Green Tea, Intermittent Sprinting Exercise, and Fat Oxidation
Abstract
:1. Introduction
2. Experimental Section
2.1. Participants
2.2. Preliminary Testing
2.3. General Study Design
2.4. Diet and Capsule Content
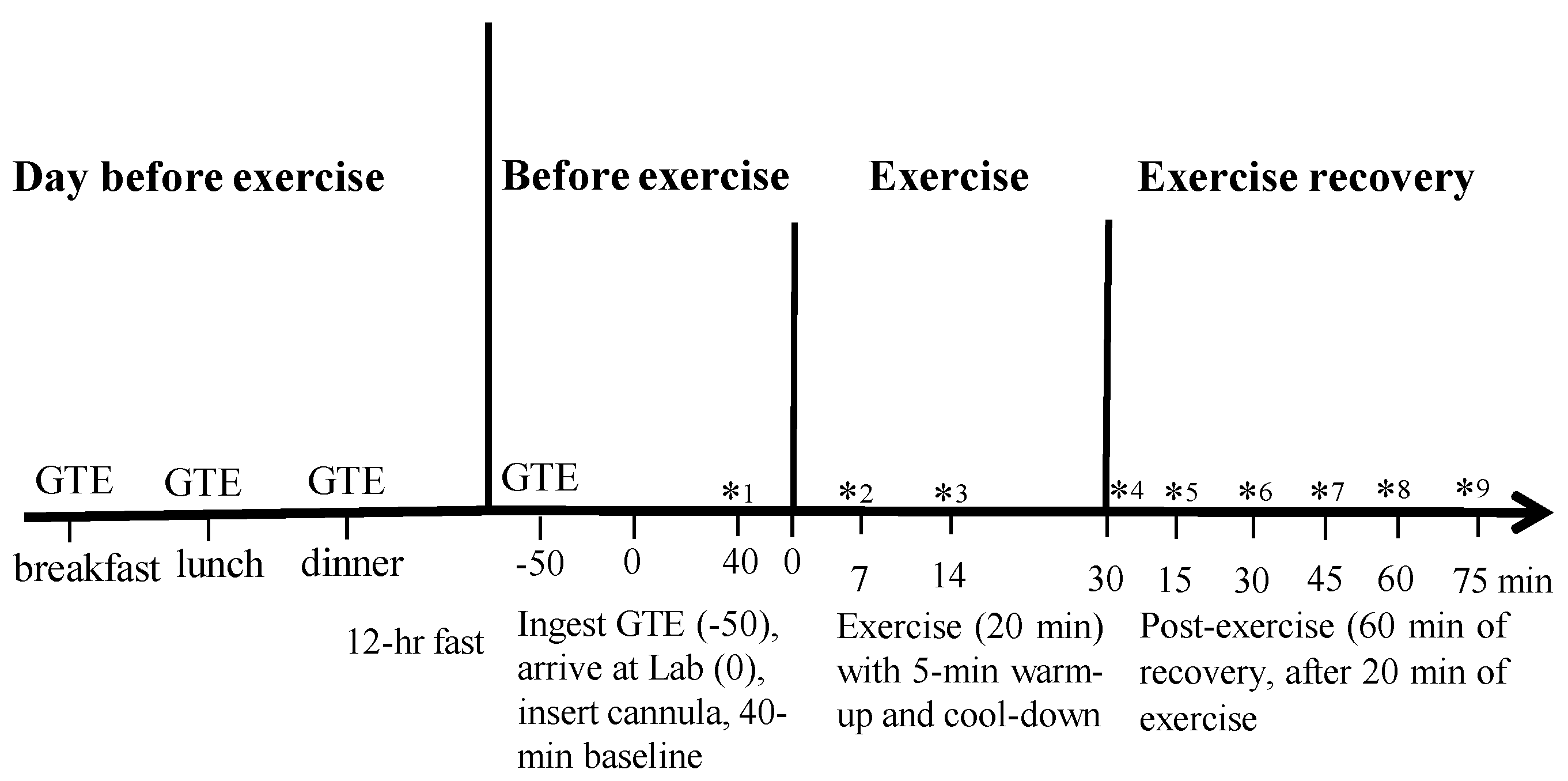
2.5. Experimental Protocol
2.6. Rest
2.7. Interval Sprinting Exercise and Post Exercise
2.8. Blood Variables and Oxidation Rates
2.9. Statistical Analysis
3. Results
3.1. Blood Testing
3.2. Workload and Exercise Intensity
| Placebo | GTE | |
|---|---|---|
| Mean power output (W) during 8 s sprint | 99.2 ± 5.7 | 99.4 ± 6.1 |
| Revolutions per minute during 8 s sprint | 111.2 ± 1.1 | 111.3 ± 1.5 |
| Pedal resistance (kg) during 8 s sprint | 0.9 ± 0.10 | 0.9 ± 0.10 |
| Mean power output (W) during 12 s recovery | 33.4 ± 3.4 | 33.8 ± 3.7 |
| Revolutions per minute during 12 s recovery | 37.2 ± 0.66 | 38.4 ± 0.70 |
| Pedal resistance (kg) during 12 s recovery | 0.9 ± 0.10 | 0.9 ± 0.10 |
| Rating of perceived exertion throughout | 13.8 ± 0.7 | 13.4 ± 0.5 |
| Lactate (mmol/L) at 7 min | 3.8 ± 0.3 | 4.1 ± 0.2 |
| Lactate (mmol/L) at 20 min | 5.3 ± 0.5 | 5.1 ± 0.3 |
| Variable | Condition | Rest | Exercise | Post Exercise | ||
|---|---|---|---|---|---|---|
| 35 min | 55 min | 75 min | ||||
| Heart rate (bpm) | GTE | 65.50 ± 3.25 | 156.31 ± 2.7 | 77.21 ± 3.7 | 73.71 ± 3.6 | 74.00 ± 3.6 |
| Placebo | 64.84 ± 2.8 | 153.95 ± 2.7 | 75.95 ± 3.8 | 72.68 ± 3.5 | 71.28 ± 3.6 | |
| O2 (L/min) | GTE | 0.209 ± 0.005 | 1.513 ± 0.047 | 0.235 ± 0.005 * | 0.217 ± 0.005 * | 0.217 ± 0.005 * |
| Placebo | 0.207 ± 0.005 | 1.463 ± 0.043 | 0.225 ± 0.005 | 0.209 ± 0.005 | 0.208 ± 0.005 | |
| CO2 (L/min) | GTE | 0.173 ± 0.007 | 1.438 ± 0.041 | 0.176 ± 0.006 | 0.168 ± 0.005 | 0.177 ± 0.006 |
| Placebo | 0.179 ± 0.005 | 1.403 ± 0.035 | 0.181 ± 0.005 | 0.172 ± 0.006 | 0.177 ± 0.005 | |
| RER | GTE | 0.83 ± 0.03 * | 0.95 ± 0.03 * | 0.75 ± 0.03 * | 0.78 ± 0.02 * | 0.81 ± 0.02 * |
| Placebo | 0.86 ± 0.03 | 0.97 ± 0.02 | 0.80 ± 0.03 | 0.82 ± 0.03 | 0.85 ± 0.03 | |
| EE (kcal/min) | GTE | 1.01 ± 0.08 | 7.48 ± 0.24 | 1.11 ± 0.08 | 1.03 ± 0.08 | 1.04 ± 0.08 |
| Placebo | 1.00 ± 0.05 | 7.25 ± 0.21 | 1.08 ± 0.05 | 1.00 ± 0.08 | 1.01 ± 0.08 | |
3.3. Lactate
3.4. Respiratory Exchange Ratio (RER)
