A High-Fat Diet Containing Lard Accelerates Prostate Cancer Progression and Reduces Survival Rate in Mice: Possible Contribution of Adipose Tissue-Derived Cytokines
Abstract
:1. Introduction
| Control Diet (10 kcal % Fat) | High-Fat Diet (60 kcal % Fat) | |||
|---|---|---|---|---|
| Product # | D12450B | D12492 | ||
| Ingredient | g | kcal | g | kcal |
| Casein, 80 Mesh | 200 | 800 | 200 | 800 |
| l-Cystine | 3 | 12 | 3 | 12 |
| Corn starch | 315 | 1260 | 0 | 0 |
| Maltodextrin 10 | 35 | 140 | 125 | 500 |
| Sucrose | 350 | 1400 | 68.8 | 275.2 |
| Cellulose, BW200 | 50 | 0 | 50 | 0 |
| Soybean Oil | 25 | 225 | 25 | 225 |
| Lard * | 20 | 180 | 245 | 2205 |
| Mineral Mix S10026 | 10 | 0 | 10 | 0 |
| Dicalcium Phosphate | 13 | 0 | 13 | 0 |
| Calcium Carbonate | 5.5 | 0 | 5.5 | 0 |
| Potassium Citrate, 1 H2O | 16.5 | 0 | 16.5 | 0 |
| Vitamin Mix V10001 | 10 | 40 | 10 | 40 |
| Choline Bitartrate | 2 | 0 | 2 | 0 |
| FD & C Yellow Dye #5 | 0.05 | 0 | ||
| FD & C Blue Dye #1 | 0.05 | 0 | ||
| Total | 1055.05 | 4057 | 773.85 | 4057 |
2. Experimental Section
2.1. Reagents
2.2. Cell Culture
2.3. Animal Studies
2.3.1. TRAMP-C2 Allograft Tumor Model
2.3.2. TRAMP Model
2.4. Immunohistochemical (IHC) and Immunofluorescence (IF) Analysis
2.5. Preparation of Adipose Tissue-Conditioned Media (ATCM)
2.6. Cytokine Array and Enzyme Linked Immunosorbent Assay (ELISA)
2.7. MTT Assay and Western Blot Analysis
2.8. Transwell Migration Assay
2.9. Tube Formation Assay and Aorta Ring Assay
2.10. Statistical Analysis
3. Results
3.1. HFD Feeding Increases Solid Tumor Growth in the TRAMP-C2 Allograft Model

| Body | Liver | Tumor | Epididymal Fat | Mesenteric Fat | ||
|---|---|---|---|---|---|---|
| CD | (g) | 27.87 ± 0.80 | 1.16 ± 0.04 | 1.54 ± 0.21 | 0.50 ± 0.06 | 0.10 ± 0.02 |
| (% of BW) | 4.18 ± 0.12 | 5.45 ± 0.69 | 1.71 ± 0.18 | 0.35 ± 0.06 | ||
| HFD | (g) | 36.87 ± 1.53 * | 1.55 ± 0.06 * | 2.60 ± 0.29 * | 1.01 ± 0.12 * | 0.38 ± 0.07 * |
| (% of BW) | 4.30 ± 0.18 | 7.21 ± 0.78 | 2.58 ± 0.21 * | 0.90 ± 0.15 * | ||
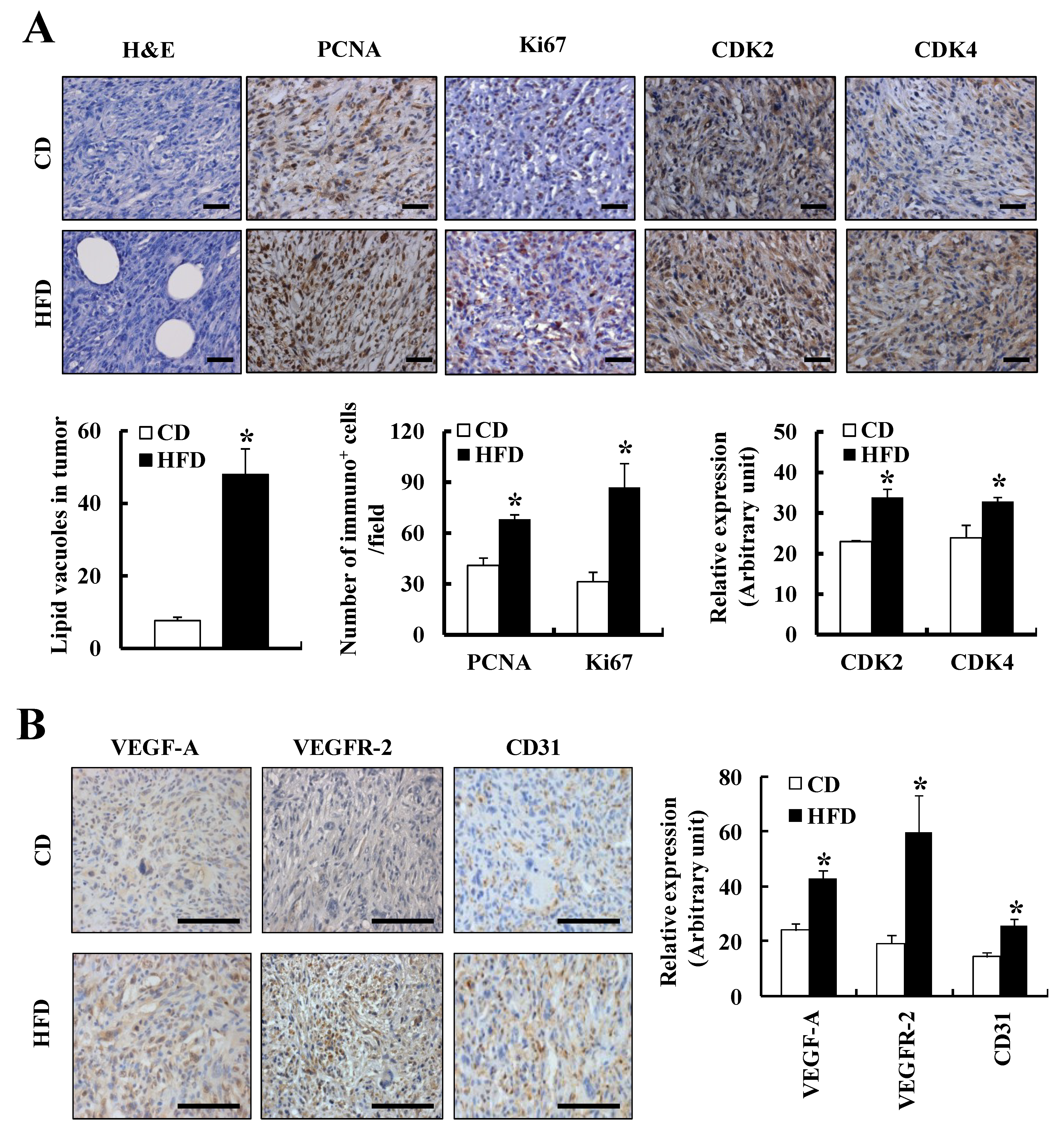

3.2. HFD Feeding Increases Prostate Cancer Development and Progression in TRAMP Mice
| Body | Liver | GU Tract | Epididymal Fat | Mesenteric Fat | |
|---|---|---|---|---|---|
| TRAMP Model (24 Weeks of Age) | |||||
| Normal mice-CD | 28.49 ± 0.50 | 0.95 ± 0.11 | 0.37 ± 0.04 | 0.84 ± 0.13 | 0.23 ± 0.02 |
| Normal mice-HFD | 42.87 ± 1.73 * | 2.08 ± 0.08 * | 0.44 ± 0.02 | 2.54 ± 0.22 * | 1.46 ± 0.05 * |
| TRAMP-CD | 27.44 ± 0.40 | 0.97 ± 0.05 | 0.66 ± 0.06 * | 0.73 ± 0.09 | 0.19 ± 0.03 |
| TRAMP-HFD | 41.42 ± 0.93 f | 1.95 ± 0.19 f | 0.93 ± 0.08 f | 2.16 ± 0.15 f | 1.10 ± 0.10 f |
| TRAMP Model (32 Weeks of Age) | |||||
| Normal mice-CD | 31.19 ± 1.13 | 1.20 ± 0.08 | 0.53 ± 0.03 | 1.10 ± 0.18 | ND |
