Higher Serum 25-Hydroxyvitamin D Concentrations Associate with a Faster Recovery of Skeletal Muscle Strength after Muscular Injury
Abstract
:1. Introduction
2. Experimental Section
2.1. Exercise Protocol
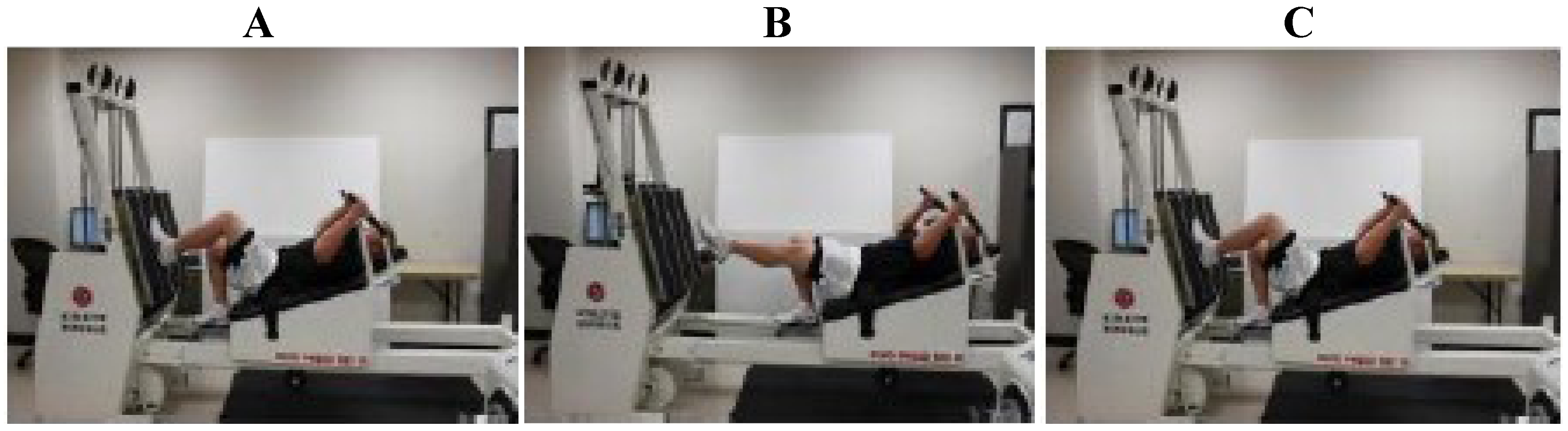
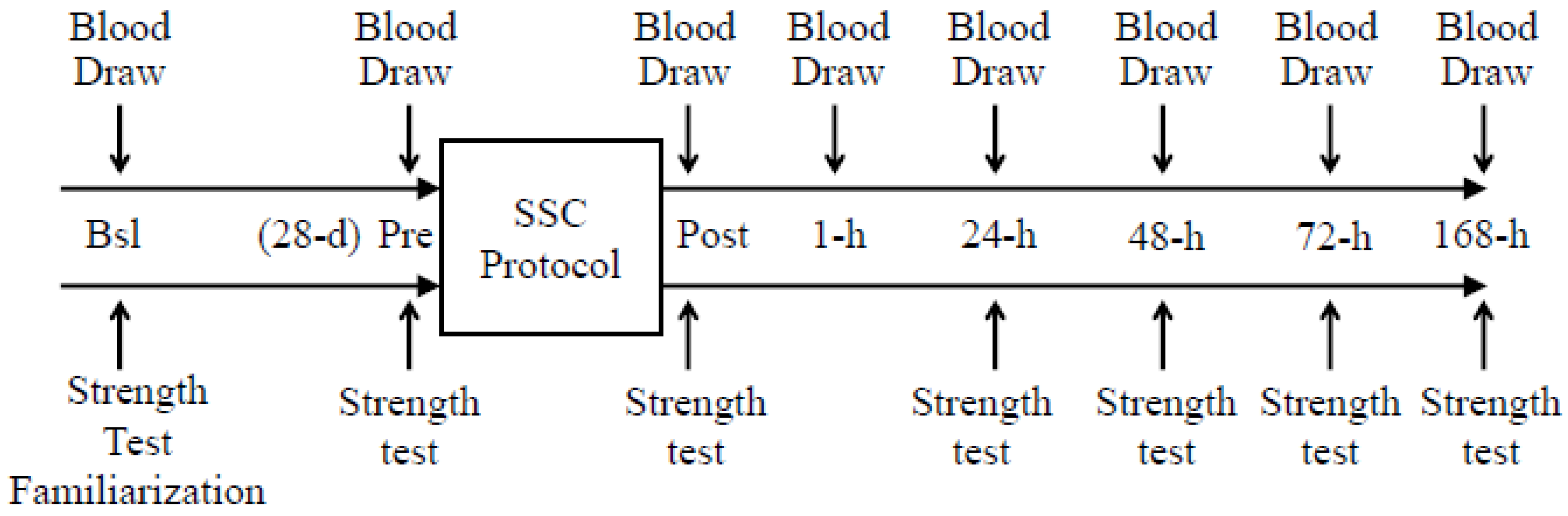
2.2. Analytical Procedures
2.2.1. Serum 25(OH)D Concentration
2.2.2. Serum Cytokine Concentrations
2.2.3. Clinical Chemistries
2.2.4. Single-Leg Strength Testing
2.3. Statistical Analyses
3. Results
3.1. Subject Characteristics
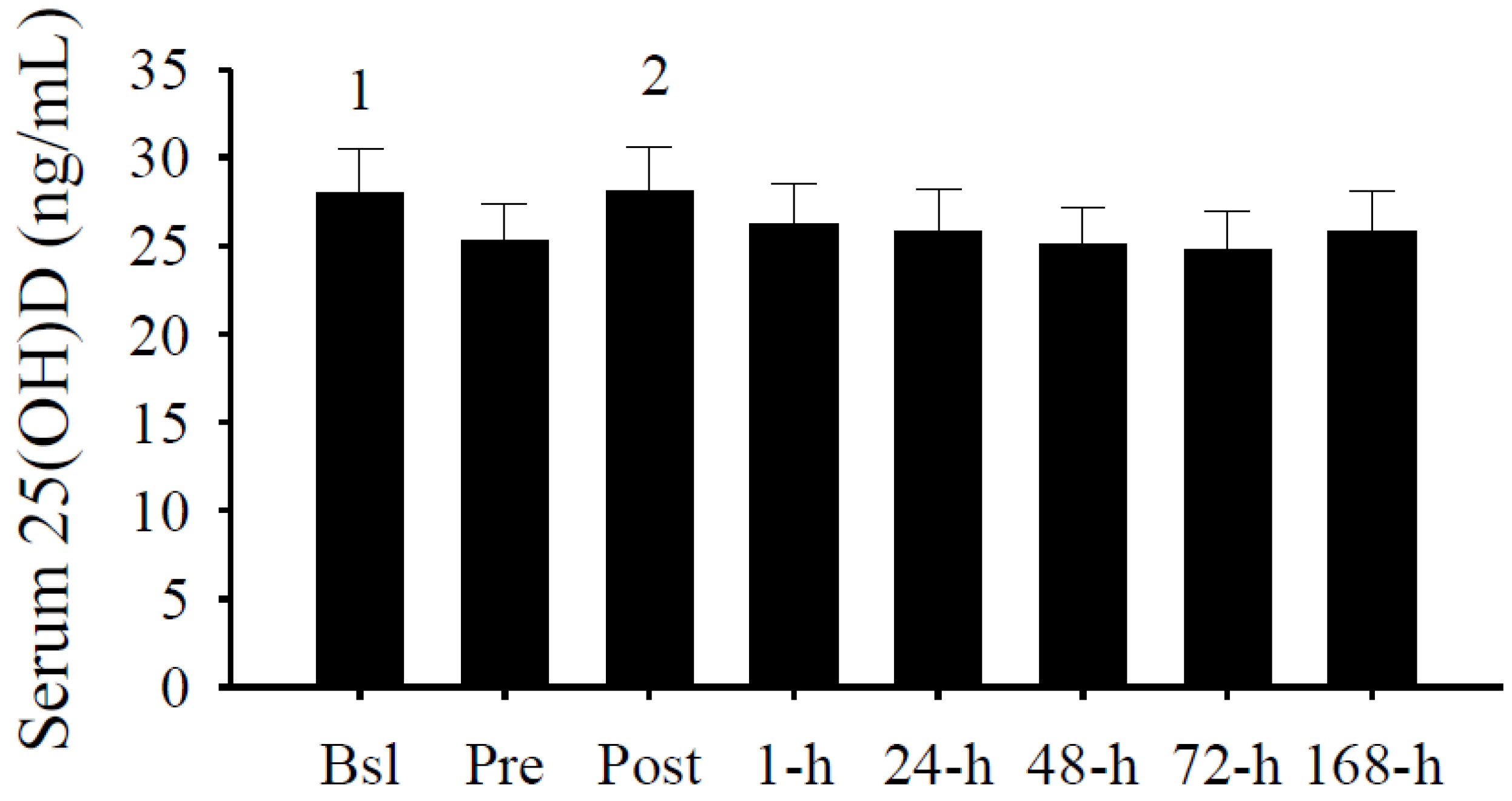
3.2. Serum 25(OH)D Concentrations
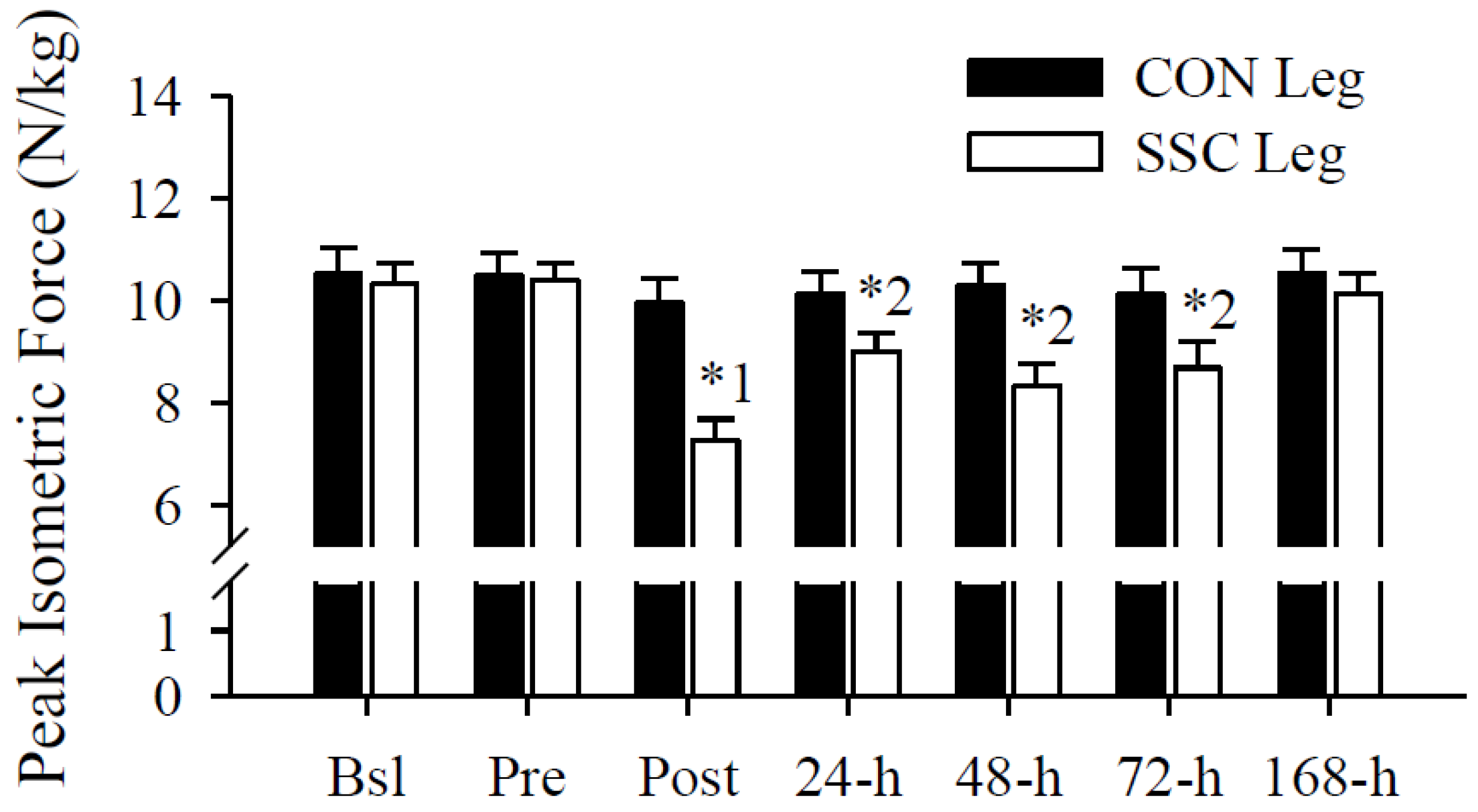
3.3. Peak Isometric Force
3.4. Serum 25(OH)D Predicts Immediate and Persistent Muscular Weakness after a Muscle-Damaging Event
| Coefficient | |||
|---|---|---|---|
| Unstandardized (±SE) | Standardized | p | |
| Post | |||
| Constant | 6.23 (±4.12) | ||
| Pre serum 25(OH)D | −0.28 (±0.11) | −0.68 | 0.03 |
| Δ serum 25(OH)D | −0.11 (±0.10) | −0.27 | 0.29 |
| Age | −0.06 (±0.08) | −0.17 | 0.50 |
| BMI | −0.28 (±0.13) | −0.57 | 0.06 |
| r2 = 0.51, adjusted r2 = 0.30 | |||
| 24-h | |||
| Constant | 2.41 (±4.00) | ||
| Pre serum 25(OH)D | −0.17 (±0.10) | −0.52 | 0.14 |
| Δ Serum 25(OH)D | −0.04 (±0.10) | −0.12 | 0.70 |
| Age | −0.03 (±0.07) | −0.13 | 0.67 |
| BMI | −0.04 (±0.13) | −0.10 | 0.77 |
| r2 = 0.24, adjusted r2 = 0.00 | |||
| 48-h | |||
| Constant | 7.24 (±4.43) | ||
| Pre serum 25(OH)D | −0.28 (±0.12) | −0.68 | 0.04 |
| Δ Serum 25(OH)D | −0.14 (±0.11) | −0.33 | 0.23 |
| Age | −0.07 (±0.08) | −0.23 | 0.40 |
| BMI | −0.10 (±0.11) | −0.29 | 0.33 |
| r2 = 0.45, adjusted r2 = 0.21 | |||
| 72-h | |||
| Constant | 6.40 (±3.34) | ||
| Pre serum 25(OH)D | −0.21 (±0.09) | −0.63 | 0.04 |
| Δ Serum 25(OH)D | −0.15 (±0.08) | −0.45 | 0.09 |
| Age | −0.08 (±0.06) | −0.29 | 0.26 |
| BMI | −0.10 (±0.11) | −0.26 | 0.36 |
| r2 = 0.52, adjusted r2 = 0.31 | |||
3.5. Plasma AST and ALT
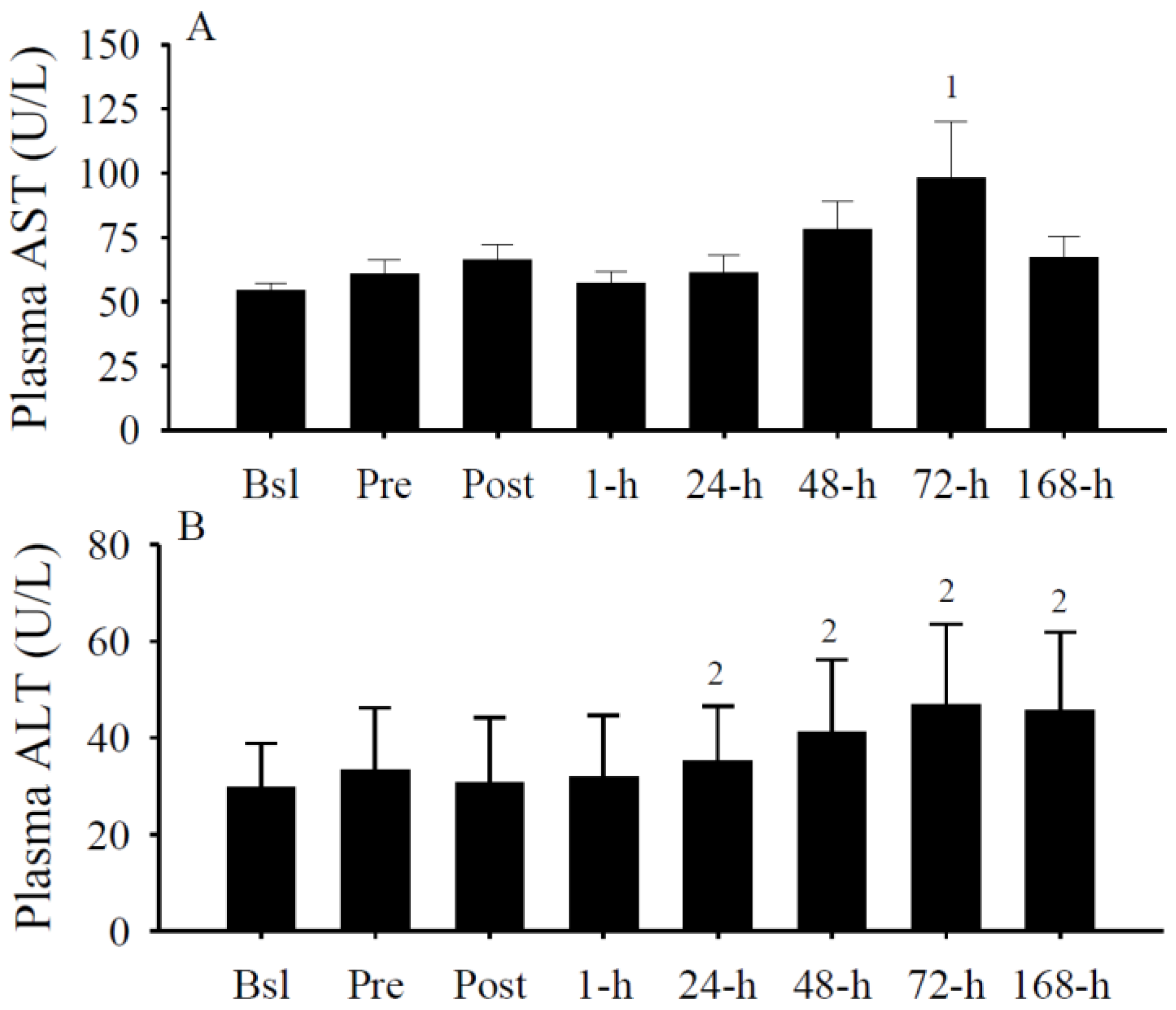
3.6. Serum Cytokine Concentrations
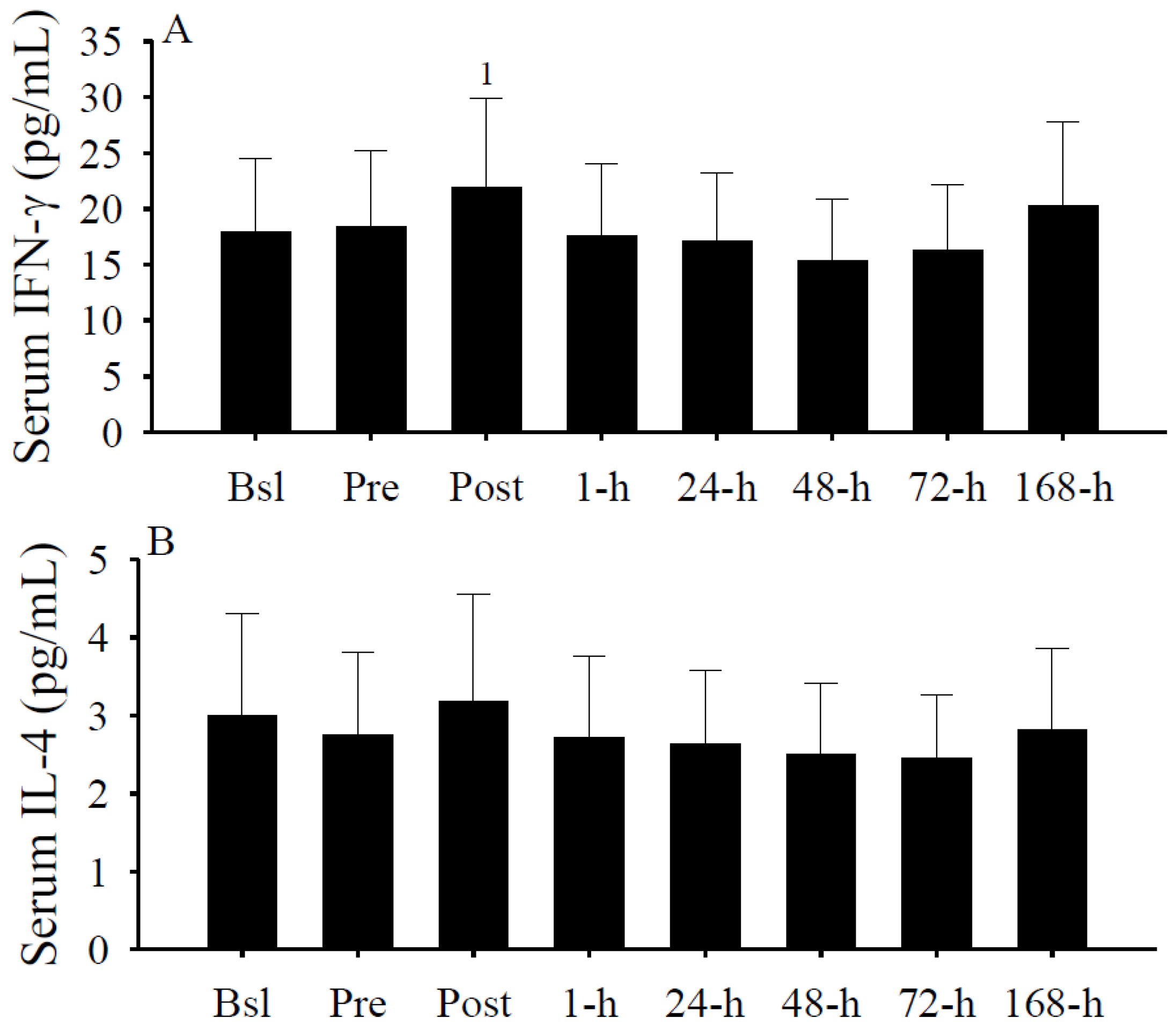
3.7. Plasma Calcium, PTH, and Albumin Concentrations
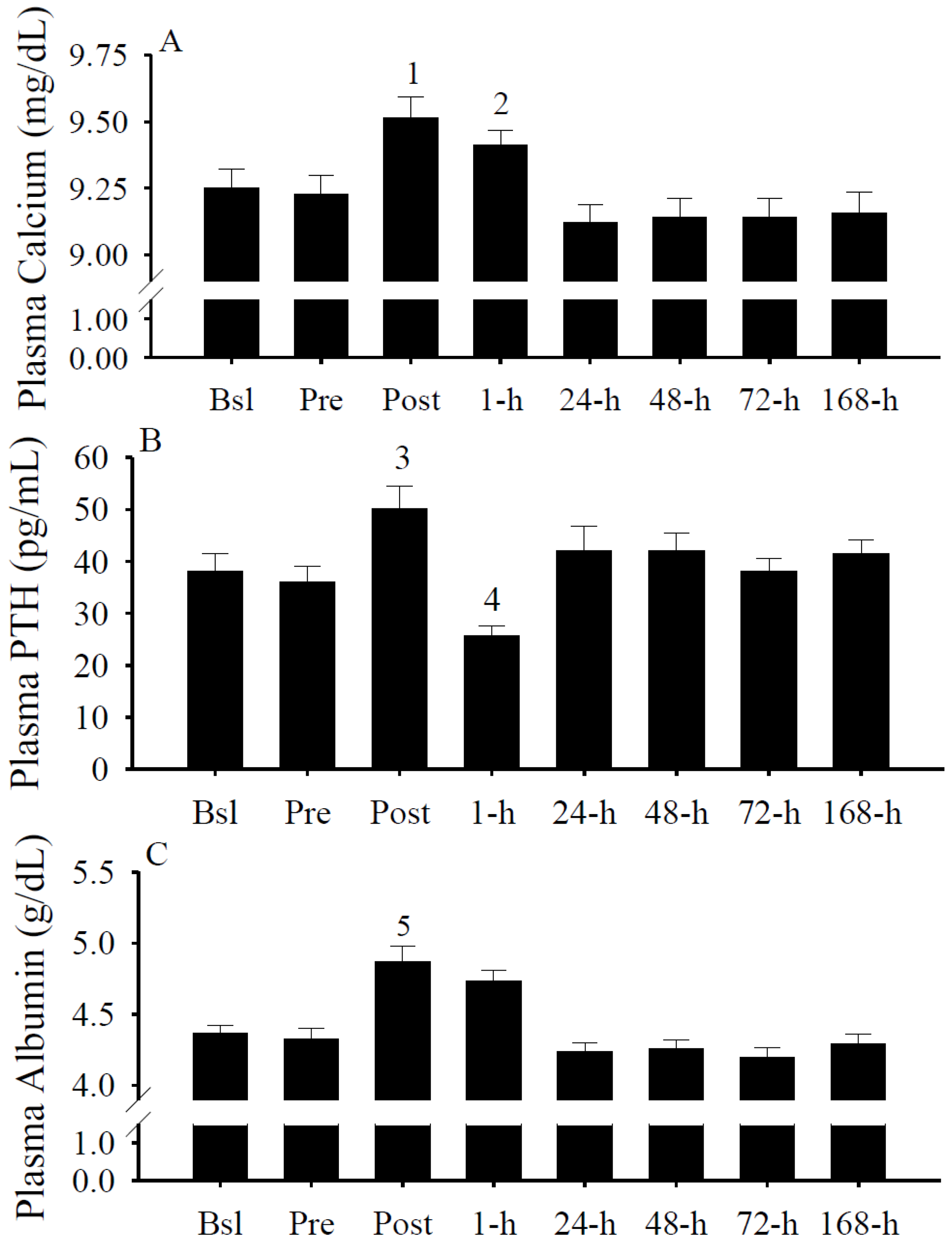
3.8. IFN-γ and Albumin Predict Serum 25(OH)D Changes
| Coefficient | |||
|---|---|---|---|
| Unstandardized (± SE) | Standardized | p | |
| Constant | 75.99 (±6.32) | ||
| Δ IFN-γ | −0.60 (±0.09) | −0.60 | <0.001 |
| Δ iPTH | 0.06 (±0.09) | 0.06 | 0.48 |
| Δ Calcium | 19.7 (±10.6) | 0.19 | 0.07 |
| Δ Albumin | 28.1 (±9.3) | 0.32 | <0.005 |
| r2 = 0.36, adjusted r2 = 0.33 | |||
3.9. Gender Differences
4. Discussion
5. Conclusions and Implications
Acknowledgments
Conflict of Interest
References
- Jones, G. Pharmacokinetics of vitamin D toxicity. Am. J. Clin. Nutr. 2008, 88, 582S–586S. [Google Scholar]
- Girgis, C.M.; Clifton-Bligh, R.J.; Hamrick, M.W.; Holick, M.F.; Gunton, J.E. The roles of vitamin D in skeletal muscle: form, function, and metabolism. Endocr. Rev. 2013, 34, 33–83. [Google Scholar] [CrossRef]
- Ceglia, L.; Harris, S.S. Vitamin D and its role in skeletal muscle. Calcif. Tissue Int. 2013, 92, 151–162. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.; Stahelin, H.B.; Walter, P. Vitamin D effects on bone and muscle. Int. J. Vitam. Nutr. Res. 2011, 81, 264–272. [Google Scholar] [CrossRef]