3.5. Fat Oxidation

3.6. O2, O2, and Energy Expenditure
3.7. Glycerol and Catecholamine Levels

4. Discussion

5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Basu, A.; Lucas, E.A. Mechanisms and effects of green tea on cardiovascular health. Nutr. Rev. 2007, 65, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, S.; Shimazu, T.; Ohmori, K.; Kikuchi, N.; Nakaya, N.; Nishino, Y.; Tsubono, Y.; Tsuji, I. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: The Ohsaki study. JAMA 2006, 296, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Wolfram, S. Effects of green tea and EGCG on cardiovascular and metabolic health. J. Am. Coll. Nutr. 2007, 26, 373S–388S. [Google Scholar] [CrossRef] [PubMed]
- Boschmann, M.; Thielecke, F. The effects of epigallocatechin-3-gallate on thermogenesis and fat oxidation in obese men: A pilot study. J. Am. Coll. Nutr. 2007, 26, 389S–395S. [Google Scholar] [CrossRef] [PubMed]
- Chantre, P.; Lairon, D. Recent findings of green tea extract AR25 (Exolise) and its activity for the treatment of obesity. Phytomedicine 2002, 9, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Mann, T.; Tomiyama, A.J.; Westling, E.; Lew, A.M.; Samuels, B.; Chapman, J. Medicare’s search for effective obesity treatments: Diets are not the answer. Am. Psych. 2007, 62, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Hursel, R.; Viechtbauer, W.; Westerterp-Plantenga, M.S. The effects of green tea on weight loss and weight maintenance: A meta-analysis. Int. J. Obes. 2009, 33, 956–961. [Google Scholar] [CrossRef] [PubMed]
- Boutcher, S.H. High-intensity intermittent exercise and fat loss. J Obes. 2011. [Google Scholar] [CrossRef] [PubMed]
- Sano, M.; Tabata, M.; Suzuki, M.; Degawa, M.; Miyase, T.; Maeda-Yamamoto, M. Simultaneous determination of twelve tea catechins by high-performance liquid chromatography with electrochemical detection. Analyst 2001, 126, 816–820. [Google Scholar] [CrossRef] [PubMed]