| Normal mice-HFD | 48.90 ± 1.63 * | 1.90 ± 0.22 * | 0.73 ± 0.09 * | 1.80 ± 0.20 * | ND |
| TRAMP-CD | 27.67 ± 0.49 | 1.04 ± 0.04 | 0.87 ± 0.08 * | 0.63 ± 0.05 | ND |
| TRAMP-HFD | 41.60 ± 2.25 f | 1.67 ± 0.17 f | 1.99 ± 0.16 f | 1.79 ± 0.18 f | ND |


3.3. HFD Feeding Reduces Survival Rate in TRAMP Mice

3.4. HFD Feeding Induces Changes in Serum Cytokine Levels

3.5. Soluble Factors Released by Adipose Tissues Stimulate Tumor Progression


4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wolin, K.Y.; Carson, K.; Colditz, G.A. Obesity and cancer. Oncologist 2010, 15, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Calle, E.E.; Kaaks, R. Overweight, obesity and cancer: Epidemiological evidence and proposed mechanisms. Nat. Rev. Cancer 2004, 4, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Basen-Engquist, K.; Chang, M. Obesity and cancer risk: Recent review and evidence. Curr. Oncol. Rep. 2011, 13, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Buschemeyer, W.C., 3rd; Freedland, S.J. Obesity and prostate cancer: Epidemiology and clinical implications. Eur. Urol. 2007, 52, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Llaverias, G.; Danilo, C.; Wang, Y.; Witkiewicz, A.K.; Daumer, K.; Lisanti, M.P.; Frank, P.G. A Western-type diet accelerates tumor progression in an autochthonous mouse model of prostate cancer. Am. J. Pathol. 2010, 177, 3180–3191. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Barnard, R.J.; Said, J.; Hong-Gonzalez, J.; Corman, D.M.; Ku, M.; Doan, N.B.; Gui, D.; Elashoff, D.; Cohen, P.; et al. Effect of low-fat diet on development of prostate cancer and Akt phosphorylation in the Hi-Myc transgenic mouse model. Cancer Res. 2008, 68, 3066–3073. [Google Scholar] [CrossRef] [PubMed]
- Bonorden, M.J.; Grossmann, M.E.; Ewing, S.A.; Rogozina, O.P.; Ray, A.; Nkhata, K.J.; Liao, D.J.; Grande, J.P.; Cleary, M.P. Growth and progression of TRAMP prostate tumors in relationship to diet and obesity. Prostate Cancer 2012, 2012. [Google Scholar] [CrossRef]
- Roberts, D.L.; Dive, C.; Renehan, A.G. Biological mechanisms linking obesity and cancer risk: New perspectives. Annu. Rev. Med. 2010, 61, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Van Kruijsdijk, R.C.; van der Wall, E.; Visseren, F.L. Obesity and cancer: The role of dysfunctional adipose tissue. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2569–2578. [Google Scholar]
