Effects of Diets Supplemented with Branched-Chain Amino Acids on the Performance and Fatigue Mechanisms of Rats Submitted to Prolonged Physical Exercise
Abstract
:1. Introduction
2. Experimental Section
2.1. Animals
2.2. Experimental Diets
| Ingredients | C ** | S1 ** | S2 ** |
|---|---|---|---|
| Starch | 620.692 | 584.992 | 573.092 |
| Casein (>85% protein) | 140.000 | 140.000 | 140.000 |
| Saccharose | 100.000 | 100.000 | 100.000 |
| Soy oil | 40.000 | 40.000 | 40.000 |
| Fiber (cellulose) | 50.000 | 50.000 | 50.000 |
| Mineral mix | 35.000 | 35.000 | 35.000 |
| Vitamin mix | 10.000 | 10.000 | 10.000 |
| l-Cystine | 1.800 | 1.800 | 1.800 |
| Choline bitartrate (41.1% de colina) | 2.500 | 2.500 | 2.500 |
| Tert-butylhydroquinone | 0.008 | 0.008 | 0.008 |
| l-Isoleucine | - *** | 8.85 | 11.8 |
| l-Leucine l | - *** | 16.35 | 21.8 |
| l-Valine | - *** | 10.5 | 14.0 |
| BCAA total addition | - | 35.7 | 47.6 |
2.3. Training Protocol
2.4. Animal Sacrifice and Sample Collection
2.5. Biochemical Analyses
2.6. Statistical Methods
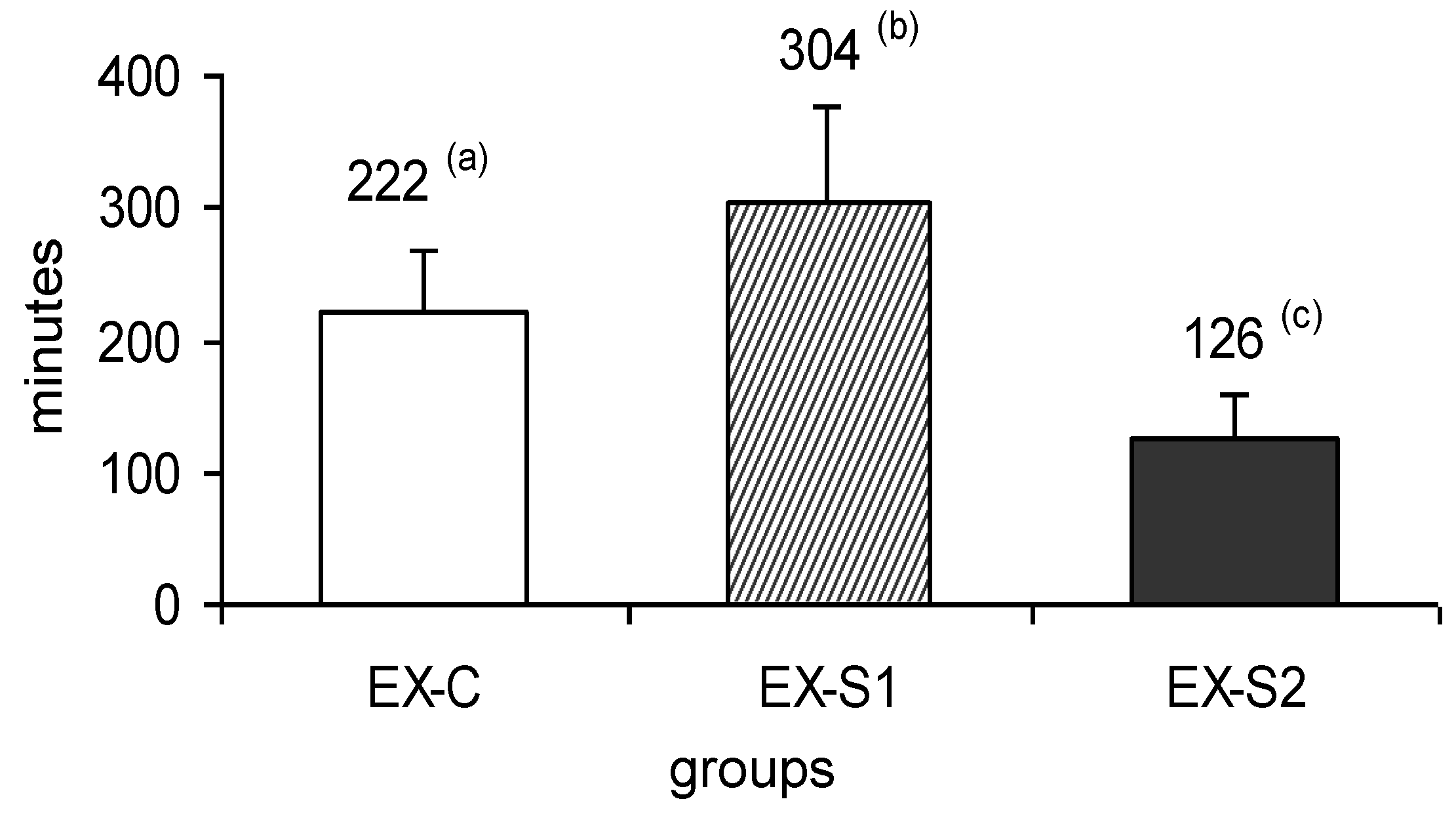
3. Results
| Variables 1 | Control | S1 | S2 | |||
|---|---|---|---|---|---|---|
| 1H | EX | 1H | EX | 1H | EX | |
| Initial body weight (g) | 238.9 ± 17.1 | 240.5 ± 14.4 | 242.5 ± 7.4 | 240.8 ± 11.9 | 235.9 ± 29.9 | 236.07 ± 26.5 |
| Final body weight (g) | 336.6 ± 9.9 | 337.6 ± 27.4 | 340.5 ± 2.5 | 340.5 ± 21.5 | 336.2 ± 20.9 | 337.0 ± 22.9 |
| Diet intake (g/day) | 21.6 ± 1.1 | 21.1 ± 0.9 | 21.6 ± 1.0 | 20.7 ± 2.0 | 20.7 ± 1.6 | 21.5 ± 1.5 |
| BCAA (mg/day) | 515.5 ± 26.8 a | 502.2 ± 22.4 a | 769.9 ± 35.3 b | 738.6 ± 70.4 b | 985.3 ± 74.8 c | 1022.8 ± 70.5 c |

| Variables 1 | Control | S1 | S2 | p 2 | p 3 | p 4 | |||
|---|---|---|---|---|---|---|---|---|---|
| 1H | EX | 1H | EX | 1H | EX | ||||
| Plasma glucose (mg/dL) | 144.9 a ± 34.2 | 55.6 b ± 14.0 | 148.2 a ± 12.5 | 60.8 b ± 13.2 | 144.0 a± 15.2 | 55.0 b ± 12.9 | 0.962 | <0.001 | - |
| Plasma free fatty acids (mmol/L) | 0.27 ± 0.05 | 0.26 ± 0.04 | 0.29 ± 0.07 | 0.27 ± 0.05 | 0.25 ± 0.04 | 0.26 ± 0.04 | 0.525 | 0.669 | - |
| Serum insulin (ng/dL) | 3.03 b,c ± 0.73 | 1.48 a ± 0.30 | 2.89 b,c ± 0.53 | 1.43 a ± 0.28 | 3.58 c ± 0.35 | 2.14 a,b ± 0.27 | 0.002 | <0.001 | - |
| Blood lactate (mmol/L) | 14.37 a ± 1.31 | 18.99 b ± 2.59 | 13.97 a ± 1.84 | 18.64 b ± 5.04 | 16.02 a ± 3.19 | 19.21 b ± 4.80 | 0.742 | <0.001 | - |
