Antiproliferative and Antiangiogenic Effects of Punica granatum Juice (PGJ) in Multiple Myeloma (MM)
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents and Compounds
2.2. Pomegranate Juice
2.3. Phenolic Compounds by HPLC Analysis
2.4. Cell Cultures and Cell Viability Assay
2.5. Cell Cycle Analysis
2.6. Cell Invasion Assay
2.7. Tube Formation Assay
2.8. Aortic Ring Assay
2.9. Wound Healing Assay
2.10. Gene Expression Analysis
2.11. Statistical Analysis
3. Results
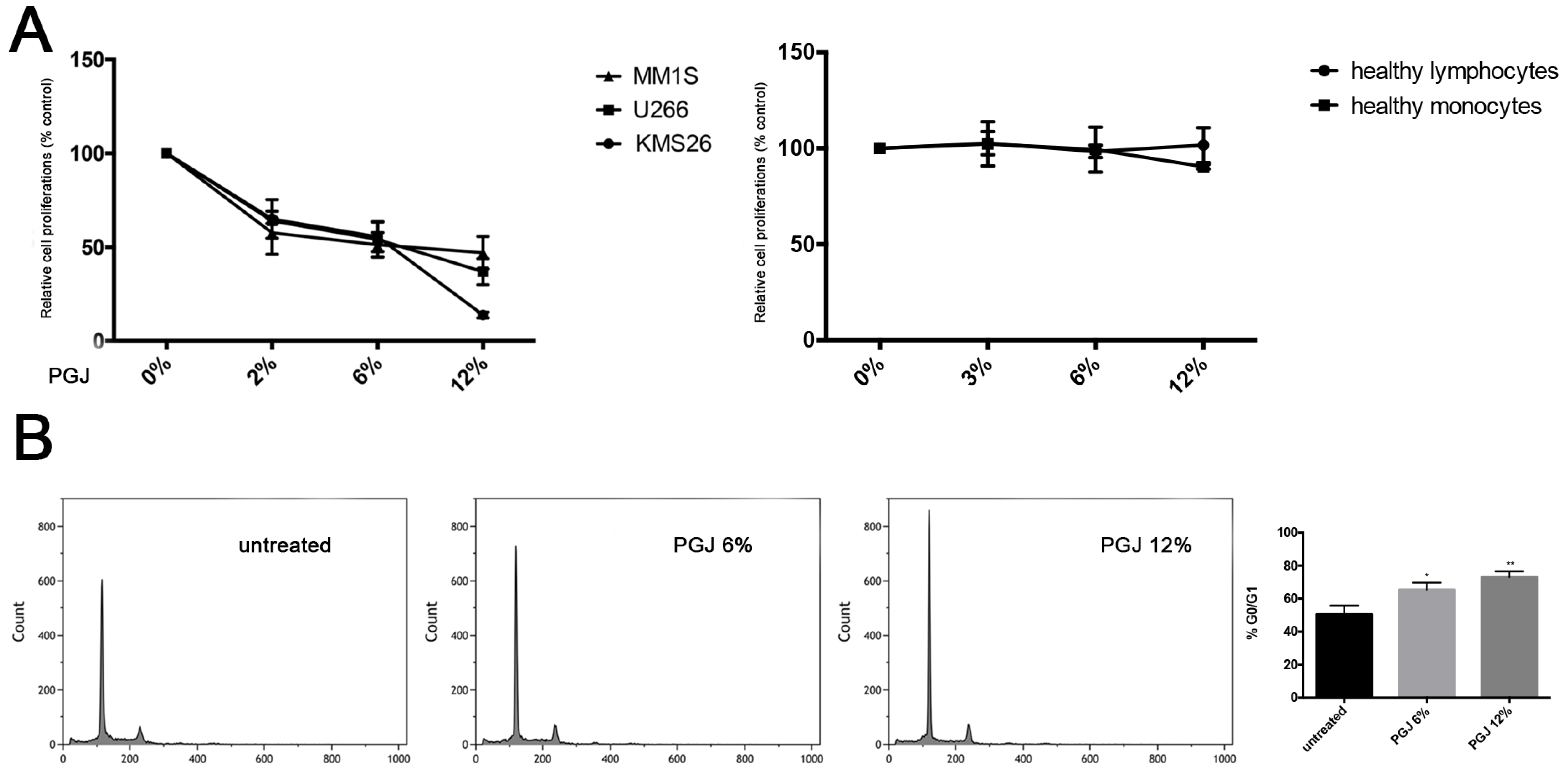
3.1. Effect of Pomegranate Juice on Multiple Myeloma Cell Viability
3.2. Pomegranate Juice Induces PPARγ Expression in Multiple Myeloma Cells
3.3. Pomegranate Juice Inhibits Angiogenesis
3.4. Effect of Pomegranate Juice on Cell Invasion and Migration
3.5. Effect of Pomegranate Juice on Angiogenic Genes Expression
3.6. Combination of Pomegranate Juice with Proteosome Inhibitor Bortezomib
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Tibullo, D.; Di Rosa, M.; Giallongo, C.; La Cava, P.; Parrinello, N.L.; Romano, A.; Conticello, C.; Brundo, M.V.; Saccone, S.; Malaguarnera, L.; et al. Bortezomib modulates CHIT1 and YKL40 in monocyte-derived osteoclast and in myeloma cells. Front. Pharmacol. 2015, 6, 226. [Google Scholar] [CrossRef] [PubMed]
- Gozzetti, A.; Candi, V.; Papini, G.; Bocchia, M. Therapeutic advancements in multiple myeloma. Front. Oncol. 2014, 4, 241. [Google Scholar] [CrossRef] [PubMed]
- Giallongo, C.; Tibullo, D.; Parrinello, N.L.; La Cava, P.; Di Rosa, M.; Bramanti, V.; Di Raimondo, C.; Conticello, C.; Chiarenza, A.; Palumbo, G.A.; et al. Granulocyte-like myeloid derived suppressor Cells (G-MDSC) are increased in multiple myeloma and are driven by dysfunctional mesenchymal stem cells (MSC). Oncotarget 2016. [Google Scholar] [CrossRef]
- Romano, A.; Conticello, C.; Di Raimondo, F. Bortezomib for the treatment of previously untreated multiple myeloma. Immunotherapy 2013, 5, 327–352. [Google Scholar] [CrossRef] [PubMed]
- Gambella, M.; Rocci, A.; Passera, R.; Gay, F.; Omede, P.; Crippa, C.; Corradini, P.; Romano, A.; Rossi, D.; Ladetto, M.; et al. High XBP1 expression is a marker of better outcome in multiple myeloma patients treated with bortezomib. Haematologica 2014, 99, e14–e16. [Google Scholar] [CrossRef] [PubMed]
- Botta, C.; Gulla, A.; Correale, P.; Tagliaferri, P.; Tassone, P. Myeloid-derived suppressor cells in multiple myeloma: Pre-clinical research and translational opportunities. Front. Oncol. 2014, 4, 348. [Google Scholar] [CrossRef] [PubMed]
- Kryukov, F.; Nemec, P.; Dementyeva, E.; Kubiczkova, L.; Ihnatova, I.; Budinska, E.; Jarkovsky, J.; Sevcikova, S.; Kuglik, P.; Hajek, R. Molecular heterogeneity and centrosome-associated genes in multiple myeloma. Leuk. Lymphoma 2013, 54, 1982–1988. [Google Scholar] [CrossRef] [PubMed]
