Impact of Pre-Pregnancy BMI on B Vitamin and Inflammatory Status in Early Pregnancy: An Observational Cohort Study
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Population
2.2. Ethics
2.3. Blood Sampling and Laboratory Analyses
2.4. Covariates
2.5. Statistical Analyses
3. Results
3.1. Characteristics of the Study Population According to Pre-Pregnancy BMI
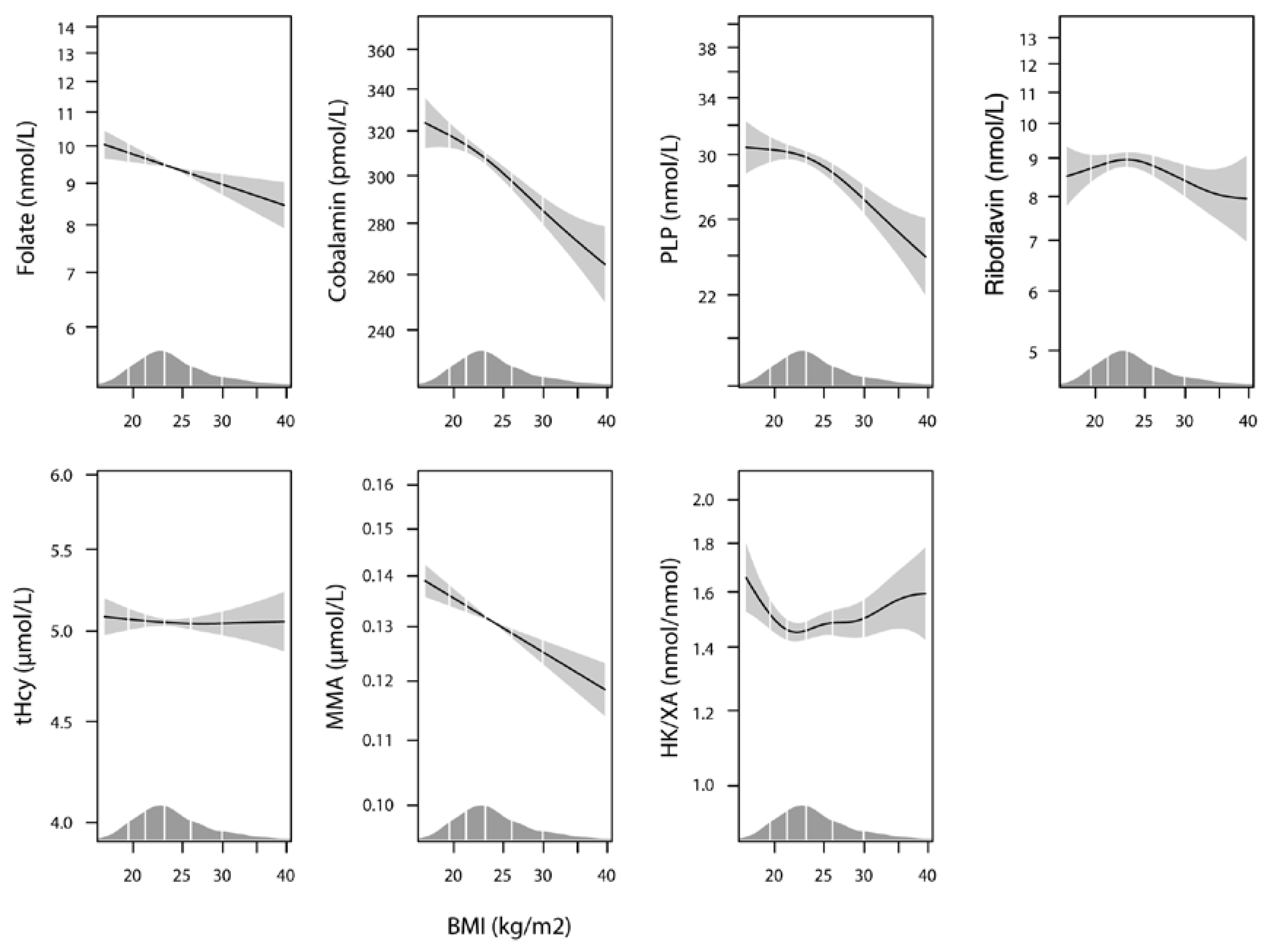
3.2. Plasma B Vitamin Status According to Pre-Pregnancy BMI
3.3. Inflammation Markers According to Pre-Pregnancy BMI
3.4. Maternal Factors and Inflammation Markers as Determinants of Plasma B Vitamin Status in Pregnancy Week 18
4. Discussion
4.1. Main Findings
4.2. Strength and Limitations
4.3. Interpretation
4.3.1. Effects of Pre-Pregnancy BMI on Gestational Weight Gain and Birth Weight
4.3.2. Pregnancy Related Changes in Biochemical Parameters
4.3.3. Maternal Predictors for B Vitamin Status
4.3.4. The Complex Relation between Pre-pregnancy BMI, Inflammation, and Vitamin Levels
5. Conclusions
Acknowledgments
Authors Contributions
Conflicts of Interest
Abbreviations
References
- Swinburn, B.A.; Sacks, G.; Hall, K.D.; McPherson, K.; Finegood, D.T.; Moodie, M.L.; Gortmaker, S.L. The global obesity pandemic: Shaped by global drivers and local environments. Lancet 2011, 378, 804–814. [Google Scholar] [CrossRef]
- Catalano, P.M.; Ehrenberg, H.M. The short- and long-term implications of maternal obesity on the mother and her offspring. Br. J. Obstet. Gynaecol. 2006, 113, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Stothard, K.J.; Tennant, P.W.; Bell, R.; Rankin, J. Maternal overweight and obesity and the risk of congenital anomalies: A systematic review and meta-analysis. J. Am. Med. Assoc. 2009, 301, 636–650. [Google Scholar] [CrossRef] [PubMed]
- Boney, C.M.; Verma, A.; Tucker, R.; Vohr, B.R. Metabolic syndrome in childhood: Association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 2005, 115, e290–e296. [Google Scholar] [CrossRef] [PubMed]
- Kimmons, J.E.; Blanck, H.M.; Tohill, B.C.; Zhang, J.; Khan, L.K. Associations between body mass index and the prevalence of low micronutrient levels among US adults. MedGenMed 2006, 8, 59. [Google Scholar] [PubMed]
- Mojtabai, R. Body mass index and serum folate in childbearing age women. Eur. J. Epidemiol. 2004, 19, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Samuel, L.; Borrell, L.N. The effect of body mass index on optimal vitamin D status in U.S. adults: The National Health and Nutrition Examination Survey 2001–2006. Ann. Epidemiol. 