Antiapoptotic and Antiautophagic Effects of Eicosapentaenoic Acid in Cardiac Myoblasts Exposed to Palmitic Acid
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Cell Cultures
2.3. Fatty Acid Supplementation
2.4. Cell Viability
2.5. Determination of Caspase Activity
2.6. DNA Breaks Detection by in Situ-Labeling and Nuclear Staining
2.7. Immunoblot Analysis
2.8. Statistical Analysis
3. Results
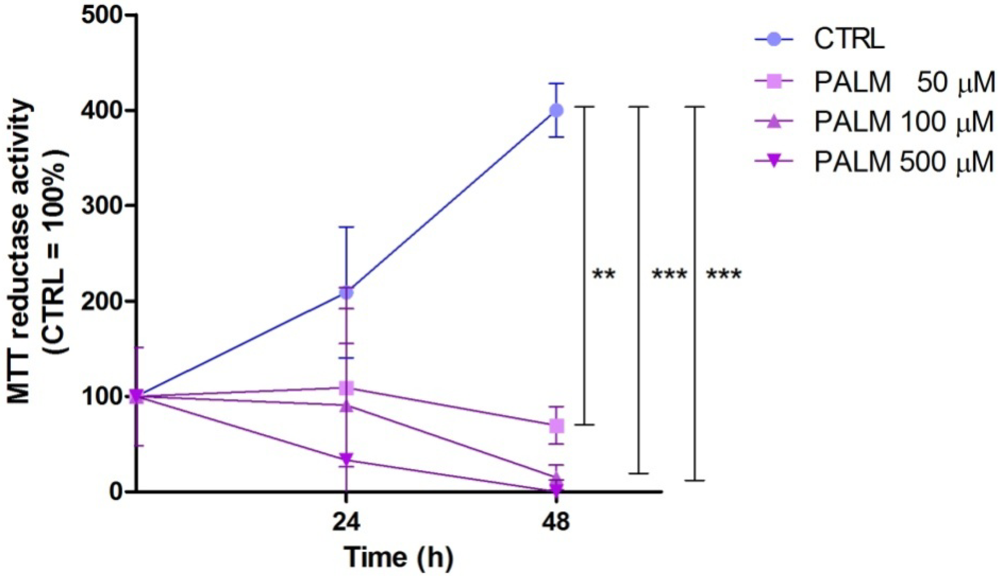
3.1. Palmitate Induces Cell Death in H9c2: Effect of EPA

3.2. Protective Effect of EPA on Palmitate-Induced Apoptosis

3.3. Palmitate Treatment Induces Autophagy in H9c2 Cardiac Cells: Effect of EPA


4. Discussion
5. Conclusions
Acknowledgements
Conflict of Interest
References
- Hooper, L.; Summerbell, C.D.; Thompson, R.; Sills, D.; Roberts, F.G.; Moore, H.; Davey Smith, G. Reduced or modified dietary fat for preventing cardiovascular disease. Cochrane Database Syst. Rev. 2011, CD002137. [Google Scholar]
- Kelley, D.E.; Goodpaster, B.H.; Storlien, L. Muscle triglyceride and insulin resistance. Annu. Rev. Nutr. 2002, 22, 325–346. [Google Scholar] [CrossRef]
- Zhou, Y.T.; Grayburn, P.; Karim, A.; Shimabukuro, M.; Higa, M.; Bactens, D.; Orci, I.; Unger, R.H. Lipotoxic heart disease in obese rats: implications for human obesity. Proc. Natl. Acad. Sci. USA 2000, 97, 1784–1789. [Google Scholar]
- Chiu, H.C.; Kovacs, A.; Ford, D.A.; Hsu, F.F.; Garcia, R.; Herrero, P.; Saffitz, J.E.; Schaffer, J.E. A novel mouse model of lipotoxic cardiomyopathy. J. Clin. Invest. 2001, 107, 813–822. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Wu, J.H. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef]
- Russo, G.L. Dietary n-6 and n-3 polyunsaturated fatty acids: from biochemistry to clinical implications in cardiovascular prevention. Biochem. Pharmacol. 2009, 77, 937–946. [Google Scholar] [CrossRef]
- van Empel, V.P.; Bertrand, A.T.; Hofstra, L.; Crijns, H.J.; Doevendans, P.A.; de Windt, L.J. Myocyte apoptosis in heart failure. Cardiovasc. Res. 2005, 67, 21–29. [Google Scholar] [CrossRef]
- Bordoni, A.; Astolfi, A.; Morandi, L.; Pession, A.; Danesi, F.; Di Nunzio, M.; Franzoni, M.; Biagi, P.; Pession, A. N-3 PUFAs modulate global gene expression profile in cultured rat cardiomyocytes. Implications in cardiac hypertrophy and heart failure. FEBS Lett. 2007, 581, 923–929. [Google Scholar]
- Leroy, C.; Tricot, S.; Lacour, B.; Grynberg, A. Protective effect of eicosapentaenoic acid on palmitate-induced apoptosis in neonatal cardiomyocytes. Biochim. Biophys. Acta 1781, 685–693. [Google Scholar]
- Mizushima, N.; Levine, B.; Cuervo, A.M.; Klionsky, D.J. Autophagy fights disease through cellular self-digestion. Nature 2008, 451, 1069–1075. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Vandenabeele, P.; Abrams, J.; Alnemri, E.S.; Baehrecke, E.H.; Blagosklonny, M.V.; El-Deiry, W.S.; Golstein, P.; Green, D.R.; et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009, 16, 3–11. [Google Scholar] [CrossRef]
- Liang, C. Negative regulation of autophagy. Cell Death Differ. 2010, 17, 1807–1815. [Google Scholar] [CrossRef]
- Amelio, I.; Melino, G.; Knight, R.A. Cell death pathology: Cross-talk with autophagy and its clinical implications. Biochem. Biophys. Res. Commun. 2011, 414, 277–281. [Google Scholar] [CrossRef]
- Singh, R.; Xiang, Y.; Wang, Y.; Baikati, K.; Cuervo, A.M.; Luu, Y.K.; Tang, Y.; Pessin, J.E.; Schwartz, G.J.; Czaja, M.J. Autophagy regulates adipose mass and differentiation in mice. J. Clin. Invest. 2009, 119, 3329–3339. [Google Scholar]
