Miles, B.J.; Tangen, C.M.; Minasian, L.M.; Pisters, L.L.; Caton, J.R.; Basler, J.W.; et al. Selenium accumulation in prostate tissue during a randomized, controlled short-term trial of L-selenomethionine: A Southwest Oncology Group Study. Clin. Cancer Res. 2006, 12, 2178–2184. [Google Scholar] [CrossRef]
- Gianduzzo, T.R.; Holmes, E.G.; Tinggi, U.; Shahin, M.; Mactaggart, P.; Nicol, D. Prostatic and peripheral blood selenium levels after oral supplementation. J. Urol. 2003, 170, 870–873. [Google Scholar] [CrossRef]
- Klein, E.A. Selenium: Epidemiology and basic science. J. Urol. 2004, 171, S50–S53. [Google Scholar] [CrossRef]
- McGuire, M.K.; Burgert, S.L.; Milner, J.A.; Glass, L.; Kummer, R.; Deering, R.; Boucek, R.; Picciano, M.F. Selenium status of infants is influenced by supplementation of formula or maternal diets. Am. J. Clin. Nutr. 1993, 58, 643–648. [Google Scholar]
- Zeng, H.; Jackson, M.I.; Cheng, W.H.; Combs, G.F., Jr. Chemical form of selenium affects its uptake, transport, and glutathione peroxidase activity in the human intestinal caco-2 cell model. Biol. Trace Elem. Res. 2011, 143, 1209–1218. [Google Scholar] [CrossRef]
- Waters, D.J.; Wildasin, K. Cancer clues from pet dogs. Sci. Am. 2006, 295, 94–101. [Google Scholar]
- Waters, D.J. Aging research 2011: Exploring the pet dog paradigm. ILAR J. 2011, 52, 97–105. [Google Scholar]
- Méplan, C.; Crosley, L.K.; Nicol, F.; Beckett, G.J.; Howie, A.F.; Hill, K.E.; Horgan, G.; Mathers, J.C.; Arthur, J.R.; Hesketh, J.E. Genetic polymorphisms in the human selenoprotein P gene determine the response of selenoprotein markers to selenium supplementation in a gender-specific manner (the SELGEN study). FASEB J. 2007, 21, 3063–3074. [Google Scholar] [CrossRef]
- Cooper, M.L.; Adami, H.O.; Grönberg, H.; Wiklund, F.; Green, F.R.; Rayman, M.P. Interaction between single nucleotide polymorphisms in selenoprotein P and mitochondrial superoxide dismutase determines prostate cancer risk. Cancer Res. 2008, 68, 10171–10177. [Google Scholar] [CrossRef]
- Ferguson, L.R.; Karunasinghe, N.; Zhu, S.; Han, D.Y.; Masters, J.G.; Wang, A.H.; Triggs, C.M. Understanding heterogeneity in supplementation effects of selenium in men: A study of stratification variables and human genetics in a prospective sample from New Zealand. Curr. Pharmacogenomics Pers. Med. 2012, 10, 204–216. [Google Scholar] [CrossRef]
- Duffield-Lillico, A.J.; Dalkin, B.L.; Reid, M.E.; Turnbull, B.W.; Slate, E.H.; Jacobs, E.T.; Marshall, J.R.; Clark, L.C.; Nutritional Prevention of Cancer Study Group. Selenium supplementation, baseline plasma selenium status and incidence of prostate cancer: An analysis of the complete treatment period of the Nutritional Prevention Cancer Trial. BJU Int. 2003, 91, 608–612. [Google Scholar]
- Algotar, A.M.; Stratton, M.S.; Xu, M.J.; Dalkin, B.L.; Nagle, R.B.; Hsu, C.H.; Ahmann, F.R.; Clark, L.C.; Stratton, S.P. Dose-dependent effects of selenized yeast on total selenium levels in prostatic tissue of men with prostate cancer. Nutr. Cancer 2011, 63, 1–5. [Google Scholar]
- Russell, R.M. The enigma of beta-carotene in carcinogenesis: What can be learned from animal studies? J. Nutr. 2004, 134, 262S–268S. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Waters, D.J.; Shen, S.; Kengeri, S.S.; Chiang, E.C.; Combs, G.F., Jr.; Morris, J.S.; Bostwick, D.G. Prostatic Response to Supranutritional Selenium Supplementation: Comparison of the Target Tissue Potency of Selenomethionine vs. Selenium-Yeast on Markers of Prostatic Homeostasis. Nutrients 2012, 4, 1650-1663. https://doi.org/10.3390/nu4111650
Waters DJ, Shen S, Kengeri SS, Chiang EC, Combs GF Jr., Morris JS, Bostwick DG. Prostatic Response to Supranutritional Selenium Supplementation: Comparison of the Target Tissue Potency of Selenomethionine vs. Selenium-Yeast on Markers of Prostatic Homeostasis. Nutrients. 2012; 4(11):1650-1663. https://doi.org/10.3390/nu4111650
Chicago/Turabian StyleWaters, David J., Shuren Shen, Seema S. Kengeri, Emily C. Chiang, Gerald F. Combs, Jr., J. Steven Morris, and David G. Bostwick. 2012. "Prostatic Response to Supranutritional Selenium Supplementation: Comparison of the Target Tissue Potency of Selenomethionine vs. Selenium-Yeast on Markers of Prostatic Homeostasis" Nutrients 4, no. 11: 1650-1663. https://doi.org/10.3390/nu4111650