- Berube-Parent, S.; Pelletier, C.; Dore, J.; Tremblay, A. Effects of encapsulated green tea and Guarana extracts containing a mixture of epigallocatechin-3-gallate and caffeine on 24 h energy expenditure and fat oxidation in men. Br. J. Nutr. 2005, 94, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Dulloo, A.G.; Duret, C.; Rohrer, D.; Girardier, L.; Mensi, N.; Fathi, M.; Chantre, P.; Vandermander, J. Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. Am. J. Clin. Nutr. 1999, 70, 1040–1045. [Google Scholar] [PubMed]
- Klaus, S.; Pultz, S.; Thone-Reineke, C.; Wolfram, S. Epigallocatechin gallate attenuates diet-induced obesity in mice by decreasing energy absorption and increasing fat oxidation. Int. J. Obes. 2005, 29, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, T.; Nakamori, M.; Komatsu, K.; Hosoda, K.; Okamura, M.; Toyama, K.; Ishikura, Y.; Sakai, T.; Kunii, D.; Yamamoto, S. Oolong tea increases energy metabolism in Japanese females. J. Med. Investig. 2003, 50, 170–175. [Google Scholar]
- Rumpler, W.; Seale, J.; Clevidence, B.; Judd, J.; Wiley, E.; Yamamoto, S.; Komatsu, T.; Sawaki, T.; Ishikura, Y.; Hosoda, K. Oolong tea increases metabolic rate and fat oxidation in men. J. Nutr. 2001, 131, 2848–2852. [Google Scholar] [PubMed]
- Kao, Y.H.; Chang, H.H.; Lee, M.J.; Chen, C.L. Tea, obesity, and diabetes. Mol. Nutr. Food Res. 2006, 50, 188–210. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Meng, X.; Yang, C.S. Enzymology of methylation of tea catechins and inhibition of catechol-O-methyltransferase by (−)-epigallocatechin gallate. Drug. Metab. Dispos. 2003, 31, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Wolfram, S.; Wang, Y.; Thielecke, F. Anti-obesity effects of green tea: From bedside to bench. Mol. Nutr. Food Res. 2006, 50, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Jezova, D.; Vigas, M.; Tatar, P.; Kvetnansky, R.; Nazar, K.; Kaciuba-Uscilko, H.; Kozlowski, S. Plasma testerone and catecholamine response to physcial exercise of different intensities in men. Eur. J. Appl. Occup. Physiol. 1985, 54, 62–66. [Google Scholar] [CrossRef]
- Zouhal, H.; Jacob, C.; Delamarche, P.; Gratas-Delamarche, A. Catecholamines and the effects of exercise, training and gender. Sports Med. 2008, 38, 401–423. [Google Scholar] [CrossRef] [PubMed]
- Trapp, E.G.; Chisholm, D.J.; Boutcher, S.H. Metabolic response of trained and untrained women during high-intensity intermittent cycle exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R2370–R2375. [Google Scholar] [CrossRef] [PubMed]