- Ceglia, L. Vitamin D and skeletal muscle tissue and function. Mol. Aspects Med. 2008, 29, 407–414. [Google Scholar] [CrossRef]
- Boland, R.L. VDR activation of intracellular signaling pathways in skeletal muscle. Mol. Cell. Endocrinol. 2011, 347, 11–16. [Google Scholar] [CrossRef]
- Boland, R. Role of vitamin D in skeletal muscle function. Endocr. Rev. 1986, 7, 434–448. [Google Scholar] [CrossRef]
- Boland, R.; de Boland, A.R.; Marinissen, M.J.; Santillan, G.; Vazquez, G.; Zanello, S. Avian muscle cells as targets for the secosteroid hormone 1,25-dihydroxy-vitamin D3. Mol. Cell. Endocrinol. 1995, 114, 1–8. [Google Scholar] [CrossRef]
- Birge, S.J.; Haddad, J.G. 25-Hydroxycholecalciferol stimulation of muscle metabolism. J. Clin. Invest. 1975, 56, 1100–1107. [Google Scholar] [CrossRef]
- De Boland, A.R.; Boland, R. In vitro cellular muscle calcium metabolism. Characterization of effects of 1,25-dihydroxy-vitamin D3 and 25-hydroxy-vitamin D3. Z. Naturforsch. C 1985, 40, 102–108. [Google Scholar]
- De Boland, A.R.; Boland, R.L. Rapid changes in skeletal muscle calcium uptake induced in vitro by 1,25-dihydroxyvitamin D3 are suppressed by calcium channel blockers. Endocrinology 1987, 120, 1858–1864. [Google Scholar] [CrossRef]
- Walters, M.R.; Ilenchuk, T.T.; Claycomb, W.C. 1,25-Dihydroxyvitamin D3 stimulates 45Ca2+ uptake by cultured adult rat ventricular cardiac muscle cells. J. Biol. Chem. 1987, 262, 2536–2541. [Google Scholar]
- Giuliani, D.L.; Boland, R.L. Effects of vitamin D3 metabolites on calcium fluxes in intact chicken skeletal muscle and myoblasts cultured in vitro. Calcif. Tissue Int. 1984, 36, 200–205. [Google Scholar] [CrossRef]
- Matthews, C.; Heimberg, K.W.; Ritz, E.; Agostini, B.; Fritzsche, J.; Hasselbach, W. Effect of 1,25-dihydroxycholecalciferol on impaired calcium transport by the sarcoplasmic reticulum in experimental uremia. Kidney Int. 1977, 11, 227–235. [Google Scholar] [CrossRef]
- Morelli, S.; Buitrago, C.; Vazquez, G.; de Boland, A.R.; Boland, R. Involvement of tyrosine kinase activity in 1alpha,25(OH)2-vitamin D3 signal transduction in skeletal muscle cells. J. Biol. Chem. 2000, 275, 36021–36028. [Google Scholar]
- Selles, J.; Boland, R. Rapid stimulation of calcium uptake and protein phosphorylation in isolated cardiac muscle by 1,25-dihydroxyvitamin D3. Mol. Cell. Endocrinol. 1991, 77, 67–73. [Google Scholar] [CrossRef]
- Buitrago, C.; Boland, R.; de Boland, A.R. The tyrosine kinase c-Src is required for 1,25(OH)2-vitamin D3 signalling to the nucleus in muscle cells. Biochim. Biophys. Acta 2001, 1541, 179–187. [Google Scholar] [CrossRef]
- Buitrago, C.G.; Arango, N.S.; Boland, R.L. 1alpha,25(OH)2D3-dependent modulation of Akt in proliferating and differentiating C2C12 skeletal muscle cells. J. Cell. Biochem. 2012, 113, 1170–1181. [Google Scholar] [CrossRef]
- Stratos, I.; Li, Z.; Herlyn, P.; Rotter, R.; Behrendt, A.K.; Mittlmeier, T.; Vollmar, B. Vitamin D increases cellular turnover and functionally restores the skeletal muscle after crush injury in rats. Am. J. Pathol. 2012, 182, 895–904. [Google Scholar]