2013, 23, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Nead, K.G.; Halterman, J.S.; Kaczorowski, J.M.; Auinger, P.; Weitzman, M. Overweight children and adolescents: A risk group for iron deficiency. Pediatrics 2004, 114, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Strauss, R.S. Comparison of serum concentrations of alpha-tocopherol and beta-carotene in a cross-sectional sample of obese and nonobese children (NHANES III). National Health and Nutrition Examination Survey. J. Pediatr. 1999, 134, 160–165. [Google Scholar] [CrossRef]
- Bodnar, L.M.; Catov, J.M.; Roberts, J.M.; Simhan, H.N. Pre-pregnancy obesity predicts poor vitamin D status in mothers and their neonates. J. Nutr. 2007, 137, 2437–2442. [Google Scholar] [PubMed]
- Kim, H.; Hwang, J.Y.; Kim, K.N.; Ha, E.H.; Park, H.; Ha, M.; Lee, K.Y.; Hong, Y.C.; Tamura, T.; Chang, N. Relationship between body-mass index and serum folate concentrations in pregnant women. Eur. J. Clin. Nutr. 2012, 66, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Goldenberg, R.L.; Johnston, K.E.; Chapman, V.R. Relationship between pre-pregnancy BMI and plasma zinc concentrations in early pregnancy. Br. J. Nutr. 2004, 91, 773–777. [Google Scholar] [CrossRef] [PubMed]
- Tomedi, L.E.; Chang, C.C.; Newby, P.K.; Evans, R.W.; Luther, J.F.; Wisner, K.L.; Bodnar, L.M. Pre-pregnancy obesity and maternal nutritional biomarker status during pregnancy: A factor analysis. Public Health Nutr. 2013, 16, 1414–1418. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.H. Multiple micronutrients in pregnancy and lactation: An overview. Am. J. Clin. Nutr. 2005, 81, 1206S–1212S. [Google Scholar] [PubMed]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory mechanisms in obesity. Ann. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef] [PubMed]
- Theofylaktopoulou, D.; Midttun, O.; Ulvik, A.; Ueland, P.M.; Tell, G.S.; Vollset, S.E.; Nygård, O.; Eussen, S.J. A community-based study on determinants of circulating markers of cellular immune activation and kynurenines: The Hordaland Health Study. Clin. Exp. Immunol. 2013, 173, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Bouter, L.M.; McQuillan, G.M.; Wener, M.H.; Harris, T.B. Elevated C-reactive protein levels in overweight and obese adults. J. Am. Med. Assoc. 1999, 282, 2131–2135. [Google Scholar] [CrossRef]
