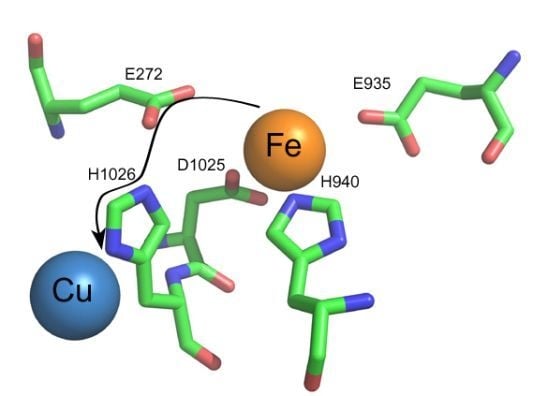
Multi-Copper Oxidases and Human Iron Metabolism
Abstract
Share and Cite
Vashchenko, G.; MacGillivray, R.T.A. Multi-Copper Oxidases and Human Iron Metabolism. Nutrients 2013, 5, 2289-2313. https://doi.org/10.3390/nu5072289
Vashchenko G, MacGillivray RTA. Multi-Copper Oxidases and Human Iron Metabolism. Nutrients. 2013; 5(7):2289-2313. https://doi.org/10.3390/nu5072289
Chicago/Turabian StyleVashchenko, Ganna, and Ross T. A. MacGillivray. 2013. "Multi-Copper Oxidases and Human Iron Metabolism" Nutrients 5, no. 7: 2289-2313. https://doi.org/10.3390/nu5072289
APA StyleVashchenko, G., & MacGillivray, R. T. A. (2013). Multi-Copper Oxidases and Human Iron Metabolism. Nutrients, 5(7), 2289-2313. https://doi.org/10.3390/nu5072289