- Tibullo, D.; Barbagallo, I.; Giallongo, C.; Vanella, L.; Conticello, C.; Romano, A.; Saccone, S.; Godos, J.; Di Raimondo, F.; Volti, L.G. Heme oxygenase-1 nuclear translocation regulates bortezomibinduced cytotoxicity and mediates genomic instability in myeloma cells. Oncotarget 2016, 7, 28868–28880. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.H.; Mulligan, G.; Fonseca, R.; Chng, W.J. A novel measure of chromosome instability can account for prognostic difference in multiple myeloma. PLoS ONE 2013, 8, e66361. [Google Scholar] [CrossRef] [PubMed]
- Safarzadeh, E.; Sandoghchian, S.S.; Baradaran, B. Herbal medicine as inducers of apoptosis in cancer treatment. Adv. Pharm. Bull. 2014, 4, 421–427. [Google Scholar] [PubMed]
- Syed, D.N.; Chamcheu, J.C.; Adhami, V.M.; Mukhtar, H. Pomegranate extracts and cancer prevention: Molecular and cellular activities. Anticancer Agents Med. Chem. 2013, 13, 1149–1161. [Google Scholar] [CrossRef] [PubMed]
- Turrini, E.; Ferruzzi, L.; Fimognari, C. Potential effects of pomegranate polyphenols in cancer prevention and therapy. Oxid. Med. Cell. Longev. 2015, 2015, 938475. [Google Scholar] [CrossRef] [PubMed]
- Lansky, E.P.; Newman, R.A. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J. Ethnopharmacol. 2007, 109, 177–206. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.R.; Newman, R.A.; Lansky, E.P. Punica granatum: Heuristic treatment for diabetes mellitus. J. Med. Food 2007, 10, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Heber, D. Pomegranate ellagitannins. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Adhami, V.M.; Khan, N.; Mukhtar, H. Cancer chemoprevention by pomegranate: Laboratory and clinical evidence. Nutr. Cancer 2009, 61, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.D.; Mehta, R.; Yu, W.; Neeman, I.; Livney, T.; Amichay, A.; Poirier, D.; Nicholls, P.; Kirby, A.; Jiang, W.; et al. Chemopreventive and adjuvant therapeutic potential of pomegranate (Punica granatum) for human breast cancer. Breast Cancer Res. Treat. 2002, 71, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Toi, M.; Bando, H.; Ramachandran, C.; Melnick, S.J.; Imai, A.; Fife, R.S.; Carr, R.E.; Oikawa, T.; Lansky, E.P. Preliminary studies on the anti-angiogenic potential of pomegranate fractions in vitro and in vivo. Angiogenesis 2003, 6, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.S.; Zhang, Y.; Seeram, N.P.; Heber, D.; Chen, S. Pomegranate ellagitannin-derived compounds exhibit antiproliferative and antiaromatase activity in breast cancer cells in vitro. Cancer Prev. Res. 2010, 3, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Nair, V.; Khan, M.; Ciolino, H.P. Pomegranate extract inhibits the proliferation and viability of MMTV-WNT-1 mouse mammary cancer stem cells in vitro. Oncol. Rep. 2010, 24, 1087–1091. [Google Scholar] [PubMed]
- Dikmen, M.; Ozturk, N.; Ozturk, Y. The antioxidant potency of Punica granatum L. Fruit peel reduces cell proliferation and induces apoptosis on breast cancer. J. Med. Food 2011, 14, 1638–1646. [Google Scholar] [CrossRef] [PubMed]
- Joseph, M.M.; Aravind, S.R.; Varghese, S.; Mini, S.; Sreelekha, T.T. Evaluation of antioxidant, antitumor and immunomodulatory properties of polysaccharide isolated from fruit rind of Punica granatum. Mol. Med. Rep. 2012, 5, 489–496. [Google Scholar] [PubMed]