- Issekutz, B., Jr. Role of beta-adrenergic receptors in mobilization of energy sources in exercising dogs. J. Appl. Physiol. 1978, 44, 869–876. [Google Scholar] [PubMed]
- Trapp, E.G.; Chisholm, D.J.; Freund, J.; Boutcher, S.H. The effects of high-intensity intermittent exercise training on fat loss and fasting insulin levels of young women. Inter. J. Obes. 2008, 32, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Heydari, M.; Freund, J.; Boutcher, S.H. The effect of high-intensity intermittent exercise on body composition of overweight young males. J. Obes. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Tomlin, D.L.; Wenger, H.A. The relationship between aerobic fitness and recovery from high intensity intermittent exercise. Sports Med. 2001, 31, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Venables, M.C.; Hulston, C.J.; Cox, H.R.; Jeukendrup, A.E. Green tea extract ingestion, fat oxidation, and glucose tolerance in healthy humans. Am. J. Clin. Nutr. 2008, 87, 778–784. [Google Scholar] [PubMed]
- Randell, R.K.; Hodgson, A.B.; Lotito, D.M.; Jacobs, D.M.; Boon, N.; Mela, D.J.; Jeukendrup, A.E. No effect of 1 or 7 d of green tea extract ingestion on fat oxidation during exercise. Med. Sci. Sports. Exerc. 2013, 45, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Achten, J.; Jeukendrup, A.E. Relation between plasma lactate concentration and fat oxidation rates over a wide range of exercise intensities. Int. J. Sports Med. 2004, 25, 32–37. [Google Scholar] [PubMed]
- Glaister, M. Multiple-sprint work: Methodological, physiological, and experimental issues. Int. J. Sports. Physiol. Perform. 2008, 3, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Maliakal, P.; Chen, L.; Meng, X.; Bondoc, F.Y.; Prabhu, S.; Lambert, G.; Mohr, S.; Yang, C.S. Pharmacokinetics of tea catechins after ingestion of green tea and (−)-epigallocatechin-3-gallate by humans: Formation of different metabolites and individual variability. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1025–1032. [Google Scholar]
- The GTE Capsule. Available online: http://www.gnc.com/product/index.jsp?productId=2545553 (accessed on 7 July 2015).
