Effects of Collagen and Collagen Hydrolysate from Jellyfish Umbrella on Histological and Immunity Changes of Mice Photoaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Preparation of JC and JCH
2.3. Experimental Design
2.4. UV Irradiation
2.5. Measurement of Water Content
2.6. Histological Analysis
2.7. Measurement of Spleen Index and Thymus Index
2.8. Statistical Analysis
3. Results and Discussion
3.1. Characterizations of JC and JCH
3.2. Determination of Water Content
| Group | Water Content (%) |
|---|---|
| NC | 72.93 ± 1.64 a |
| MC | 63.38 ± 3.99 c |
| JC-1 | 65.92 ± 2.32 bc |
| JC-1 | 67.35 ± 1.79 b |
| JCH-1 | 67.31 ± 2.68 b |
| JCH-2 | 69.54 ± 0.87 a |
3.3. The Effects of JC and JCH on Histological Changes of Photoaging Skin
3.3.1. The Effects of JC and JCH on Morphology of Photoaging Skin
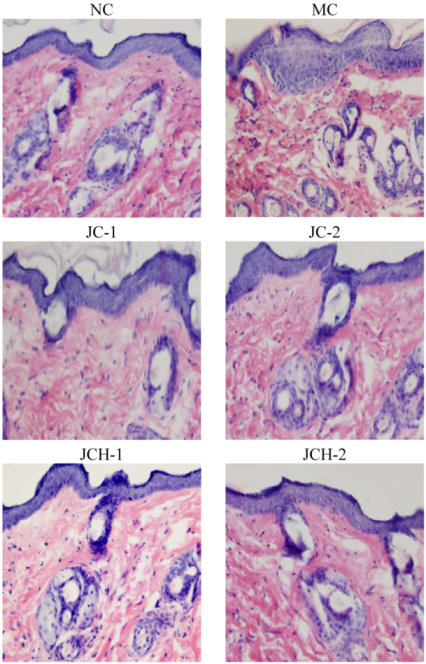
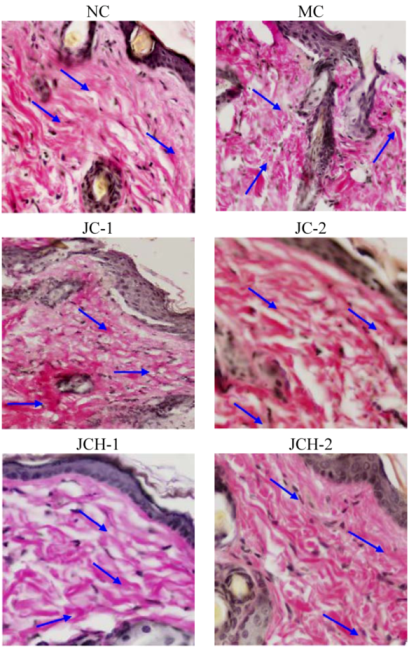
3.3.2. The Effects of JC and JCH on Endogenous Collagen of Photoaging Skin
 ).
).
 ).
).
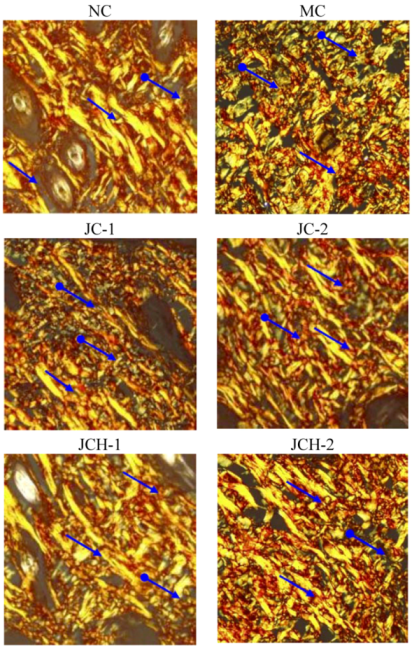
3.3.3. The Effects of JC and JCH on Collagen Type I and Collagen Type III of Photoaging Skin
 ), green: collagen type III (
), green: collagen type III (  ).