| Liver glycogen (mg/100 mg tissue) | 0.76 a ± 0.16 | 0.10 b ± 0.02 | 0.72 a ± 0.21 | 0.33 b ± 0.04 | 0.79 a ± 0.15 | 0.32 b ± 0.03 b | - | - | <0.001 |
| Gastrocnemius muscle glycogen (mg/100 mg tissue) | 0.20 a ± 0.06 | 0.11 b ± 0.03 | 0.24 a ± 0.06 | 0.13 b ± 0.03 | 0.21 a ± 0.05 | 0.10 b ± 0.02 | - | - | <0.001 |
| Soleus muscle glycogen (mg/100 mg tissue) | 0.33 a,b ± 0.08 | 0.23 c ± 0.05 | 0.41 b ± 0.02 | 0.33 a,d ± 0.05 | 0.34 a,b ± 0.03 | 0.24 c,d ± 0.03 | <0.001 | <0.001 | - |
| Hypothalamic serotonin (pg/mg tissue) | 242.3 a ± 59.6 | 330.5 a,b ± 74.5 | 256.1 a,b ± 52.2 | 321.8 a,b ± 94.9 | 272.8 a,b ± 44.9 | 384.6 b ± 108.6 | 0.359 | 0.011 | - |
4. Discussion
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Newsholme, E.A.; Blomstrand, E. The plasma level of some amino acids and physical and mental fatigue. Experientia 1996, 52, 413–415. [Google Scholar] [CrossRef]
- Jakeman, P.M. Amino acid metabolism, branched-chain amino acid feeding and brain monoamine function. Proc. Nutr. Soc. 1998, 57, 37–41. [Google Scholar]
- Ament, W.; Verkerke, G.J. Exercise and fatigue. Sports Med. 2009, 39, 389–422. [Google Scholar] [CrossRef]
- Macintosh, B.R.; Rassier, D.E. What is fatigue? Appl. Physiol. Nutr. Metab. 2002, 27, 42–55. [Google Scholar]
- Banister, E.W.; Cameron, B.J.C. Exercise-induced hyperammonemia: Peripheral and central effects. Int. J. Sports Med. 2006, 11, S129–S142. [Google Scholar]
- Mittleman, K.D.; Ricci, M.R.; Bailey, S.P. Branched-chain amino acids prolong exercise during heat stress in men and women. Med. Sci. Sports Exerc. 1998, 30, 83–91. [Google Scholar]
- Blomstrand, E. A role for branched-chain amino acids in reducing central fatigue. J. Nutr. 2006, 136, 544–547. [Google Scholar]
- Wagenmakers, A.J.M. Muscle amino acid metabolism at rest ad during exercise: Role in human physiology and metabolism. Exerc. Sports Rev. 1998, 26, 287–314. [Google Scholar]
- Jin, G.; Kataoka, Y.; Tanaka, M.; Mizuma, H.; Nozaki, S.; Tahara, T.; Mizuno, K.; Yamato, M.; Watanabe, Y. Changes in plasma and tissue amino acid levels in an animal model of complex fatigue. Nutrition 2009, 25, 597–607. [Google Scholar] [CrossRef]
- Ferrando, A.A.; Williams, B.D.; Stuart, C.A.; Lane, H.W.; Wolfe, R.R. Oral branched-chain amino acids decrease whole-body proteolysis. J. Parenter. Enteral Nutr. 1995, 19, 47–54. [Google Scholar]