- Duncan, A.; Talwar, D.; McMillan, D.C.; Stefanowicz, F.; O’Reilly, D.S. Quantitative data on the magnitude of the systemic inflammatory response and its effect on micronutrient status based on plasma measurements. Am. J. Clin. Nutr. 2012, 95, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Gori, A.M.; Sofi, F.; Corsi, A.M.; Gazzini, A.; Sestini, I.; Lauretani, F.; Bandinelli, S.; Gensini, G.F.; Ferrucci, L.; Abbate, R. Predictors of vitamin B6 and folate concentrations in older persons: The InCHIANTI study. Clin. Chem. 2006, 52, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Reyman, M.; Verrijn Stuart, A.A.; van Summeren, M.; Rakhshandehroo, M.; Nuboer, R.; de Boer, F.K.; van den Ham, H.J.; Kalkhoven, E.; Prakken, B.; Schipper, H.S. Vitamin D deficiency in childhood obesity is associated with high levels of circulating inflammatory mediators, and low insulin sensitivity. Int. J. Obes. 2014. [Google Scholar] [CrossRef] [PubMed]
- Magnus, P.; Irgens, L.M.; Haug, K.; Nystad, W.; Skjaerven, R.; Stoltenberg, C. Cohort profile: The Norwegian Mother and Child Cohort Study (MoBa). Int. J. Epidemiol. 2006, 35, 1146–1150. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, R.M.; Vollset, S.E.; Monsen, A.L.; Ulvik, A.; Haugen, M.; Meltzer, H.M.; Magnus, P.; Ueland, P.M. Infant birth size is not associated with maternal intake and status of folate during the second trimester in Norwegian pregnant women. J. Nutr. 2010, 140, 572–579. [Google Scholar] [CrossRef] [PubMed]
- O’Broin, S.; Kelleher, B. Microbiological assay on microtitre plates of folate in serum and red cells. J. Clin. Pathol. 1992, 45, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, B.P.; Broin, S.D. Microbiological assay for vitamin B12 performed in 96-well microtitre plates. J. Clin. Pathol. 1991, 44, 592–595. [Google Scholar] [CrossRef] [PubMed]
- Baril, L.; Carmel, R. Comparison of radioassay and microbiological assay for serum folate, with clinical assessment of discrepant results. Clin. Chem. 1978, 24, 2192–2196. [Google Scholar] [PubMed]
- Windelberg, A.; Arseth, O.; Kvalheim, G.; Ueland, P.M. Automated assay for the determination of methylmalonic acid, total homocysteine, and related amino acids in human serum or plasma by means of methylchloroformate derivatization and gas chromatography-mass spectrometry. Clin. Chem. 2005, 51, 2103–2109. [Google Scholar] [CrossRef] [PubMed]
- Ulvik, A.; Theofylaktopoulou, D.; Midttun, O.; Nygard, O.; Eussen, S.J.; Ueland, P.M. Substrate product ratios of enzymes in the kynurenine pathway measured in plasma as indicators of functional vitamin B-6 status. Am. J. Clin. Nutr. 2013, 98, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, D.; Weiss, G.; Wachter, H. Neopterin, biochemistry and clinical use as a marker for cellular immune reactions. Int. Arch. Allergy Immunol. 