- Osorio-Costa, F.; Rocha, G.Z.; Dias, M.M.; Carvalheira, J.B. Epidemiological and molecular mechanisms aspects linking obesity and cancer. Arq. Bras. Endocrinol. Metabol. 2009, 53, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Birmingham, J.M.; Busik, J.V.; Hansen-Smith, F.M.; Fenton, J.I. Novel mechanism for obesity-induced colon cancer progression. Carcinogenesis 2009, 30, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, T.; Endo, H.; Tomimoto, A.; Sugiyama, M.; Takahashi, H.; Saito, S.; Inamori, M.; Nakajima, N.; Watanabe, M.; Kubota, N.; et al. Adiponectin suppresses colorectal carcinogenesis under the high-fat diet condition. Gut 2008, 57, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 2003, 112, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Gruen, M.L.; Hao, M.; Piston, D.W.; Hasty, A.H. Leptin requires canonical migratory signaling pathways for induction of monocyte and macrophage chemotaxis. Am. J. Physiol. Cell. Physiol. 2007, 293, C1481–C1488. [Google Scholar] [CrossRef] [PubMed]
- Kanda, H.; Tateya, S.; Tamori, Y.; Kotani, K.; Hiasa, K.; Kitazawa, R.; Kitazawa, S.; Miyachi, H.; Maeda, S.; Egashira, K.; et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Invest. 2006, 116, 1494–1505. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Dirat, B.; Bochet, L.; Dabek, M.; Daviaud, D.; Dauvillier, S.; Majed, B.; Wang, Y.Y.; Meulle, A.; Salles, B.; le Gonidec, S.; et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011, 71, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Nieman, K.M.; Kenny, H.A.; Penicka, C.V.; Ladanyi, A.; Buell-Gutbrod, R.; Zillhardt, M.R.; Romero, I.L.; Carey, M.S.; Mills, G.B.; Hotamisligil, G.S.; et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med. 2011, 17, 1498–1503. [Google Scholar] [CrossRef] [PubMed]
- Foster, B.A.; Gingrich, J.R.; Kwon, E.D.; Madias, C.; Greenberg, N.M. Characterization of prostatic epithelial cell lines derived from transgenic adenocarcinoma of the mouse prostate (TRAMP) model. Cancer Res. 1997, 57, 3325–3330. [Google Scholar] [PubMed]
- Cho, H.J.; Park, S.Y.; Kim, E.J.; Kim, J.K.; Park, J.H. 3,3ʹ-Diindolylmethane inhibits prostate cancer development in the transgenic adenocarcinoma mouse prostate model. Mol. Carcinog. 2011, 50, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Workman, P.; Aboagye, E.O.; Balkwill, F.; Balmain, A.; Bruder, G.; Chaplin, D.J.; Double, J.A.; Everitt, J.; Farningham, D.A.; Glennie, M.J.; et al. Guidelines for the welfare and use of animals in cancer research. Br. J. Cancer 2010, 102, 1555–1577. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kim, M.; Kwon, G.T.; Lim do, Y.; Yu, R.; Sung, M.K.; Lee, K.W.; Daily, J.W., 3rd; Park, J.H. A high-fat diet increases angiogenesis, solid tumor growth, and lung metastasis of CT26 colon cancer cells in obesity-resistant BALB/c mice. Mol. Carcinog. 2012, 51, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Kim, W.K.; Kim, E.J.; Jung, K.C.; Park, S.; Lee, H.S.; Tyner, A.L.; Park, J.H. Conjugated linoleic acid inhibits cell proliferation and ErbB3 signaling in HT-29 human colon cell line. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, G996–G1005. [Google Scholar] [CrossRef] [PubMed]