- Strüder, H.K.; Hollmann, W.; Platen, P.; Wöstmann, R.; Ferrauti, A.; Weber, K. Effect of exercise intensity on free tryptophan to branched-chain amino acids ratio and plasma prolactin during endurance exercise. Appl. Physiol. Nutr. Metab. 1997, 22, 280–291. [Google Scholar]
- Holecek, M.; Kandar, R.; Sispera, L.; Koverik, M. Acute hyperammonemia activates branched-chain amino acid catabolism and decreases their extracellular concentrations: Different sensitivity of red and white muscle. Amino Acids 2010, 8, 1–10. [Google Scholar]
- Kimball, S.R.; Jefferson, L.S. Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. J. Nutr. 2006, 136, 227–231. [Google Scholar]
- Davis, J.M.; Alderson, N.A.; Welsh, R.S. Serotonin and central nervous system fatigue: Nutritional considerations. Am. J. Clin. Nutr. 2000, 72, S563–S568. [Google Scholar]
- Blomstrand, E. Amino acids and central fatigue. Amino Acids 2001, 20, 25–34. [Google Scholar] [CrossRef]
- Shimomura, Y.; Murakami, T.; Nakai, N.; Nagasaki, M.; Obayashi, M.; Li, Z.; Xu, M.; Sato, Y.; Kato, T.; Shimomura, N.; et al. Supression of glycogen consumption during acute exercise by dietary branched-chain amino acids in rats. J. Nutr. Sci. Vitamol. 2000, 46, 71–77. [Google Scholar] [CrossRef]
- Cermak, N.M.; Solheim, A.S.; Gardner, M.S.; Tarnopolsky, M.A.; Gibala, M.J. Muscle metabolism during exercise with carbohydrate or protein-carbohydrate ingestion. Med. Sci. Sports Exerc. 2009, 41, 2158–2164. [Google Scholar] [CrossRef]
- Blomstrand, E.; Hassmén, P.; Ekblom, B.; Newsholme, E.A. Administration of branched-amino acids during sustained exercise: Effects on performance and on plasma concentrations of some amino acids. Eur. J. Appl. Physiol. 1991, 63, 83–88. [Google Scholar] [CrossRef]
- Crowe, M.J.; Weatherson, J.N.; Bowden, B.F. Effects of dietary leucine supplementation on exercise performance. Eur. J. Appl. Physiol. 2006, 97, 664–672. [Google Scholar] [CrossRef]
- Galiano, F.J.; Davis, J.M.; Bailey, S.P.; Woods, J.A.; Hamilton, M.; Bartoli, W.P. Physiological, endocrine and performance effects of adding branched-chain amino acids to a 6% carbohydrate-electrolyte beverage during prolonged cycling. Med. Sci. Sports Exerc. 1991, 23, S14–S23. [Google Scholar]
- Davis, J.M.; Welsh, R.S.; de Volve, K.L.; Alderson, N.A. Effects of branched-chain amino acids and carbohydrate in fatigue during intermittent, high-intensity running. Int. J. Sports Med. 1999, 20, 309–314. [Google Scholar] [CrossRef]