1993, 101, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Raitala, A.; Pertovaara, M.; Karjalainen, J.; Oja, S.S.; Hurme, M. Association of interferon-gamma +874(T/A) single nucleotide polymorphism with the rate of tryptophan catabolism in healthy individuals. Scand. J. Immunol. 2005, 61, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Midttun, O.; Hustad, S.; Ueland, P.M. Quantitative profiling of biomarkers related to B-vitamin status, tryptophan metabolism and inflammation in human plasma by liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2009, 23, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2006. [Google Scholar]
- Nilsen, R.M.; Vollset, S.E.; Gjessing, H.K.; Skjaerven, R.; Melve, K.K.; Schreuder, P.; Alsaker, E.R.; Haug, K.; Daltveit, A.K.; Magnus, P. Self-selection and bias in a large prospective pregnancy cohort in Norway. Paediatr. Perinat. Epidemiol. 2009, 23, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Visscher, T.L.; Viet, A.L.; Kroesbergen, I.H.; Seidell, J.C. Underreporting of BMI in adults and its effect on obesity prevalence estimations in the period 1998 to 2001. Obesity 2006, 14, 2054–2063. [Google Scholar] [CrossRef] [PubMed]
- Hustad, S.; Eussen, S.; Midttun, O.; Ulvik, A.; van de Kant, P.M.; Morkrid, L.; Gislefoss, R.; Ueland, P.M. Kinetic modeling of storage effects on biomarkers related to B vitamin status and one-carbon metabolism. Clin. Chem. 2012, 58, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Stamnes Koepp, U.M.; Frost Andersen, L.; Dahl-Joergensen, K.; Stigum, H.; Nass, O.; Nystad, W. Maternal pre-pregnant body mass index, maternal weight change and offspring birthweight. Acta Obstet. Gynecol. Scand. 2012, 91, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Metz, J.; McGrath, K.; Bennett, M.; Hyland, K.; Bottiglieri, T. Biochemical indices of vitamin B12 nutrition in pregnant patients with subnormal serum vitamin B12 levels. Am. J. Hematol. 1995, 48, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Bruinse, H.W.; van den Berg, H. Changes of some vitamin levels during and after normal pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 1995, 61, 31–37. [Google Scholar] [CrossRef]
- Pietrzik, K.F.; Thorand, B. Folate economy in pregnancy. Nutrition 1997, 13, 975–977. [Google Scholar] [CrossRef]
- Milman, N.; Byg, K.E.; Bergholt, T.; Eriksen, L.; Hvas, A.M. Cobalamin status during normal pregnancy and postpartum: A longitudinal study comprising 406 Danish women. Eur. J. Haematol. 2006, 76, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Shibata, K.; Tachiki, A.; Mukaeda, K.; Fukuwatari, T.; Sasaki, S.; Jinno, Y. Changes in plasma pyridoxal 5’-phosphate concentration during pregnancy stages in Japanese women. J. Nutr. Sci. Vitaminol. 