- Dimsdale, J.E.; Ziegler, M.G. What do plasma and urinary measures of catecholamines tell us about human response to stressors? Circulation 1991, 83, 36–42. [Google Scholar]
- Weir, J.B.; de, V. New methods for calculating metabolic rate with special reference to protein metabolism. J. Physiol. Lond. 1949, 109, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.A.; Watras, A.C.; O’Brien, M.J.; Luke, A.; Dobratz, J.R.; Earthman, C.P.; Schoeller, D.A. Assessing validity and reliability of resting metabolic rate in six gas analysis systems. J. Am. Diet Assoc. 2009, 109, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Malatesta, D.; Werlen, C.; Bulfaro, S.; Cheneviere, X.; Borrani, F. Effect of high-intensity interval exercise on lipid oxidation during postexercise recovery. Med. Sci. Sports Exerc. 2009, 41, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Borg, G. Psychological basis of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Duncan, M.W.; Compton, P.; Lazarus, L.; Smythe, G.A. Measurement of norepinephrine and 3,4-dihydroxyphenylglycol in urine and plasma for the diagnosis of pheochromocytoma. N. Engl. J. Med. 1988, 319, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Peronnet, F.; Massicotte, D. Table of nonprotein respiratory quotient: An update. Can. J. Sport Sci. 1991, 16, 23–29. [Google Scholar] [PubMed]
- Gregersen, N.T.; Bitz, C.; Krog-Mikkelsen, I.; Hels, O.; Kovacs, E.M.; Rycroft, J.A.; Frandsen, E.; Mela, D.J.; Astrup, A. Effect of moderate intakes of different tea catechins and caffeine on acute measures of energy metabolism under sedentary conditions. Br. J. Nutr. 2009, 102, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Kimber, N.E.; Cameron-Smith, D.; McGee, S.L.; Hargreaves, M. Skeletal muscle fat metabolism after exercise in humans: Influence of fat availability. J. Appl. Physiol. 2013, 114, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Glaister, M. Multiple sprint work: Physiological responses, mechanisms of fatigue and the influence of aerobic fitness. Sports Med. 2005, 35, 757–777. [Google Scholar] [CrossRef] [PubMed]
- Borsheim, E.; Bahr, R. Effect of exercise intensity, duration and mode on post-exercise oxygen consumption. Sports Med. 2003, 33, 1037–1060. [Google Scholar] [CrossRef] [PubMed]
- Christmass, M.A.; Dawson, B.; Arthur, P.G. Effect of work and recovery duration on skeletal muscle oxygenation and fuel use during sustained intermittent exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1999, 80, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Ichinose, T.; Nomura, S.; Someya, Y.; Akimoto, S.; Tachiyashiki, K.; Imaizumi, K. Effect of endurance training supplemented with green tea extract on substrate metabolism during exercise in humans. Scand. J. Med. Sci. Sports 2011, 21, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.C.; Lonac, M.C.; Johnson, T.K.; Schweder, M.M.; Bell, C. Epigallocatechin-3-gallate increases maximal oxygen uptake in adult humans. Med. Sci. Sports Exerc. 2010, 42, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Dean, S.; Braakhuis, A.; Paton, C. The effects of EGCG on fat oxidation and endurance performance in male cyclists. Int. J. Sport. Nutr. Exerc. Metab. 2009, 19, 624–644. [Google Scholar] [PubMed]
- Eichenberger, P.; Mettler, S.; Arnold, M.; Colombani, P.C. No effects of three-week consumption of a green tea extract on time trial performance in endurance-trained men. Int. J. Vitam. Nutr. Res. 2010, 80, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.C.; Fattor, J.A.; Henderson, G.C.; Brooks, G.A. Lipid oxidation in fit young adults during postexercise recovery. J. Appl. Physiol. 2005, 99, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Malatesta, D.; Vismara, L.; Menegoni, F.; Galli, M.; Romei, M.; Capodaglio, P. Mechanical external work and recovery at preferred walking speed in obese subjects. Med. Sci. Sports Exerc. 2009, 41, 426–434. [Google Scholar] [CrossRef] [PubMed]