- Alami-Durante, H.; Cluzeaud, M.; Bazin, D.; Mazurais, D.; Zambonino-Infante, J.L. Dietary cholecalciferol regulates the recruitment and growth of skeletal muscle fibers and the expressions of myogenic regulatory factors and myosin heavy chain in European sea bass larvae. J. Nutr. 2011, 141, 2146–2151. [Google Scholar] [CrossRef]
- Srikuea, R.; Zhang, X.; Park-Sarge, O.K.; Esser, K.A. VDR and CYP27B1 are expressed in C2C12 cells and regenerating skeletal muscle: Potential role in suppression of myoblast proliferation. Am. J. Physiol. Cell. Physiol. 2012, 303, C396–C405. [Google Scholar] [CrossRef]
- Garcia, L.A.; Ferrini, M.G.; Norris, K.C.; Artaza, J.N. 1,25(OH)(2)vitamin D(3) enhances myogenic differentiation by modulating the expression of key angiogenic growth factors and angiogenic inhibitors in C(2)C(12) skeletal muscle cells. J. Steroid. Biochem. Mol. Biol. 2013, 133, 1–11. [Google Scholar] [CrossRef]
- Ward, K.A.; Das, G.; Berry, J.L.; Roberts, S.A.; Rawer, R.; Adams, J.E.; Mughal, Z. Vitamin D status and muscle function in post-menarchal adolescent girls. J. Clin. Endocrinol. Metab. 2009, 94, 559–563. [Google Scholar] [CrossRef]
- Ward, K.A.; Das, G.; Roberts, S.A.; Berry, J.L.; Adams, J.E.; Rawer, R.; Mughal, M.Z. A randomized, controlled trial of vitamin D supplementation upon musculosketal health in postmenarchal females. J. Clin. Endocrinol. Metab. 2010, 95, 4643–4651. [Google Scholar]
- Foo, L.H.; Zhang, Q.; Zhu, K.; Ma, G.; Hu, X.; Greenfield, H.; Fraser, D.R. Low vitamin D status has an adverse influence on bone mass, bone turnover, and muscle strength in Chinese adolescent girls. J. Nutr. 2009, 139, 1002–1007. [Google Scholar] [CrossRef]
- Ceglia, L.; Chiu, G.R.; Harris, S.S.; Araujo, A.B. Serum 25-hydroxyvitamin D concentration and physical function in adult men. Clin. Endocrinol. (Oxf.) 2011, 74, 370–376. [Google Scholar]
- Ziambaras, K.; Dagogo-Jack, S. Reversible muscle weakness in patients with vitamin D deficiency. West. J. Med. 1997, 167, 435–439. [Google Scholar]
- Russell, J.A. Osteomalacic myopathy. Muscle Nerve 1994, 17, 578–580. [Google Scholar] [CrossRef]
- Stockton, K.A.; Kandiah, D.A.; Paratz, J.D.; Bennell, K.L. Fatigue, muscle strength and vitamin D status in women with systemic lupus erythematosus compared to healthy controls. Lupus 2011, 21, 271–278. [Google Scholar]
- Glerup, H.; Mikkelsen, K.; Poulsen, L.; Hass, E.; Overbeck, S.; Andersen, H.; Charles, P.; Eriksen, E.F. Hypovitaminosis D myopathy without biochemical signs of osteomalacic bone involvement. Calcif. Tissue Int. 2000, 66, 419–424. [Google Scholar]
- Barker, T.; Martins, T.B.; Hill, H.R.; Kjeldsberg, C.R.; Trawick, R.H.; Weaver, L.K.; Traber, M.G. Low vitamin D impairs strength recovery after anterior cruciate ligament surgery. J. Evid. Based Complement. Altern. Med. 2011, 16, 201–209. [Google Scholar] [CrossRef]
- Prineas, J.W.; Mason, A.S.; Henson, R.A. Myopathy in metabolic bone disease. Br. Med. J. 1965, 1, 1034–1036. [Google Scholar] [CrossRef]