2013, 59, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Cikot, R.J.; Steegers-Theunissen, R.P.; Thomas, C.M.; de Boo, T.M.; Merkus, H.M.; Steegers, E.A. Longitudinal vitamin and homocysteine levels in normal pregnancy. Br. J. Nutr. 2001, 85, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, P.; McDermott, L. Long chain PUFA transport in human term placenta. J. Nutr. 2009, 139, 636–639. [Google Scholar] [CrossRef] [PubMed]
- Ueland, P.M.; Refsum, H.; Schneede, J. Determinants of plasma homocysteine. In Homocysteine and Vascular Disease; Robinson, K., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 59–84. [Google Scholar]
- Velzing-Aarts, F.V.; Holm, P.I.; Fokkema, M.R.; van der Dijs, F.P.; Ueland, P.M.; Muskiet, F.A. Plasma choline and betaine and their relation to plasma homocysteine in normal pregnancy. Am. J. Clin. Nutr. 2005, 81, 1383–1389. [Google Scholar] [PubMed]
- Savage, D.G.; Lindenbaum, J.; Stabler, S.P.; Allen, R.H. Sensitivity of serum methylmalonic acid and total homocysteine determinations for diagnosing cobalamin and folate deficiencies. Am. J. Med. 1994, 96, 239–246. [Google Scholar] [CrossRef]
- Greibe, E.; Andreasen, B.H.; Lildballe, D.L.; Morkbak, A.L.; Hvas, A.M.; Nexo, E. Uptake of cobalamin and markers of cobalamin status: A longitudinal study of healthy pregnant women. Clin. Chem. Lab. Med. 2011, 49, 1877–1882. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.M.; Molloy, A.M.; Ueland, P.M.; Fernandez-Ballart, J.D.; Schneede, J.; Arija, V.; Scott, J.M. Longitudinal study of the effect of pregnancy on maternal and fetal cobalamin status in healthy women and their offspring. J. Nutr. 2007, 137, 1863–1867. [Google Scholar] [PubMed]
- Bjorke-Monsen, A.L.; Roth, C.; Magnus, P.; Midttun, O.; Nilsen, R.M.; Reichborn-Kjennerud, T.; Stoltenberg, C.; Susser, E.; Vollset, S.E.; Ueland, P.M. Maternal B vitamin status in pregnancy week 18 according to reported use of folic acid supplements. Mol. Nutr. Food Res. 2013, 57, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.H.; Bjorke-Monsen, A.L.; Teixeira, A.L.; Silverman, M.N. Maternal stress, nutrition and physical activity: Impact on immune function, CNS development and psychopathology. Brain Res. 2015. [CrossRef] [PubMed]
- Schrocksnadel, K.; Widner, B.; Bergant, A.; Neurauter, G.; Schrocksnadel, H.; Fuchs, D. Tryptophan degradation during and after gestation. Adv. Exp. Med. Biol. 2003, 527, 77–83. [Google Scholar] [PubMed]
- Fuith, L.C.; Fuchs, D.; Hausen, A.; Hetzel, H.; Reibnegger, G.; Werner, E.R.; Wachter, H. Neopterin, a marker of cell-mediated immune activation in human pregnancy. Int. J. Fertil. 1991, 36, 372–375. [Google Scholar] [PubMed]