- Banerjee, N.; Talcott, S.; Safe, S.; Mertens-Talcott, S.U. Cytotoxicity of pomegranate polyphenolics in breast cancer cells in vitro and vivo: Potential role of MIRNA-27a and MIRNA-155 in cell survival and inflammation. Breast Cancer Res. Treat. 2012, 136, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Sreeja, S.; Santhosh Kumar, T.R.; Lakshmi, B.S.; Sreeja, S. Pomegranate extract demonstrate a selective estrogen receptor modulator profile in human tumor cell lines and in vivo models of estrogen deprivation. J. Nutr. Biochem. 2012, 23, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Shirode, A.B.; Kovvuru, P.; Chittur, S.V.; Henning, S.M.; Heber, D.; Reliene, R. Antiproliferative effects of pomegranate extract in MCF-7 breast cancer cells are associated with reduced DNA repair gene expression and induction of double strand breaks. Mol. Carcinog. 2014, 53, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.; Lansky, E.P. Breast cancer chemopreventive properties of pomegranate (Punica granatum) fruit extracts in a mouse mammary organ culture. Eur. J. Cancer Prev. 2004, 13, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, A.; Mandal, A.; Bhattacharyya, P.; Bhatia, D. Pomegranate exerts chemoprevention of experimentally induced mammary tumorigenesis by suppression of cell proliferation and induction of apoptosis. Nutr. Cancer 2016, 68, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Di Rosa, M.; Tibullo, D.; Vecchio, M.; Nunnari, G.; Saccone, S.; Di Raimondo, F.; Malaguarnera, L. Determination of chitinases family during osteoclastogenesis. Bone 2014, 61, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Henning, S.M.; Heber, D. Limitations of MTT and MTS-based assays for measurement of antiproliferative activity of green tea polyphenols. PLoS ONE 2010, 5, e10202. [Google Scholar] [CrossRef] [PubMed]
- Cree, I.A.; Andreotti, P.E. Measurement of cytotoxicity by ATP-based luminescence assay in primary cell cultures and cell lines. Toxicol. In Vitro 1997, 11, 553–556. [Google Scholar] [CrossRef]
- Petty, R.D.; Sutherland, L.A.; Hunter, E.M.; Cree, I.A. Comparison of MTT and ATP-based assays for the measurement of viable cell number. J. Biolumin. Chemilumin. 1995, 10, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Motta, C.; D’Angeli, F.; Scalia, M.; Satriano, C.; Barbagallo, D.; Naletova, I.; Anfuso, C.D.; Lupo, G.; Spina-Purrello, V. Pj-34 inhibits parp-1 expression and erk phosphorylation in glioma-conditioned brain microvascular endothelial cells. Eur. J. Pharmacol. 