- Gordon, P.L.; Doyle, J.W.; Johansen, K.L. Association of 1,25-dihydroxyvitamin D levels with physical performance and thigh muscle cross-sectional area in chronic kidney disease stage 3 and 4. J. Ren. Nutr. 2012, 22, 423–433. [Google Scholar] [CrossRef]
- Grimaldi, A.S.; Parker, B.A.; Capizzi, J.A.; Clarkson, P.M.; Pescatello, L.S.; White, C.M.; Thompson, P.D. 25(OH) vitamin D is associated with greater muscle strength in healthy men and women. Med. Sci. Sports Exerc. 2013, 45, 157–162. [Google Scholar] [CrossRef]
- Von Hurst, P.R.; Conlon, C.; Foskett, A. Vitamin D status predicts hand-grip strength in young adult women living in Auckland, New Zealand. J. Steroid. Biochem. Mol. Biol. 2012. [Google Scholar] [CrossRef]
- Wicherts, I.S.; van Schoor, N.M.; Boeke, A.J.; Visser, M.; Deeg, D.J.; Smit, J.; Knol, D.L.; Lips, P. Vitamin D status predicts physical performance and its decline in older persons. J. Clin. Endocrinol. Metab. 2007, 92, 2058–2065. [Google Scholar] [CrossRef]
- Barker, T.; Martins, T.B.; Hill, H.R.; Kjeldsberg, C.R.; Dixon, B.M.; Schneider, E.D.; Henridsen, V.T.; Weaver, L.K. Circulating pro-inflammatory cytokines are elevated and peak power output correlates with 25-hydroxyvitamin D in vitamin D insufficient adults. Eur. J. Appl. Physiol. 2013. [Google Scholar] [CrossRef]
- Carrillo, A.E.; Flynn, M.G.; Pinkston, C.; Markofski, M.M.; Jiang, Y.; Donkin, S.S.; Teegarden, D. Impact of vitamin D supplementation during a resistance training intervention on body composition, muscle function, and glucose tolerance in overweight and obese adults. Clin. Nutr. 2012. [Google Scholar] [CrossRef]
- Barker, T.; Martins, T.B.; Hill, H.R.; Kjeldsberg, C.R.; Henriksen, V.T.; Dixon, B.M.; Schneider, E.D.; Dern, A.; Weaver, L.K. Different doses of supplemental vitamin D maintain interleukin-5 without altering skeletal muscle strength: A randomized, double-blind, placebo-controlled study in vitamin D sufficient adults. Nutr. Metab. (Lond.) 2012, 9, 16. [Google Scholar] [CrossRef]
- Sato, Y.; Iwamoto, J.; Kanoko, T.; Satoh, K. Low-dose vitamin D prevents muscular atrophy and reduces falls and hip fractures in women after stroke: A randomized controlled trial. Cerebrovasc. Dis. 2005, 20, 187–192. [Google Scholar] [CrossRef]
- Janssen, H.C.; Samson, M.M.; Verhaar, H.J. Muscle strength and mobility in vitamin D-insufficient female geriatric patients: A randomized controlled trial on vitamin D and calcium supplementation. Aging Clin. Exp. Res. 2010, 22, 78–84. [Google Scholar]
- Verhaar, H.J.; Samson, M.M.; Jansen, P.A.; de Vreede, P.L.; Manten, J.W.; Duursma, S.A. Muscle strength, functional mobility and vitamin D in older women. Aging (Milano) 2000, 12, 455–460. [Google Scholar]
- Floyd, M.; Ayyar, D.R.; Barwick, D.D.; Hudgson, P.; Weightman, D. Myopathy in chronic renal failure. Q. J. Med. 1974, 43, 509–524. [Google Scholar]
- Warren, G.L.; Lowe, D.A.; Armstrong, R.B. Measurement tools used in the study of eccentric contraction-induced injury. Sports Med. 1999, 27, 43–59. [Google Scholar] [CrossRef]
- Komi, P.V. Stretch-shortening cycle: A powerful model to study normal and fatigued muscle. J. Biomech. 2000, 33, 1197–1206. [Google Scholar] [CrossRef]