- Von Versen-Hoeynck, F.M.; Hubel, C.A.; Gallaher, M.J.; Gammill, H.S.; Powers, R.W. Plasma levels of inflammatory markers neopterin, sialic acid, and C-reactive protein in pregnancy and preeclampsia. Am. J. Hypertens. 2009, 22, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Navolan, D.B.; Vladareanu, S.; Lahdou, I.; Ciohat, I.; Kleist, C.; Grigoras, D.; Vladareanu, R.; Terness, P.; Sas, I. Early pregnancy serum neopterin concentrations predict spontaneous preterm birth in asymptomatic pregnant women. J. Perinat. Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, R.M.; Vollset, S.E.; Gjessing, H.K.; Magnus, P.; Meltzer, H.M.; Haugen, M.; Ueland, P.M. Patterns and predictors of folic acid supplement use among pregnant women: The Norwegian Mother and Child Cohort Study. Am. J. Clin. Nutr. 2006, 84, 1134–1141. [Google Scholar] [PubMed]
- Theofylaktopoulou, D.; Ulvik, A.; Midttun, O.; Ueland, P.M.; Vollset, S.E.; Nygard, O.; Hustad, S.; Tell, G.S.; Eussen, S.J. Vitamins B2 and B6 as determinants of kynurenines and related markers of interferon-gamma-mediated immune activation in the community-based Hordaland Health Study. Br. J. Nutr. 2014, 112, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Lepski, S.; Brockmeyer, J. Impact of dietary factors and food processing on food allergy. Mol. Nutr. Food Res. 2013, 57, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Ulvik, A.; Midttun, O.; Pedersen, E.R.; Eussen, S.J.; Nygard, O.; Ueland, P.M. Evidence for increased catabolism of vitamin B-6 during systemic inflammation. Am. J. Clin. Nutr. 2014, 100, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Chiang, E.P.; Smith, D.E.; Selhub, J.; Dallal, G.; Wang, Y.C.; Roubenoff, R. Inflammation causes tissue-specific depletion of vitamin B6. Arthritis Res. Ther. 2005, 7, R1254–R1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torsvik, I.K.; Ueland, P.M.; Markestad, T.; Midttun, O.; Monsen, A.L. Motor development related to duration of exclusive breastfeeding, B vitamin status and B12 supplementation in infants with a birth weight between 2000–3000 g, results from a randomized intervention trial. BMC Pediatr. 2015, 15, 218. [Google Scholar] [CrossRef] [PubMed]

| Pre-pregnancy BMI, Categories | p Value | |||||
|---|---|---|---|---|---|---|
| <18.5 | 18.5–24.9 | 25.0–29.9 | 30.0–34.9 | ≥35.0 | ||
| n = 80 (3%) | n = 1827 (65%) | n = 587 (21%) | n = 213 (8%) | n = 90 (3%) | ||
| Primipara, n (%) | 47 (59%) | 785 (43%) | 231 (39%) | 86 (40%) | 26 (29%) | 0.001 b |