- Verger, P.H.; Aymard, P.; Cynobert, L.; Anton, G.; Luigi, R. Effects of administration of branched-chain amino acids versus glucose during acute exercise in the rat. Physiol. Behav. 1994, 55, 523–526. [Google Scholar] [CrossRef]
- Calder, P.; Pannier, J.P.; Matthys, D.M.; Lacroix, E.M. Pre-exercise branched-chain amino acid administration increases endurance performance in rats. Med. Sci. Sports Exerc. 1997, 29, 1182–1186. [Google Scholar] [CrossRef]
- Calder, P.; Matthys, D.; Derave, W.; Pannier, J.L. Effect of branched-chain amino acids, glucose, and glucose plus branched-chain amino acids on endurance performance in rats. Med. Sci. Sports Exerc. 1999, 31, 583–587. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 Purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing Committee on the Reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar]
- Vieira, R.; Haebisch, H.; Kokubun, E.; Hell, N.S.; Curi, R. Swimming system for physical exercise of rats. Arq. Biol. Tecnol. 1988, 31, 387–394. [Google Scholar]
- Lancha, A.H., Jr.; Recco, M.B.; Abdalla, D.S.P.; Curi, R. Effect of aspartate, asparagine and carnitine supplementation in the diet on metabolism of skeletal muscle during a moderate exercise. Physiol. Behav. 1995, 57, 367–371. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Proteins measurements with folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Alp, P.R.; Newsholme, E.A.; Zammit, V.A. Activities of citrate synthase and NAD linked and NADP linked isocitrate dehydrogenase in muscle from vertebrates and invertebates. Biochem. J. 1976, 154, 689–700. [Google Scholar]
- Ribeiro, E.B.; Bettiker, R.L.; Bogdanov, M.; Wurtman, R.J. Effects of systemic nicotine on serotonin release in rat brain. Brain Res. 1993, 621, 311–318. [Google Scholar] [CrossRef]
- Cipolla-Neto, J.; Bartol, I.; Seraphim, P.M.; Afeche, S.C.; Scialfa, J.H.; Peracoli, A.M. The effects of lesions of the thalamic intergeniculate leaflet on the pineal metabolism. Brain Res. 1995, 691, 133–141. [Google Scholar] [CrossRef]
- Regouw, B.J.; Cornelissen, P.J.; Helder, R.A.; Spijkers, J.B.; Weeber, Y.M. Specific determination of free fatty acid in plasma. Clin. Chim. Acta 1971, 31, 187–195. [Google Scholar] [CrossRef]
- Statistica Software, version 7.1, StatSoft Inc.: Tulsa, OK, USA, 2005.