- Kwon, G.T.; Cho, H.J.; Chung, W.Y.; Park, K.K.; Moon, A.; Park, J.H. Isoliquiritigenin inhibits migration and invasion of prostate cancer cells: Possible mediation by decreased JNK/AP-1 signaling. J. Nutr. Biochem. 2009, 20, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.; Patel, A.V.; Calle, E.E.; Jacobs, E.J.; Chao, A.; Thun, M.J. Body mass index, height, and prostate cancer mortality in two large cohorts of adult men in the United States. Cancer Epidemiol. Biomark. Prev. 2001, 10, 345–353. [Google Scholar]
- Gong, Z.; Neuhouser, M.L.; Goodman, P.J.; Albanes, D.; Chi, C.; Hsing, A.W.; Lippman, S.M.; Platz, E.A.; Pollak, M.N.; Thompson, I.M.; et al. Obesity, diabetes, and risk of prostate cancer: Results from the prostate cancer prevention trial. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1977–1983. [Google Scholar] [CrossRef]
- Rodriguez, C.; Freedland, S.J.; Deka, A.; Jacobs, E.J.; McCullough, M.L.; Patel, A.V.; Thun, M.J.; Calle, E.E. Body mass index, weight change, and risk of prostate cancer in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol. Biomark. Prev. 2007, 16, 63–69. [Google Scholar] [CrossRef]
- Wright, M.E.; Chang, S.C.; Schatzkin, A.; Albanes, D.; Kipnis, V.; Mouw, T.; Hurwitz, P.; Hollenbeck, A.; Leitzmann, M.F. Prospective study of adiposity and weight change in relation to prostate cancer incidence and mortality. Cancer 2007, 109, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Banez, L.L.; Hamilton, R.J.; Partin, A.W.; Vollmer, R.T.; Sun, L.; Rodriguez, C.; Wang, Y.; Terris, M.K.; Aronson, W.J.; Presti, J.C., Jr.; et al. Obesity-Related plasma hemodilution and PSA concentration among men with prostate cancer. JAMA 2007, 298, 2275–2280. [Google Scholar] [CrossRef] [PubMed]
- Hammarsten, J.; Hogstedt, B. Hyperinsulinaemia as a risk factor for developing benign prostatic hyperplasia. Eur. Urol. 2001, 39, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Freedland, S.J.; Wen, J.; Wuerstle, M.; Shah, A.; Lai, D.; Moalej, B.; Atala, C.; Aronson, W.J. Obesity is a significant risk factor for prostate cancer at the time of biopsy. Urology 2008, 72, 1102–1105. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Adelaiye, R.M.; Rastelli, A.L.; Miles, K.M.; Ciamporcero, E.; Longo, V.D.; Nguyen, H.; Vessella, R.; Pili, R. Dietary protein restriction inhibits tumor growth in human xenograft models. Oncotarget 2013, 4, 2451–2461. [Google Scholar] [PubMed]