2015, 761, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Lupo, G.; Motta, C.; Salmeri, M.; Spina-Purrello, V.; Alberghina, M.; Anfuso, C.D. An in vitro retinoblastoma human triple culture model of angiogenesis: A modulatory effect of TGF-beta. Cancer Lett. 2014, 354, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Giurdanella, G.; Motta, C.; Muriana, S.; Arena, V.; Anfuso, C.D.; Lupo, G.; Alberghina, M. Cytosolic and calcium-independent phospholipase a(2) mediate glioma-enhanced proangiogenic activity of brain endothelial cells. Microvasc. Res. 2011, 81, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Giallongo, C.; Tibullo, D.; La Cava, P.; Branca, A.; Parrinello, N.; Spina, P.; Stagno, F.; Conticello, C.; Chiarenza, A.; Vigneri, P.; et al. Brit1/mcph1 expression in chronic myeloid leukemia and its regulation of the g2/m checkpoint. Acta Haematol. 2011, 126, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Khateeb, J.; Gantman, A.; Kreitenberg, A.J.; Aviram, M.; Fuhrman, B. Paraoxonase 1 (pon1) expression in hepatocytes is upregulated by pomegranate polyphenols: A role for ppar-gamma pathway. Atherosclerosis 2010, 208, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Aouali, N.; Broukou, A.; Bosseler, M.; Keunen, O.; Schlesser, V.; Janji, B.; Palissot, V.; Stordeur, P.; Berchem, G. Epigenetic activity of peroxisome proliferator-activated receptor gamma agonists increases the anticancer effect of histone deacetylase inhibitors on multiple myeloma cells. PLoS ONE 2015, 10, e0130339. [Google Scholar] [CrossRef] [PubMed]
- Vacca, A.; Ria, R.; Reale, A.; Ribatti, D. Angiogenesis in multiple myeloma. Chem. Immunol. Allergy 2014, 99, 180–196. [Google Scholar] [PubMed]
- Paller, C.J.; Ye, X.; Wozniak, P.J.; Gillespie, B.K.; Sieber, P.R.; Greengold, R.H.; Stockton, B.R.; Hertzman, B.L.; Efros, M.D.; Roper, R.P.; et al. A randomized phase II study of pomegranate extract for men with rising psa following initial therapy for localized prostate cancer. Prostate Cancer Prostatic Dis. 2013, 16, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Sartippour, M.R.; Seeram, N.P.; Rao, J.Y.; Moro, A.; Harris, D.M.; Henning, S.M.; Firouzi, A.; Rettig, M.B.; Aronson, W.J.; Pantuck, A.J.; et al. Ellagitannin-rich pomegranate extract inhibits angiogenesis in prostate cancer in vitro and in vivo. Int. J. Oncol. 2008, 32, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Rettig, M.B.; Heber, D.; An, J.; Seeram, N.P.; Rao, J.Y.; Liu, H.; Klatte, T.; Belldegrun, A.; Moro, A.; Henning, S.M.; et al. Pomegranate extract inhibits androgen-independent prostate cancer growth through a nuclear factor-kappab-dependent mechanism. Mol. Cancer Ther. 2008, 7, 2662–2671. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Aronson, W.J.; Zhang, Y.; Henning, S.M.; Moro, A.; Lee, R.P.; Sartippour, M.; Harris, D.M.; Rettig, M.; Suchard, M.A.; et al. Pomegranate ellagitannin-derived metabolites inhibit prostate cancer growth and localize to the mouse prostate gland. J. Agric. Food Chem. 2007, 55, 7732–7737. [Google Scholar] [CrossRef] [PubMed]
- Costantini, S.; Rusolo, F.; De Vito, V.; Moccia, S.; Picariello, G.; Capone, F.; Guerriero, E.; Castello, G.; Volpe, M.G. Potential anti-inflammatory effects of the hydrophilic fraction of pomegranate (Punica granatum L.) seed oil on breast cancer cell lines. Molecules 2014, 19, 8644–8660. [Google Scholar] [CrossRef] [PubMed]