- Eisenberg-Lerner, A.; Bialik, S.; Simon, H.U.; Kimchi, A. Life and death partners: apoptosis, autophagy and the cross-talk between them. Cell Death Differ. 2009, 16, 966–975. [Google Scholar] [CrossRef]
- Gurusamy, N.; Lekli, I.; Mukherjee, S.; Ray, D.; Ahsan, M.K.; Gherghiceanu, M.; Popescu, L.M.; Das, D.K. Cardioprotection by resveratrol: A novel mechanism via autophagy involving the mTORC2 pathway. Cardiovasc. Res. 2010, 86, 103–112. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Stefanelli, C.; Pignatti, C.; Tantini, B.; Fattori, M.; Stanic, I.; Mackintosh, C.A.; Flamigni, F.; Guarnieri, C.; Caldarera, C.M.; Pegg, A.E. Effect of polyamine depletion on caspase activation: a study with spermine synthase-deficient cells. Biochem. J. 2001, 355, 199–206. [Google Scholar] [CrossRef]
- Stanic, I.; Facchini, A.; Borzì, R.M.; Vitellozzi, R.; Stefanelli, C.; Goldring, M.B.; Guarnieri, C.; Facchini, A.; Flamigni, F. Polyamine depletion inhibits apoptosis following blocking of survival pathways in human chondrocytes stimulated by tumor necrosis factor-alpha. J. Cell. Physiol. 2006, 206, 138–146. [Google Scholar] [CrossRef]
- Tantini, B.; Fiumana, E.; Cetrullo, S.; Pignatti, C.; Bonavita, F.; Shantz, L.M.; Giordano, E.; Muscari, C.; Flamigni, F.; Guarnieri, C.; et al. Involvement of polyamines in apoptosis of cardiac myoblasts in a model of simulated ischemia. J. Mol. Cell. Cardiol. 2006, 40, 775–782. [Google Scholar]
- Rousseau, D.; Héliès-Toussaint, C.; Moreau, D.; Raederstorff, D.; Grynberg, A. Dietary n-3 PUFAs affect the blood pressure rise and cardiac impairments in a hyperinsulinemia rat model in vivo. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H1294–H1302. [Google Scholar]
- Xiao, Y.F.; Ma, L.; Wang, S.Y.; Josephson, M.E.; Wang, G.K.; Morgan, J.P.; Leaf, A. Potent block of inactivation-deficient Na+ channels by n-3 polyunsaturated fatty acids. Am. J. Physiol. Cell. Physiol. 2006, 290, C362–C370. [Google Scholar]
- Mizushima, N.; Yoshimori, T. How to interpret LC3 immunoblotting. Autophagy 2007, 3, 542–545. [Google Scholar]
- Mei, S.; Ni, H.M.; Manley, S.; Bockus, A.; Kassel, K.M.; Luyendyk, J.P.; Copple, B.L.; Ding, W.X. Differential roles of unsaturated and saturated Fatty acids on autophagy and apoptosis in hepatocytes. J. Pharmacol. Exp. Ther. 2011, 339, 487–498. [Google Scholar] [CrossRef]
- Ghafourifar, P.; Klein, S.D.; Schucht, O.; Schenk, U.; Pruschy, M.; Rocha, S.; Richter, C. Ceramide induces cytochrome c release from isolated mitochondria. Importance of mitochondrial redox state. J. Biol. Chem. 1999, 10, 6080–6084. [Google Scholar]
- Ghosh, S.; Rodrigues, B. Cardiac cell death in early diabetes and its modulation by dietary fatty acids. Biochim. Biophys. Acta 1761, 1148–1162. [Google Scholar]
- Ostrander, D.B.; Sparagna, G.C.; Amoscato, A.A.; McMillin, J.B.; Dowhan, W. Decreased cardiolipin synthesis corresponds with cytochrome c release in palmitate-induced cardiomyocyte apoptosis. J. Biol. Chem. 2001, 276, 38061–38067. [Google Scholar]
- Gambert, S.; Vergely, C.; Filomenko, R.; Moreau, D.; Bettaieb, A.; Opie, L.H.; Rochette, L. Adverse effects of free fatty acid associated with increased oxidative stress in postischemic isolated rat hearts. Mol. Cell. Biochem. 2006, 283, 147–152. [Google Scholar] [CrossRef]