- Allen, N.E.; Key, T.J.; Appleby, P.N.; Travis, R.C.; Roddam, A.W.; Tjonneland, A.; Johnsen, N.F.; Overvad, K.; Linseisen, J.; Rohrmann, S.; et al. Animal foods, protein, calcium and prostate cancer risk: The European Prospective Investigation into Cancer and Nutrition. Br. J. Cancer 2008, 98, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Corr, J.G.; Thaler, H.T.; Tao, Y.; Fair, W.R.; Heston, W.D. Decreased growth of established human prostate LNCaP tumors in nude mice fed a low-fat diet. J. Natl. Cancer Inst. 1995, 87, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.H.; Barnard, R.J.; Cohen, P.; Freedland, S.; Tran, C.; deGregorio, F.; Elshimali, Y.I.; Heber, D.; Aronson, W.J. Effect of isocaloric low-fat diet on human LAPC-4 prostate cancer xenografts in severe combined immunodeficient mice and the insulin-like growth factor axis. Clin. Cancer Res. 2003, 9, 2734–2743. [Google Scholar] [PubMed]
- Ngo, T.H.; Barnard, R.J.; Anton, T.; Tran, C.; Elashoff, D.; Heber, D.; Freedland, S.J.; Aronson, W.J. Effect of isocaloric low-fat diet on prostate cancer xenograft progression to androgen independence. Cancer Res. 2004, 64, 1252–1254. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Yokoyama, W.; Davis, P.A. TRAMP prostate tumor growth is slowed by walnut diets through altered IGF-1 levels, energy pathways, and cholesterol metabolism. J. Med. Food 2014, 17, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, J.C.; Masko, E.M.; Wu, C.; Keenan, M.M.; Pilla, D.M.; Aronson, W.J.; Chi, J.T.; Freedland, S.J. Fish oil slows prostate cancer xenograft growth relative to other dietary fats and is associated with decreased mitochondrial and insulin pathway gene expression. Prostate Cancer Prostatic Dis. 2013, 16, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Escobar, E.L.; Gomes-Marcondes, M.C.; Carvalho, H.F. Dietary fatty acid quality affects AR and PPARgamma levels and prostate growth. Prostate 2009, 69, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Ma, J. Body mass index, prostate cancer-specific mortality, and biochemical recurrence: A systematic review and meta-analysis. Cancer Prev. Res. (Phila) 2011, 4, 486–501. [Google Scholar] [CrossRef]
- Akinsete, J.A.; Ion, G.; Witte, T.R.; Hardman, W.E. Consumption of high omega-3 fatty acid diet suppressed prostate tumorigenesis in C3(1) Tag mice. Carcinogenesis 2012, 33, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Berquin, I.M.; Min, Y.; Wu, R.; Wu, J.; Perry, D.; Cline, J.M.; Thomas, M.J.; Thornburg, T.; Kulik, G.; Smith, A.; et al. Modulation of prostate cancer genetic risk by omega-3 and omega-6 fatty acids. J. Clin. Invest. 2007, 117, 1866–1875. [Google Scholar] [CrossRef] [PubMed]
- Haque, R.; van Den Eeden, S.K.; Wallner, L.P.; Richert-Boe, K.; Kallakury, B.; Wang, R.; Weinmann, S. Association of body mass index and prostate cancer mortality. Obes. Res. Clin. Pract. 2014, 8, e374–e381. [Google Scholar] [CrossRef] [PubMed]