- Amakura, Y.; Okada, M.; Tsuji, S.; Tonogai, Y. High-performance liquid chromatographic determination with photodiode array detection of ellagic acid in fresh and processed fruits. J. Chromatogr. A 2000, 896, 87–93. [Google Scholar] [CrossRef]
- Elfalleh, W.; Tlili, N.; Nasri, N.; Yahia, Y.; Hannachi, H.; Chaira, N.; Ying, M.; Ferchichi, A. Antioxidant capacities of phenolic compounds and tocopherols from tunisian pomegranate (Punica granatum) fruits. J. Food Sci. 2011, 76, C707–C713. [Google Scholar] [CrossRef] [PubMed]
- Fanali, C.; Dugo, L.; D’Orazio, G.; Lirangi, M.; Dacha, M.; Dugo, P.; Mondello, L. Analysis of anthocyanins in commercial fruit juices by using nano-liquid chromatography-electrospray-mass spectrometry and high-performance liquid chromatography with UV-Vis detector. J. Sep. Sci. 2011, 34, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Pande, G.; Akoh, C.C. Antioxidant capacity and lipid characterization of six georgia-grown pomegranate cultivars. J. Agric. Food Chem. 2009, 57, 9427–9436. [Google Scholar] [CrossRef] [PubMed]
- Kohno, H.; Suzuki, R.; Yasui, Y.; Hosokawa, M.; Miyashita, K.; Tanaka, T. Pomegranate seed oil rich in conjugated linolenic acid suppresses chemically induced colon carcinogenesis in rats. Cancer Sci. 2004, 95, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Kiraz, Y.; Neergheen-Bhujun, V.S.; Rummun, N.; Baran, Y. Apoptotic effects of non-edible parts of Punica granatum on human multiple myeloma cells. Tumour Biol. 2016, 37, 1803–1815. [Google Scholar] [CrossRef] [PubMed]
- Dahlawi, H.; Jordan-Mahy, N.; Clench, M.; McDougall, G.J.; Maitre, C.L. Polyphenols are responsible for the proapoptotic properties of pomegranate juice on leukemia cell lines. Food Sci. Nutr. 2013, 1, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Aouali, N.; Palissot, V.; El-Khoury, V.; Moussay, E.; Janji, B.; Pierson, S.; Brons, N.H.; Kellner, L.; Bosseler, M.; Van Moer, K.; et al. Peroxisome proliferator-activated receptor gamma agonists potentiate the cytotoxic effect of valproic acid in multiple myeloma cells. Br. J. Haematol. 2009, 147, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Eucker, J.; Bangeroth, K.; Zavrski, I.; Krebbel, H.; Zang, C.; Heider, U.; Jakob, C.; Elstner, E.; Possinger, K.; Sezer, O. Ligands of peroxisome proliferator-activated receptor gamma induce apoptosis in multiple myeloma. Anticancer Drugs 2004, 15, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Malki, A.; Cao, Y.; Li, Y.; Qian, Y.; Wang, X.; Chen, X. Glucose- and triglyceride-lowering dietary penta-O-galloyl-alpha-d-glucose reduces expression of ppargamma and c/ebpalpha, induces p21-mediated g1 phase cell cycle arrest, and inhibits adipogenesis in 3t3-l1 preadipocytes. Exp. Clin. Endocrinol. Diabetes 2015, 123, 308–316. [Google Scholar] [PubMed]
- Di Raimondo, F. Angiogenesis in hematology: A field of active research. Leuk. Res. 2003, 27, 571–573. [Google Scholar] [CrossRef]
- Wang, L.; Martins-Green, M. Pomegranate and its components as alternative treatment for prostate cancer. Int. J. Mol. Sci. 2014, 15, 14949–14966. [Google Scholar] [CrossRef] [PubMed]