- Burgi, A.A.; Gorham, E.D.; Garland, C.F.; Mohr, S.B.; Garland, F.C.; Zeng, K.; Thompson, K.; Lappe, J.M. High serum 25-hydroxyvitamin D is associated with a low incidence of stress fractures. J. Bone Miner. Res. 2011, 26, 2371–2377. [Google Scholar] [CrossRef]
- Lappe, J.; Cullen, D.; Haynatzki, G.; Recker, R.; Ahlf, R.; Thompson, K. Calcium and vitamin D supplementation decreases incidence of stress fractures in female navy recruits. J. Bone Miner. Res. 2008, 23, 741–749. [Google Scholar] [CrossRef]
- Snijder, M.B.; van Schoor, N.M.; Pluijm, S.M.; van Dam, R.M.; Visser, M.; Lips, P. Vitamin D status in relation to one-year risk of recurrent falling in older men and women. J. Clin. Endocrinol. Metab. 2006, 91, 2980–2985. [Google Scholar] [CrossRef]
- Broe, K.E.; Chen, T.C.; Weinberg, J.; Bischoff-Ferrari, H.A.; Holick, M.F.; Kiel, D.P. A higher dose of vitamin D reduces the risk of falls in nursing home residents: A randomized, multiple-dose study. J. Am. Geriatr. Soc. 2007, 55, 234–239. [Google Scholar] [CrossRef]
- Bischoff, H.A.; Stahelin, H.B.; Dick, W.; Akos, R.; Knecht, M.; Salis, C.; Nebiker, M.; Theiler, R.; Pfeifer, M.; Begerow, B.; et al. Effects of vitamin D and calcium supplementation on falls: A randomized controlled trial. J. Bone Miner. Res. 2003, 18, 343–351. [Google Scholar] [CrossRef]
- Visser, M.; Deeg, D.J.; Lips, P. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): The Longitudinal Aging Study Amsterdam. J. Clin. Endocrinol. Metab. 2003, 88, 5766–5772. [Google Scholar] [CrossRef]
- Chapuy, M.C.; Arlot, M.E.; Delmas, P.D.; Meunier, P.J. Effect of calcium and cholecalciferol treatment for three years on hip fractures in elderly women. BMJ 1994, 308, 1081–1082. [Google Scholar] [CrossRef]
- Dawson-Hughes, B.; Harris, S.S.; Krall, E.A.; Dallal, G.E. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N. Engl. J. Med. 1997, 337, 670–676. [Google Scholar] [CrossRef]
- Flicker, L.; Mead, K.; MacInnis, R.J.; Nowson, C.; Scherer, S.; Stein, M.S.; Thomasx, J.; Hopper, J.L.; Wark, J.D. Serum vitamin D and falls in older women in residential care in Australia. J. Am. Geriatr. Soc. 2003, 51, 1533–1538. [Google Scholar] [CrossRef]
- Ring, S.M.; Dannecker, E.A.; Peterson, C.A. Vitamin D status is not associated with outcomes of experimentally-induced muscle weakness and pain in young, healthy volunteers. J. Nutr. Metab. 2010, 2010, 674240. [Google Scholar]
- Peake, J.M.; Nosaka, K.; Muthalib, M.; Suzuki, K. Systemic inflammatory responses to maximal versus submaximal lengthening contractions of the elbow flexors. Exerc. Immunol. Rev. 2006, 12, 72–85. [Google Scholar]
- Louw, J.A.; Werbeck, A.; Louw, M.E.; Kotze, T.J.; Cooper, R.; Labadarios, D. Blood vitamin concentrations during the acute-phase response. Crit. Care Med. 1992, 20, 934–941. [Google Scholar] [CrossRef]
- Reid, D.; Toole, B.J.; Knox, S.; Talwar, D.; Harten, J.; O’Reilly, D.S.; Blackwell, S.; Kinsella, J.; McMillan, D.C.; Wallace, A.M. The relation between acute changes in the systemic inflammatory response and plasma 25-hydroxyvitamin D concentrations after elective knee arthroplasty. Am. J. Clin. Nutr. 2011, 93, 1006–1011. [Google Scholar] [CrossRef]