| Age, years, mean (SD) | 27.8 (4.9) | 29.9 (4.5) | 29.9 (4.6) | 29.8 (4.6) | 29.5 (4.8) | 0.003 a |
| Education | ||||||
| Primary school, n (%) | 9 (12%) | 39 (2%) | 15 (3%) | 11 (5%) | 6 (7%) | <0.001 b |
| Secondary school, n (%) | 30 (41%) | 623 (36%) | 273 (48%) | 97 (47%) | 48 (55%) | |
| University or college, n (%) | 35 (47%) | 1077 (62%) | 286 (50%) | 98 (48%) | 34 (39%) | |
| Use of supplements anytime from four weeks before pregnancy up to pregnancy week 18 | 63 (79%) | 1523 (83%) | 470 (79%) | 156 (72%) | 61 (68%) | <0.001 b |
| Use of alcohol (≥1/month), n (%) | 5 (0.2%) | 83 (3%) | 15 (0.6%) | 1 (0%) | 3 (0.1%) | 0.009 b |
| Daily smoking, n (%) | 13 (16%) | 140 (8%) | 47 (8%) | 22 (10%) | 9 (10%) | 0.04 b |
| Weight increase, kg, mean (SD), (% of pre-pregnancy weight) | ||||||
| to pregnancy week 18 | 4.5 (3.1) (9%) | 3.3 (2.9) (5%) | 2.4 (3.2) (3%) | 1.1 (3.5) (1%) | −0.1 (4.3) (−0.1%) | <0.001 a <0.001 a |
| to birth | 17.3 (6.3) (35%) | 15.5 (5.9) (25%) | 14.8 (6.7) (20%) | 11.5 (7.3) (13%) | 8.7 (8.9) (8%) | <0.001 a <0.001 a |
| Birth weight, g, mean (SD) | 3343 (635) | 3575 (588) | 3727 (600) | 3696 (648) | 3758 (755) | <0.001 a |
| Pre-pregnancy BMI; Categories | p Value | |||||
|---|---|---|---|---|---|---|
| <18.5 | 18.5–24.9 | 25.0–29.9 | 30.0–34.9 | ≥35.0 | ||
| n = 80 | n = 1827 | n = 587 | n = 213 | n = 90 | ||
| Plasma folate, nmol/L a | 8.8 (6.0–15.7) | 9.2 (6.3–16.0) | 8.1 (5.7–12.8) | 7.3 (5.1–12.2) | 7.3 (5.0–12.1) | <0.001 |
| Plasma cobalamin, pmol/L a | 328 (243–383) | 314 (254–386) | 301 (240–363) | 271 (220–338) | 268 (204–317) | <0.001 |
| Plasma PLP, nmol/L a | 27.0 (21.4–39.7) | 28.3 (21.8–40.7) | 26.6 (20.6–35.6) | 23.8 (17.4–33.7) | 21.5 (17.0–30.1) | <0.001 |
| Plasma riboflavin, nmol/L a | 7.8 (4.8–14.4) | 8.1 (5.6–13.2) | 7.8 (5.2–13.9) | 7.2 (5.2–11.0) | 6.9 (4.4–10.1) | 0.004 |
| B vitamin status a,b | 12.2 (3.7) | 12.5 (3.6) | 11.7 (3.7) | 10.6 (3.7) | 10.1 (3.4) | <0.001 |
| Plasma tHcy, µmol/L a | 5.00 (4.22–5.92) | 4.91 (4.27–5.79) | 4.94 (4.23–5.78) | 5.04 (4.44–6.21) | 5.33 (4.43–6.13) | 0.03 |
| Plasma MMA, µmol/L a | 0.13 (0.11–0.16) | 0.13 (0.11–0.16) | 0.13 (0.10–0.16) | 0.12 (0.10–0.15) | 0.12 (0.10–0.15) | 0.009 |
| Plasma HK/XA a | 1.6 (1.2–2.1) | 1.4 (1.0–2.0) | 1.5 (1.1–2.1) | 1.7 (1.2–2.2) | 1.8 (1.2–2.4) | <0.001 |
| Plasma neopterine, µmol/L a | 6.7 (6.1–8.1) | 7.0 (6.1–8.1) | 7.3 (6.3–8.6) | 7.9 (7.0–9.1) | 8.5 (7.31–9.9) | <0.001 |
| KTR, nmol/µmol a | 18.1 (16.0–21.5) | 18.3 (16.2–20.6) | 18.8 (16.8–21.2) | 19.6 (17.5–22.8) | 21.2 (18.5–24.0) | <0.001 |