- Teng, K.T.; Chang, C.Y.; Chang, L.F.; Nesaretnam, K. Modulation of obesity-induced inflammation by dietary fats: Mechanisms and clinical evidence. Nutr. J. 2014, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, S.J.; Feskens, E.J.; Bos, M.B.; Hoelen, D.W.; Heijligenberg, R.; Bromhaar, M.G.; de Groot, L.C.; de Vries, J.H.; Muller, M.; Afman, L.A. A saturated fatty acid-rich diet induces an obesity-linked proinflammatory gene expression profile in adipose tissue of subjects at risk of metabolic syndrome. Am. J. Clin. Nutr. 2009, 90, 1656–1664. [Google Scholar]
- Di Sebastiano, K.M.; Mourtzakis, M. The role of dietary fat throughout the prostate cancer trajectory. Nutrients 2014, 6, 6095–6109. [Google Scholar]
- Jung, J.I.; Cho, H.J.; Jung, Y.J.; Kwon, S.H.; Her, S.; Choi, S.S.; Shin, S.H.; Lee, K.W.; Park, J.H. High-fat diet-induced obesity increases lymphangiogenesis and lymph node metastasis in the B16F10 melanoma allograft model: Roles of adipocytes and M2-macrophages. Int. J. Cancer 2015, 136, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Venkatasubramanian, P.N.; Brendler, C.B.; Plunkett, B.A.; Crawford, S.E.; Fitchev, P.S.; Morgan, G.; Cornwell, M.L.; McGuire, M.S.; Wyrwicz, A.M.; Doll, J.A. Periprostatic adipose tissue from obese prostate cancer patients promotes tumor and endothelial cell proliferation: A functional and MR imaging pilot study. Prostate 2014, 74, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.; Monteiro, C.; Cunha, V.; Oliveira, M.J.; Freitas, M.; Fraga, A.; Principe, P.; Lobato, C.; Lobo, F.; Morais, A.; et al. Human periprostatic adipose tissue promotes prostate cancer aggressiveness in vitro. J. Exp. Clin. Cancer Res. 2012, 31, 32. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Mizuarai, S.; Araki, H.; Mashiko, S.; Ishihara, A.; Kanatani, A.; Itadani, H.; Kotani, H. Adiposity elevates plasma MCP-1 levels leading to the increased CD11b-positive monocytes in mice. J. Biol. Chem. 2003, 278, 46654–46660. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Cai, Z.; Galson, D.L.; Xiao, G.; Liu, Y.; George, D.E.; Melhem, M.F.; Yao, Z.; Zhang, J. Monocyte chemotactic protein-1 (MCP-1) acts as a paracrine and autocrine factor for prostate cancer growth and invasion. Prostate 2006, 66, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.H.; Frost, E.; To, V.; Robinson, S.; Ffrench-Constant, C.; Geertman, R.; Ransohoff, R.M.; Miller, R.H. The chemokine receptor CXCR2 controls positioning of oligodendrocyte precursors in developing spinal cord by arresting their migration. Cell 2002, 110, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Pelus, L.M.; Fukuda, S. Peripheral blood stem cell mobilization: The CXCR2 ligand GRObeta rapidly mobilizes hematopoietic stem cells with enhanced engraftment properties. Exp. Hematol. 