- Barker, T.; Martins, T.B.; Kjeldsberg, C.R.; Trawick, R.H.; Hill, H.R. Circulating interferon-gamma correlates with 1,25(OH)D and the 1,25(OH)D-to-25(OH)D ratio. Cytokine 2012, 60, 23–26. [Google Scholar] [CrossRef]
- Koeffler, H.P.; Reichel, H.; Bishop, J.E.; Norman, A.W. Gamma-interferon stimulates production of 1,25-dihydroxyvitamin D3 by normal human macrophages. Biochem. Biophys. Res. Commun. 1985, 127, 596–603. [Google Scholar] [CrossRef]
- Reichel, H.; Koeffler, H.P.; Barbers, R.; Norman, A.W. Regulation of 1,25-dihydroxyvitamin D3 production by cultured alveolar macrophages from normal human donors and from patients with pulmonary sarcoidosis. J. Clin. Endocrinol. Metab. 1987, 65, 1201–1209. [Google Scholar] [CrossRef]
- Stoffels, K.; Overbergh, L.; Giulietti, A.; Verlinden, L.; Bouillon, R.; Mathieu, C. Immune regulation of 25-hydroxyvitamin-D3-1alpha-hydroxylase in human monocytes. J. Bone Miner. Res. 2006, 21, 37–47. [Google Scholar]
- Stoffels, K.; Overbergh, L.; Bouillon, R.; Mathieu, C. Immune regulation of 1alpha-hydroxylase in murine peritoneal macrophages: unravelling the IFNgamma pathway. J. Steroid. Biochem. Mol. Biol. 2007, 103, 567–571. [Google Scholar] [CrossRef]
- Overbergh, L.; Stoffels, K.; Valckx, D.; Giulietti, A.; Bouillon, R.; Mathieu, C. Regulation of 25-hydroxyvitamin D-1alpha-hydroxylase by IFNgamma in human monocytic THP1 cells. J. Steroid. Biochem. Mol. Biol. 2004, 89–90, 453–455. [Google Scholar]
- Edfeldt, K.; Liu, P.T.; Chun, R.; Fabri, M.; Schenk, M.; Wheelwright, M.; Keegan, C.; Krutzik, S.R.; Adams, J.S.; Hewison, M.; Modlin, R.L. T-cell cytokines differentially control human monocyte antimicrobial responses by regulating vitamin D metabolism. Proc. Natl. Acad. Sci. USA 2010, 107, 22593–22598. [Google Scholar] [CrossRef]
- Weinstock, C.; Konig, D.; Harnischmacher, R.; Keul, J.; Berg, A.; Northoff, H. Effect of exhaustive exercise stress on the cytokine response. Med. Sci. Sports Exerc. 1997, 29, 345–354. [Google Scholar] [CrossRef]
- Malm, C.; Sjodin, B.; Sjoberg, B.; Lenkei, R.; Renstrom, P.; Lundberg, I.E.; Ekblom, B. Leukocytes, cytokines, growth factors and hormones in human skeletal muscle and blood after uphill or downhill running. J. Physiol. 2004, 556, 983–1000. [Google Scholar]
- Barker, T.; Henriksen, V.T.; Martins, T.B.; Kjeldsberg, C.R.; Hill, H.R. Fluctuations in the skeletal muscle power-velocity relationship and interferon-gamma after a muscle-damaging event in humans. Extrem. Physiol. Med. 2012, 1, 1–6. [Google Scholar]
- Peake, J.M.; Suzuki, K.; Hordern, M.; Wilson, G.; Nosaka, K.; Coombes, J.S. Plasma cytokine changes in relation to exercise intensity and muscle damage. Eur. J. Appl. Physiol. 2005, 95, 514–521. [Google Scholar] [CrossRef]
- Hirose, L.; Nosaka, K.; Newton, M.; Laveder, A.; Kano, M.; Peake, J.; Suzuki, K. Changes in inflammatory mediators following eccentric exercise of the elbow flexors. Exerc. Immunol. Rev. 2004, 10, 75–90. [Google Scholar]