- Faria, A.; Calhau, C. The bioactivity of pomegranate: Impact on health and disease. Crit. Rev. Food Sci. Nutr. 2011, 51, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Glynn, S.J.; Gaffney, K.J.; Sainz, M.A.; Louie, S.G.; Petasis, N.A. Molecular characterization of the boron adducts of the proteasome inhibitor bortezomib with epigallocatechin-3-gallate and related polyphenols. Org. Biomol. Chem. 2015, 13, 3887–3899. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Liu, F.T. Why bortezomib cannot go with ‘green’? Cancer Biol. Med. 2013, 10, 206–213. [Google Scholar] [PubMed]





| Compound | Concentration (mg·L−1) |
|---|---|
| gallic acid | 18.2 |
| ellagic acid | 97.5 |
| ellagic acid glucoside | 11.7 |
| α-punicalagin | 3.1 |
| β-punicalagin | 6.5 |
| delphinidin 3,5-diglucoside | 110.5 |
| cyanidin 3,5-diglucoside | 242.8 |
| pelargonidin 3,5-diglucoside | 9.3 |
| delphinidin 3-diglucoside | 60.4 |
| cyanidin 3-diglucoside | 180.6 |
| pelargonidin 3-diglucoside | 12.1 |
| Genes | VEGF | PGJ + VEGF | p Value |
|---|---|---|---|
| VEGF | 43 | 0.036 | p < 0.001 |
| ADAMST1 | 23 | 0.041 | p < 0.001 |
| CXCL12 | 16 | 0.136 | p < 0.001 |
| CXCL2 | 9 | 0.038 | p < 0.001 |
| FGF2 | 32 | 0.011 | p < 0.001 |
| FIGF | 12 | 0.065 | p < 0.05 |
| IL12A | 9 | 0.030 | p < 0.001 |
| IL8 | 21 | 0.007 | p < 0.01 |
| MMP2 | 5 | 0.031 | p < 0.001 |
| PDGFB | 15 | 0.116 | p < 0.001 |
| VEGFB | 19 | 0.028 | p < 0.001 |
| VEGFC | 18 | 0.008 | p < 0.001 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tibullo, D.; Caporarello, N.; Giallongo, C.; Anfuso, C.D.; Genovese, C.; Arlotta, C.; Puglisi, F.; Parrinello, N.L.; Bramanti, V.; Romano, A.; et al. Antiproliferative and Antiangiogenic Effects of Punica granatum Juice (PGJ) in Multiple Myeloma (MM). Nutrients 2016, 8, 611. https://doi.org/10.3390/nu8100611
Tibullo D, Caporarello N, Giallongo C, Anfuso CD, Genovese C, Arlotta C, Puglisi F, Parrinello NL, Bramanti V, Romano A, et al. Antiproliferative and Antiangiogenic Effects of Punica granatum Juice (PGJ) in Multiple Myeloma (MM). Nutrients. 2016; 8(10):611. https://doi.org/10.3390/nu8100611
Chicago/Turabian StyleTibullo, Daniele, Nunzia Caporarello, Cesarina Giallongo, Carmelina Daniela Anfuso, Claudia Genovese, Carmen Arlotta, Fabrizio Puglisi, Nunziatina L. Parrinello, Vincenzo Bramanti, Alessandra Romano, and et al. 2016. "Antiproliferative and Antiangiogenic Effects of Punica granatum Juice (PGJ) in Multiple Myeloma (MM)" Nutrients 8, no. 10: 611. https://doi.org/10.3390/nu8100611
APA StyleTibullo, D., Caporarello, N., Giallongo, C., Anfuso, C. D., Genovese, C., Arlotta, C., Puglisi, F., Parrinello, N. L., Bramanti, V., Romano, A., Lupo, G., Toscano, V., Avola, R., Brundo, M. V., Di Raimondo, F., & Raccuia, S. A. (2016). Antiproliferative and Antiangiogenic Effects of Punica granatum Juice (PGJ) in Multiple Myeloma (MM). Nutrients, 8(10), 611. https://doi.org/10.3390/nu8100611