- McGarvey, W.; Jones, R.; Petersen, S. Excess post-exercise oxygen consumption following continuous and interval cycling exercise. Int. J. Sport Nutr. Exerc. Metab. 2005, 15, 28–37. [Google Scholar] [PubMed]
- Arner, P.; Kriegholm, E.; Engfeldt, P.; Bolinder, J. Adrenergic regulation of lipolysis in situ at rest and during exercise. J. Clin. Investig. 1990, 85, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.L.; Rennie, C.; Tarnopolskym, M.A. Substrate utilization during endurance exercise in men and women after endurance training. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E898–E907. [Google Scholar]
- Burguera, B.; Proctor, D.; Dietz, N.; Guo, Z.; Joyner, M.; Jensen, M.D. Leg free fatty acid kinetics during exercise in men and women. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E113–E117. [Google Scholar] [PubMed]
- Blatchford, F.K.; Knowlton, R.G.; Schneider, D.A. Plasma FFA responses to prolonged walking in untrained men and women. Eur. J. Appl. Physiol. Occup. Physiol. 1985, 53, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, J.F.; Klein, S. Oxidation of nonplasma fatty acids during exercise is increased in women with abdominal obesity. J. Appl. Physiol. 2000, 89, 2276–2282. [Google Scholar] [PubMed]
- Horowitz, J.F.; Klein, S. Lipid metabolism during endurance exercise. Am. J. Clin Nutr. 2000, 72, 558S–563S. [Google Scholar]
- Horton, T.J.; Pagliassotti, M.J.; Hobbs, K.; Hill, J.O. Fuel metabolism in men and women during and after long-duration exercise. J. Appl. Physiol. 1998, 85, 1823–1832. [Google Scholar] [PubMed]
- Bracco, D.; Ferrarra, J.M.; Arnaud, M.J.; Jequier, E.; Schutz, Y. Effects of caffeine on energy metabolism, heart rate, and methylxanthine metabolism in lean and obese women. Am. J. Physiol. 1995, 269, E671–E678. [Google Scholar] [PubMed]
- Dulloo, A.G.; Geissler, C.A.; Horton, T.; Collins, A.; Miller, D.S. Normal caffeine consumption: Influence on thermogenesis and daily energy expenditure in lean and postobese human volunteers. Am. J. Clin. Nutr. 1989, 49, 44–50. [Google Scholar] [PubMed]
- Strott, C.A. Sulfonation and molecular action. Endocr. Rev. 2002, 23, 703–732. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, A.B.; Randell, R.K.; Boon, N.; Garczarek, U.; Mela, D.J.; Jeukendrup, A.E.; Jacobs, D.M. Metabolic response to green tea extract during rest and moderate-intensity exercise. J. Nutr. Biochem. 2013, 24, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, A.B.; Randell, R.K.; Jeukendrup, A.E. The effect of green tea on fat oxidation at rest and during exercise: Evidence of efficacy and proposed mechanisms. Adv. Nutr. 2013, 42, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Dunn, S.L.; Siu, W.; Freund, J.; Boutcher, S.H. The effect of a lifestyle intervention on metabolic health in young women. Diabetes Metab. Syn. Obes. 2014, 7, 437–444. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gahreman, D.; Wang, R.; Boutcher, Y.; Boutcher, S. Green Tea, Intermittent Sprinting Exercise, and Fat Oxidation. Nutrients 2015, 7, 5646-5663. https://doi.org/10.3390/nu7075245
Gahreman D, Wang R, Boutcher Y, Boutcher S. Green Tea, Intermittent Sprinting Exercise, and Fat Oxidation. Nutrients. 2015; 7(7):5646-5663. https://doi.org/10.3390/nu7075245
Chicago/Turabian StyleGahreman, Daniel, Rose Wang, Yati Boutcher, and Stephen Boutcher. 2015. "Green Tea, Intermittent Sprinting Exercise, and Fat Oxidation" Nutrients 7, no. 7: 5646-5663. https://doi.org/10.3390/nu7075245