- Duncan, A.; Talwar, D.; McMillan, D.C.; Stefanowicz, F.; O’Reilly, D.S. Quantitative data on the magnitude of the systemic inflammatory response and its effect on micronutrient status based on plasma measurements. Am. J. Clin. Nutr. 2012, 95, 64–71. [Google Scholar] [CrossRef]
- Martins, T.B.; Pasi, B.M.; Pickering, J.W.; Jaskowski, T.D.; Litwin, C.M.; Hill, H.R. Determination of cytokine responses using a multiplexed fluorescent microsphere immunoassay. Am. J. Clin. Pathol. 2002, 118, 346–353. [Google Scholar] [CrossRef]
- Martins, T.B.; Anderson, J.L.; Muhlestein, J.B.; Horne, B.D.; Carlquist, J.F.; Roberts, W.L.; Carlquist, J.F. Risk factor analysis of plasma cytokines in patients with coronary artery disease by a multiplexed fluorescent immunoassay. Am. J. Clin. Pathol. 2006, 125, 906–913. [Google Scholar] [CrossRef]
- Barker, T.; Leonard, S.W.; Hansen, J.; Trawick, R.H.; Ingram, R.; Burdett, G.; Lebold, K.M.; Walker, J.A.; Traber, M.G. Vitamin E and C supplementation does not ameliorate muscle dysfunction following anterior cruciate ligament surgery. Free Radic. Biol. Med. 2009, 47, 1611–1618. [Google Scholar] [CrossRef]
- Webb, A.R.; Kline, L.; Holick, M.F. Influence of season and latitude on the cutaneous synthesis of vitamin D3: Exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J. Clin. Endocrinol. Metab. 1988, 67, 373–378. [Google Scholar] [CrossRef]
- Brancaccio, P.; Limongelli, F.M.; Maffulli, N. Monitoring of serum enzymes in sport. Br. J. Sports Med. 2006, 40, 96–97. [Google Scholar] [CrossRef]
- Nie, J.; Tong, T.K.; George, K.; Fu, F.H.; Lin, H.; Shi, Q. Resting and post-exercise serum biomarkers of cardiac and skeletal muscle damage in adolescent runners. Scand. J. Med. Sci. Sports 2011, 21, 625–629. [Google Scholar] [CrossRef]
- Dusso, A.S.; Kamimura, S.; Gallieni, M.; Zhong, M.; Negrea, L.; Shapiro, S.; Slatopolsky, E. Gamma-interferon-induced resistance to 1,25-(OH)2 D3 in human monocytes and macrophages: A mechanism for the hypercalcemia of various granulomatoses. J. Clin. Endocrinol. Metab. 1997, 82, 2222–2232. [Google Scholar]
- Adams, J.S.; Gacad, M.A. Characterization of 1 alpha-hydroxylation of vitamin D3 sterols by cultured alveolar macrophages from patients with sarcoidosis. J. Exp. Med. 1985, 161, 755–765. [Google Scholar] [CrossRef]
- Reiss, A.B.; Awadallah, N.W.; Malhotra, S.; Montesinos, M.C.; Chan, E.S.; Javitt, N.B.; Cronstein, B.N. Immune complexes and IFN-gamma decrease cholesterol 27-hydroxylase in human arterial endothelium and macrophages. J. Lipid Res. 2001, 42, 1913–1922. [Google Scholar]
- Overbergh, L.; Decallonne, B.; Valckx, D.; Verstuyf, A.; Depovere, J.; Laureys, J.; Rutgeerts, O.; Saint-Arnaud, R.; Bouillon, R.; Mathieu, C. Identification and immune regulation of 25-hydroxyvitamin D-1-alpha-hydroxylase in murine macrophages. Clin. Exp. Immunol. 2000, 120, 139–146. [Google Scholar] [CrossRef]
- Monkawa, T.; Yoshida, T.; Hayashi, M.; Saruta, T. Identification of 25-hydroxyvitamin D3 1alpha-hydroxylase gene expression in macrophages. Kidney Int. 2000, 58, 559–568. [Google Scholar] [CrossRef]