).
 ), green: collagen type III (
), green: collagen type III (  ).
).
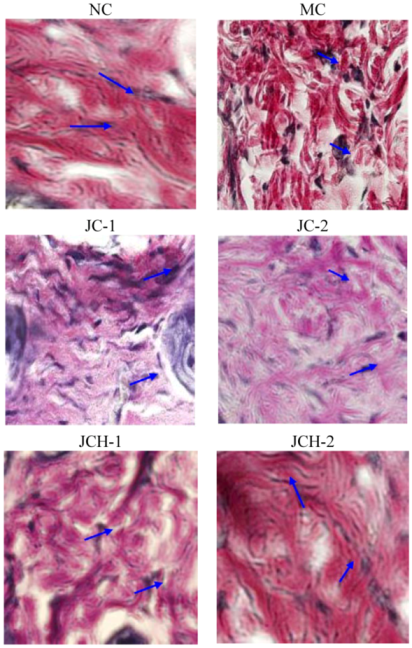
3.3.4. The Effect of JC and JCH on Elastin Protein Fiber of Photoaging Skin
 ).
).
 ).
).
3.4. Thymus Index and Spleen Index
| Group | Thymus Index (mg/g) | Spleen Index (mg/g) |
|---|---|---|
| NC | 0.57 ± 0.09 a | 3.91 ± 0.08 a |
| MC | 0.38 ± 0.10 d | 2.95 ± 0.09 b |
| JC-1 | 0.47 ± 0.16 c | 3.93 ± 0.31 a |
| JC-2 | 0.52 ± 0.13 b | 3.94 ± 0.48 a |
| JCH-1 | 0.56 ± 0.18 a | 3.77 ± 0.59 a |
| JCH-2 | 0.59 ± 0.15 a | 3.82 ± 0.44 a |
4. Conclusion
References
- Agar, N.S.; Halliday, G.M.; Barneston, R.; Ananthaswamy, H.N.; Wheeler, M.; Jones, A.M. The basal layer in human squamous tumors harbors more UVA than UVB fingerprint mutations: A role for UVA in human skin carcinogenesis. Proc. Natl. Acad. Sci. USA 2004, 101, 4954–4959. [Google Scholar]
- Hou, H.; Li, B.; Zhang, Z.; Xue, C.; Yu, G.; Wang, J.; Bao, Y.; Bu, L.; Sun, J.; Peng, Z.; Su, S. Moisuture absorption and retention properties, and activity in alleviating skin photodamage of collagen polypeptide from marine fish skin. Food Chem. 2012, 135, 1432–1439. [Google Scholar] [CrossRef]
- Han, Y.T.; Han, Z.W.; Yu, G.Y.; Wang, Y.J.; Cui, R.Y.; Wang, C.B. Inhibitory effect of polypeptide from Chlamys farreri on ultraviolet A-induced oxidative damage on human skin fibroblasts in vitro. Pharmacol. Res. 2004, 49, 265–274. [Google Scholar] [CrossRef]
- Prasad, N.R.; Ramachandran, S.; Pugalendi, K.V.; Menon, V.P. Ferulic acid inhibits UV-B-induced oxidative stress in human lymphocytes. Nutr. Res. 2007, 27, 559–564. [Google Scholar] [CrossRef]
- Hou, H.; Li, B.F.; Zhao, X.; Zhuang, Y.L.; Ren, G.Y.; Yan, M.Y.; Zhang, X.Q.; Chen, L.; Fan, Y. The effect of pacific cod (Gadus macrocephalus) skin gelatin polypeptides on UV radiation induced skin photoaging in ICR mice. Food Chem. 2009, 115, 945–950. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocolloid. 2011, 25, 1813–1827. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, Y.; Hou, H.; Zhao, X.; Zhang, Z.; Li, B. Effects of collagen and collagen hydrolysate from jellyfish (Rhopilema esculentum) on mice skin photoaging induced by UV irradiation. J. Food Sci. 2009, 74, 183–188. [Google Scholar] [CrossRef]
- Nagai, T.; Worawattanamateekul, W.; Suzuki, N.; Nakamura, T.; Ito, T.; Fujiki, K.; Nakao, M.; Yano, T. Isolation and characterization of collagen from rhizostomous jellyfish (Rhopilema asamushi). Food Chem. 2000, 70, 205–208. [Google Scholar] [CrossRef]
- The Minister of Health of the People’s Republic of China, National Food Safety Standard Determination of Moisture in Foods; Standards Press of China: Beijing, China, 2010.