2006, 34, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.; McGurk, M.; Pettigrew, J.; Santinelli, A.; Mazzucchelli, R.; Johnston, P.G.; Montironi, R.; Waugh, D.J. Nonapical and cytoplasmic expression of interleukin-8, CXCR1, and CXCR2 correlates with cell proliferation and microvessel density in prostate cancer. Clin. Cancer Res. 2005, 11, 4117–4127. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, R.; Sharma, P.K.; Singh, U.P.; Rai, S.N.; Chung, L.W.; Cooper, C.R.; Novakovic, K.R.; Grizzle, W.E.; Lillard, J.W., Jr. Serum CXCL13 positively correlates with prostatic disease, prostate-specific antigen and mediates prostate cancer cell invasion, integrin clustering and cell adhesion. Cancer Lett. 2009, 283, 29–35. [Google Scholar] [CrossRef] [PubMed]
- El Haibi, C.P.; Sharma, P.K.; Singh, R.; Johnson, P.R.; Suttles, J.; Singh, S.; Lillard, J.W., Jr. PI3Kp110-, Src-, FAK-dependent and DOCK2-independent migration and invasion of CXCL13-stimulated prostate cancer cells. Mol. Cancer 2010, 9, 85. [Google Scholar]
- Singh, S.; Singh, R.; Singh, U.P.; Rai, S.N.; Novakovic, K.R.; Chung, L.W.; Didier, P.J.; Grizzle, W.E.; Lillard, J.W., Jr. Clinical and biological significance of CXCR5 expressed by prostate cancer specimens and cell lines. Int. J. Cancer 2009, 125, 2288–2295. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, H.J.; Kwon, G.T.; Park, H.; Song, H.; Lee, K.W.; Kim, J.-I.; Park, J.H.Y. A High-Fat Diet Containing Lard Accelerates Prostate Cancer Progression and Reduces Survival Rate in Mice: Possible Contribution of Adipose Tissue-Derived Cytokines. Nutrients 2015, 7, 2539-2561. https://doi.org/10.3390/nu7042539
Cho HJ, Kwon GT, Park H, Song H, Lee KW, Kim J-I, Park JHY. A High-Fat Diet Containing Lard Accelerates Prostate Cancer Progression and Reduces Survival Rate in Mice: Possible Contribution of Adipose Tissue-Derived Cytokines. Nutrients. 2015; 7(4):2539-2561. https://doi.org/10.3390/nu7042539
Chicago/Turabian StyleCho, Han Jin, Gyoo Taik Kwon, Heesook Park, Hyerim Song, Ki Won Lee, Jung-In Kim, and Jung Han Yoon Park. 2015. "A High-Fat Diet Containing Lard Accelerates Prostate Cancer Progression and Reduces Survival Rate in Mice: Possible Contribution of Adipose Tissue-Derived Cytokines" Nutrients 7, no. 4: 2539-2561. https://doi.org/10.3390/nu7042539
APA StyleCho, H. J., Kwon, G. T., Park, H., Song, H., Lee, K. W., Kim, J.-I., & Park, J. H. Y. (2015). A High-Fat Diet Containing Lard Accelerates Prostate Cancer Progression and Reduces Survival Rate in Mice: Possible Contribution of Adipose Tissue-Derived Cytokines. Nutrients, 7(4), 2539-2561. https://doi.org/10.3390/nu7042539