- Quinlan, G.J.; Martin, G.S.; Evans, T.W. Albumin: Biochemical properties and therapeutic potential. Hepatology 2005, 41, 1211–1219. [Google Scholar] [CrossRef]
- Green, H.J.; Thomson, J.A.; Ball, M.E.; Hughson, R.L.; Houston, M.E.; Sharratt, M.T. Alterations in blood volume following short-term supramaximal exercise. J. Appl. Physiol. 1984, 56, 145–149. [Google Scholar]
- Gillen, C.M.; Lee, R.; Mack, G.W.; Tomaselli, C.M.; Nishiyasu, T.; Nadel, E.R. Plasma volume expansion in humans after a single intense exercise protocol. J. Appl. Physiol. 1991, 71, 1914–1920. [Google Scholar]
- Yang, R.C.; Mack, G.W.; Wolfe, R.R.; Nadel, E.R. Albumin synthesis after intense intermittent exercise in human subjects. J. Appl. Physiol. 1998, 84, 584–592. [Google Scholar] [CrossRef]
- Bikle, D.D.; Gee, E.; Halloran, B.; Kowalski, M.A.; Ryzen, E.; Haddad, J.G. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J. Clin. Endocrinol. Metab. 1986, 63, 954–959. [Google Scholar] [CrossRef]
- Garabedian, M.; Holick, M.F.; DeLuca, H.F.; Boyle, I.T. Control of 25-hydroxycholecalciferol metabolism by parathyroid glands. Proc. Natl. Acad. Sci. USA 1972, 69, 1673–1676. [Google Scholar] [CrossRef]
- Rasmussen, H.; Wong, M.; Bikle, D.; Goodman, D.B. Hormonal control of the renal conversion of 25-hydroxycholecalciferol to 1,25-dihydroxycholecalciferol. J. Clin. Invest. 1972, 51, 2502–2504. [Google Scholar] [CrossRef]
- Fraser, D.R.; Kodicek, E. Regulation of 25-hydroxycholecalciferol-1-hydroxylase activity in kidney by parathyroid hormone. Nat. New Biol. 1973, 241, 163–166. [Google Scholar]
- Boyle, I.T.; Gray, R.W.; DeLuca, H.F. Regulation by calcium of in vivo synthesis of 1,25-dihydroxycholecalciferol and 21,25-dihydroxycholecalciferol. Proc. Natl. Acad. Sci. USA 1971, 68, 2131–2134. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Barker, T.; Henriksen, V.T.; Martins, T.B.; Hill, H.R.; Kjeldsberg, C.R.; Schneider, E.D.; Dixon, B.M.; Weaver, L.K. Higher Serum 25-Hydroxyvitamin D Concentrations Associate with a Faster Recovery of Skeletal Muscle Strength after Muscular Injury. Nutrients 2013, 5, 1253-1275. https://doi.org/10.3390/nu5041253
Barker T, Henriksen VT, Martins TB, Hill HR, Kjeldsberg CR, Schneider ED, Dixon BM, Weaver LK. Higher Serum 25-Hydroxyvitamin D Concentrations Associate with a Faster Recovery of Skeletal Muscle Strength after Muscular Injury. Nutrients. 2013; 5(4):1253-1275. https://doi.org/10.3390/nu5041253
Chicago/Turabian StyleBarker, Tyler, Vanessa T. Henriksen, Thomas B. Martins, Harry R. Hill, Carl R. Kjeldsberg, Erik D. Schneider, Brian M. Dixon, and Lindell K. Weaver. 2013. "Higher Serum 25-Hydroxyvitamin D Concentrations Associate with a Faster Recovery of Skeletal Muscle Strength after Muscular Injury" Nutrients 5, no. 4: 1253-1275. https://doi.org/10.3390/nu5041253
APA StyleBarker, T., Henriksen, V. T., Martins, T. B., Hill, H. R., Kjeldsberg, C. R., Schneider, E. D., Dixon, B. M., & Weaver, L. K. (2013). Higher Serum 25-Hydroxyvitamin D Concentrations Associate with a Faster Recovery of Skeletal Muscle Strength after Muscular Injury. Nutrients, 5(4), 1253-1275. https://doi.org/10.3390/nu5041253