- Araujo, J.A., Jr.; Falavigna, G.; Rogero, M.M.; Pires, I.S.O.; Pedrosa, R.G.; Castro, I.A.; Donato, J., Jr.; Tirapegui, J. Effect of chronic supplementation with branched-chain amino acids on the performance and hepatic and muscle glycogen content in trained rats. Life Sci. 2006, 79, 1343–1348. [Google Scholar] [CrossRef]
- Marquezi, M.L.; Roschel, H.A.; Costa, A.S.; Sawada, L.A.; Lancha, A.H., Jr. Effect of aspartate and asparagines supplementation on fatigue determinants in intense exercise. Int. J. Sport Nutr. Exerc. Metab. 2003, 13, 65–75. [Google Scholar]
- Mccully, K.K.; Authier, B.; Olive, J. Muscle fatigue: The role of metabolism. Appl. Physiol. Nutr. Metab. 2002, 27, 70–82. [Google Scholar]
- Meeusen, R.; Watson, P.; Hasegawa, H.; Roelands, B.; Piacentini, M.F. Brain neurotransmitters in fatigue and overtraining. Appl. Physiol. Nutr. Metab. 2007, 32, 857–864. [Google Scholar] [CrossRef]
- Weicker, H.; Strüder, H.K. Influence of exercise on serotonergic neuromodulation in the brain. Amino Acids 2001, 20, 35–47. [Google Scholar] [CrossRef]
- Shimomura, Y.; Obayashi, M.; Murakami, T.; Harris, R.A. Regulation of branched-chain amino acid catabolism: Nutritional and hormonal regulation of activity and expression of the branched-chain α-keto acid dehydrogenase kinase. Curr. Opin. Clin. Nutr. Metab. Care 2001, 4, 419–423. [Google Scholar] [CrossRef]
- Fernstrom, J.D.; Fernstrom, M.H. Exercise, serum free tryptophan, and central fatigue. J. Nutr. 2006, 136, 553–559. [Google Scholar]
- Smriga, M.; Kameish, M.; Torii, K. Exercise-dependent preference for a mixture of branched-chain amino acids and homeostatic control of brain serotonin in exercising rats. J. Nutr. 2006, 136, 548–552. [Google Scholar]
- Shimomura, Y.; Murakami, T.; Nakai, N.; Nagasaki, M.; Harris, R.A. Exercise promotes BCAA catabolism: Effects of BCAA supplementation on skeletal muscle during exercise. J. Nutr. 2004, 134, 1583–1587. [Google Scholar]
- Gleeson, M. Interrelationship between physical activity and branched-chain amino acids. J. Nutr. 2005, 135, 1591–1595. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Falavigna, G.; Junior, J.A.d.A.; Rogero, M.M.; Pires, I.S.d.O.; Pedrosa, R.G.; Junior, E.M.; Castro, I.A.d.; Tirapegui, J. Effects of Diets Supplemented with Branched-Chain Amino Acids on the Performance and Fatigue Mechanisms of Rats Submitted to Prolonged Physical Exercise. Nutrients 2012, 4, 1767-1780. https://doi.org/10.3390/nu4111767
Falavigna G, Junior JAdA, Rogero MM, Pires ISdO, Pedrosa RG, Junior EM, Castro IAd, Tirapegui J. Effects of Diets Supplemented with Branched-Chain Amino Acids on the Performance and Fatigue Mechanisms of Rats Submitted to Prolonged Physical Exercise. Nutrients. 2012; 4(11):1767-1780. https://doi.org/10.3390/nu4111767
Chicago/Turabian StyleFalavigna, Gina, Jonas Alves de Araújo Junior, Marcelo Macedo Rogero, Ivanir Santana de Oliveira Pires, Rogério Graça Pedrosa, Eivor Martins Junior, Inar Alves de Castro, and Julio Tirapegui. 2012. "Effects of Diets Supplemented with Branched-Chain Amino Acids on the Performance and Fatigue Mechanisms of Rats Submitted to Prolonged Physical Exercise" Nutrients 4, no. 11: 1767-1780. https://doi.org/10.3390/nu4111767
APA StyleFalavigna, G., Junior, J. A. d. A., Rogero, M. M., Pires, I. S. d. O., Pedrosa, R. G., Junior, E. M., Castro, I. A. d., & Tirapegui, J. (2012). Effects of Diets Supplemented with Branched-Chain Amino Acids on the Performance and Fatigue Mechanisms of Rats Submitted to Prolonged Physical Exercise. Nutrients, 4(11), 1767-1780. https://doi.org/10.3390/nu4111767