- Jung, J.W.; Cha, S.H.; Lee, S.C.; Chun, I.K.; Kim, Y.P. Age-related changes of water content in the rat skin. J. Dermatol. Sci. 1997, 14, 12–19. [Google Scholar] [CrossRef]
- Wulf, H.C.; Sandby-Møller, J.; Kobayasi, T.; Gniadecki, R. Gniadecki skin aging and natural photoprotection. Micron 2004, 35, 185–191. [Google Scholar] [CrossRef]
- Labat-Robert, J.; Fourtanier, A.; Boyer-Lafargue, B.; Robert, L. Age dependent increase of elastase type protease activity in mouse skin: Effect of UV-irradiation. J. Photoch. Photo. B 2000, 57, 113–118. [Google Scholar] [CrossRef]
- Borges, L.F.; Gutierrez, P.S.; Marana, H.R.; Taboga, S.R. Picrosiriuspolarization staining method as an efficient histopathological tool for collagenolysis detection in vesical prolapse lesions. Micron 2007, 38, 580–583. [Google Scholar] [CrossRef]
- Liang, J.; Pei, X.; Zhang, Z.; Wang, N.; Wang, J.; Li, Y. The protective effects of long-term oral administration of marine collagen hydrolysate from chum salmon on collagen matrix homeostasis in the chronological aged skin of sprague-dawley male rats. J. Food Sci. 2010, 75, 230–238. [Google Scholar] [CrossRef]
- Venditti, E.; Scirè, A.; Tanfani, F.; Greci, L.; Damiani, E. Nitroxides are more efficient inhibitors of oxidative damage to calf skin collagen than antioxidant vitamins. Biochim. Biophys. Acta 2008, 1780, 58–68. [Google Scholar] [CrossRef]
- Tsuji, N.; Moriwaki, S.; Suzuki, Y.; Takema, Y.; Imokawa, G. The role of elastases secreted by fibroblasts in wrinkle formation: Implication through selective inhibition of elastase activity. Photochem. Photobiol. 2001, 74, 283–290. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, H. Effects of electroacupuncture on T-lymphocytes, spleen index, thymus index and lymphopoiesis levels in strenuous exercise-induced stress rats (in Chinese). Zhen Ci Yan Jiu 2012, 37, 136–139. [Google Scholar]
- Goettsch, W.; Garssen, J.; Slob, W.; de Gruijl, F.R.; Loveren, H.V. Risk assessment for the harmful effects of UVB radiation on the immunological resistance to infectious diseases. Environ. Health Perspect. 1998, 106, 71–77. [Google Scholar]
- Beissert, S.; Schwartz, T. Mechanisms involved in ultraviolet light-induced immunosuppression. J. Invest. Dermatol. Symp. Proc. 1999, 4, 61–64. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fan, J.; Zhuang, Y.; Li, B. Effects of Collagen and Collagen Hydrolysate from Jellyfish Umbrella on Histological and Immunity Changes of Mice Photoaging. Nutrients 2013, 5, 223-233. https://doi.org/10.3390/nu5010223
Fan J, Zhuang Y, Li B. Effects of Collagen and Collagen Hydrolysate from Jellyfish Umbrella on Histological and Immunity Changes of Mice Photoaging. Nutrients. 2013; 5(1):223-233. https://doi.org/10.3390/nu5010223
Chicago/Turabian StyleFan, Jian, Yongliang Zhuang, and Bafang Li. 2013. "Effects of Collagen and Collagen Hydrolysate from Jellyfish Umbrella on Histological and Immunity Changes of Mice Photoaging" Nutrients 5, no. 1: 223-233. https://doi.org/10.3390/nu5010223