- Lavie, C.J.; Milani, R.V.; Mehra, M.R.; Ventura, H.O. Omega-3 polyunsaturated fatty acids and cardiovascular diseases. J. Am. Coll. Cardiol. 2009, 54, 585–594. [Google Scholar] [CrossRef]
- Leaf, A.; Albert, C.M.; Josephson, M.; Steinhaus, D.; Kluger, J.; Kang, J.X.; Cox, B.; Zhang, H.; Schoenfeld, D. Fatty acid antiarrhythmia trial investigators. Prevention of fatal arrhythmias in high-risk subjects by fish oil n-3 fatty acid intake. Circulation 2005, 112, 2762–2768. [Google Scholar] [CrossRef]
- Raitt, M.H.; Connor, W.E.; Morris, C.; Kron, J.; Halperin, B.; Chugh, S.S.; McClelland, J.; Cook, J.; MacMurdy, K.; Swenson, R.; et al. Fish oil supplementation and risk of ventricular tachycardia and ventricular fibrillation in patients with implantable defibrillators. A randomized controlled trial. J. Am. Med. Assoc. 2005, 293, 2884–2891. [Google Scholar]
- Brouwer, I.A.; Zock, P.L.; Camm, A.J.; Böcker, D.; Hauer, R.N.; Wever, E.F.; Dullemeijer, C.; Ronden, J.E.; Katan, M.B.; Lubinski, A.; et al. Effect of fish oil on ventricular tachyarrhythmia and death in patients with implantable cardioverter defibrillators: The Study on Omega-3 Fatty Acids and Ventricular Arrhythmia (SOFA) randomized trial. J. JAMA 2006, 295, 2613–2619. [Google Scholar] [CrossRef]
- Liang, H.; Zhong, Y.; Zhou, S.; Li, Q.Q. Palmitic acid-induced apoptosis in pancreatic β-cells is increased by liver X receptor agonist and attenuated by eicosapentaenoate. In Vivo 2011, 25, 711–718. [Google Scholar]
- Cecconi, F.; Levine, B. The role of autophagy in mammalian development: Cell makeover rather than cell death. Dev. Cell 2008, 15, 344–357. [Google Scholar] [CrossRef]
- Kuma, A.; Hatano, M.; Matsui, M.; Yamamoto, A.; Nakaya, H.; Yoshimori, T.; Ohsumi, Y.; Tokuhisa, T.; Mizushima, N. The role of autophagy during the early neonatal starvation period. Nature 2004, 432, 1032–1036. [Google Scholar]
- De Meyer, G.R.; Martinet, W. Autophagy in the cardiovascular system. Biochim. Biophys. Acta 1793, 1485–1495. [Google Scholar]
- Gustafsson, A.B.; Gottlieb, R.A. Recycle or die: The role of autophagy in cardioprotection. J. Mol. Cell. Cardiol. 2008, 44, 654–661. [Google Scholar] [CrossRef]
- Elmore, S.P.; Qian, T.; Grissom, S.F.; Lemasters, J.J. The mitochondrial permeability transition initiates autophagy in rat hepatocytes. FASEB J. 2001, 15, 2286–2287. [Google Scholar]
- Rothermel, B.A.; Hill, J.A. Autophagy in load-induced heart disease. Circ. Res. 2008, 103, 1363–1369. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cetrullo, S.; Tantini, B.; Flamigni, F.; Pazzini, C.; Facchini, A.; Stefanelli, C.; Caldarera, C.M.; Pignatti, C. Antiapoptotic and Antiautophagic Effects of Eicosapentaenoic Acid in Cardiac Myoblasts Exposed to Palmitic Acid. Nutrients 2012, 4, 78-90. https://doi.org/10.3390/nu4020078
Cetrullo S, Tantini B, Flamigni F, Pazzini C, Facchini A, Stefanelli C, Caldarera CM, Pignatti C. Antiapoptotic and Antiautophagic Effects of Eicosapentaenoic Acid in Cardiac Myoblasts Exposed to Palmitic Acid. Nutrients. 2012; 4(2):78-90. https://doi.org/10.3390/nu4020078
Chicago/Turabian StyleCetrullo, Silvia, Benedetta Tantini, Flavio Flamigni, Claudia Pazzini, Annalisa Facchini, Claudio Stefanelli, Claudio M. Caldarera, and Carla Pignatti. 2012. "Antiapoptotic and Antiautophagic Effects of Eicosapentaenoic Acid in Cardiac Myoblasts Exposed to Palmitic Acid" Nutrients 4, no. 2: 78-90. https://doi.org/10.3390/nu4020078