| Plasma Folate, nmol/L | Plasma Cobalamin, pmol/L | Plasma PLP, nmol/L | Plasma Riboflavin, nmol/L | Plasma tHcy, µmol/L | Plasma MMA, µmol/L | Plasma HK/XA | B Vitamin Score a | |
|---|---|---|---|---|---|---|---|---|
| B (95% CI) | B (95% CI) | B (95% CI) | B (95% CI) | B (95% CI) | B (95% CI) | B (95% CI) | B (95% CI) | |
| Pre-pregnancy BMI c | −0.59 ** (−0.98, −0.20) | −16.7 ** (−22.0, −11.4) | −2.55 ** (−3.72, −1.38) | −0.60 (−1.25, 0.06) | −0.02 (−0.16, 0.12) | −0.006 ** (−0.008, −0.003) | 0.11 * (0.04, 0.18) | −0.47 ** (−0.60, −0.35) |
| Parity e | −1.33 ** (−1.72, −0.95) | −3.6 (−8.8, 1.6) | −1.28 * (−2.43, −0.12) | −1.08 * (−1.73, −0.43) | −0.01 (−0.15, 0.13) | −0.001 (−0.004, 0.002) | −0.03 (−0.09, 0.04) | −0.34 ** (−0.47, −0.22) |
| Age d | 2.69 ** (2.05, 3.32) | 12.3 * (3.6, 20.9) | 1.96 * (0.06, 3.86) | 0.30 (−0.77, 1.36) | 0.04 (−0.19, 0.27) | 0.002 (−0.002, 0.007) | −0.12 * (−0.24, −0.01) | 0.63 ** (0.43, 0.83) |
| Use of supplements b | 5.14 ** (4.35, 5.94) | 13.0 * (2.3, 23.7) | 8.49 ** (6.12, 10.85) | 1.80 * (0.48, 3.13) | −0.75 ** (−1.03, −0.47) | 0.001 (−0.004, 0.007) | −0.21 * (−0.35, −0.07) | 1.73 ** (1.48, 1.98) |
| Neopterin f | −0.12 (−0.35, 0.11) | −1.9 (−5.0, 1.2) | −1.00 * (−1.68, −0.31) | −0.08 (−0.47, 0.30) | 0.10 * (0.02, 0.18) | 0.003 ** (0.001, 0.004) | 0.19 ** (0.15, 0.23) | −0.12 ** (−0.20, −0.05) |
| Independent Variables | OR (95% CI) for | ||||
|---|---|---|---|---|---|
| Plasma Folate <5.45 nmol/L (Quintile 1) | Plasma Cobalamin <230 pmol/L (Quintile 1) | Plasma PLP <19.26 µmol/L (Quintile 1) | Plasma Riboflavin <4.91 µmol/L (Quintile 1) | B Vitamin Score a (Quintile 1) | |
| Pre-pregnancy BMI (vs. category 2 18.5–24.9) | |||||
| <18.5 | 1.0 (0.5–1.8) | 1.1 (0.6–1.9) | 1.0 (0.5–1.8) | 1.7 (1.0–2.8) | 1.1 (0.7–1.9) |
| 25.0–29.9 | 1.1 (0.9–1.5) | 1.3 (1.0–1.6) | 1.3 (1.0–1.6) | 1.3 (1.0–1.7) | 1.5 (1.2–1.8) |
| 30.0–34.9 | 1.5 (1.0–2.1) | 2.1 (1.5–2.9) | 2.1 (1.6–3.0) | 1.4 (1.0–2.0) | 2.7 (2.0–3.7) |
| ≥35.0 | 1.7 (1.0–2.8) | 2.3 (1.4–3.6) | 2.2 (1.4–3.5) | 1.8 (1.1–3.0) | 2.8 (1.7–4.3) |
| p trend | 0.006 | <0.001 | <0.001 | 0.001 | <0.001 |
| Parity (vs. primipara) | |||||
| Para 1 | 1.2 (1.0–1.6) | 1.1 (0.9–1.4) | 1.3 (1.0–1.6) | 1.3 (1.0–1.6) | 1.3 (1.0–1.6) |
| Para 2 | 1.7 (1.2–2.3) | 1.3 (1.0–1.7) | 1.1 (0.8–1.5) | 1.2 (0.9–1.6) | 1.6 (1.2–2.1) |
| Para 3+ | 3.2 (2.1–4.9) | 1.6 (1.0–2.4) | 1.1 (0.7–1.8) | 1.4 (0.9–2.2) | 2.1 (1.4–3.1) |
| p trend | <0.001 | 0.02 | 0.36 | 0.11 | <0.001 |
| Maternal age (vs. ≤25 years) | |||||
| 25–35 | 0.4 (0.3–0.6) | 0.7 (0.6–1.0) | 0.7 (0.7–1.0) | 0.7 (0.6–1.0) | 0.5 (0.4–0.7) |
| ≥35 | 0.3 (0.2–0.5) | 0.8 (0.5–1.2) | 0.8 (0.5–1.1) | 0.6 (0.4–0.9) | 0.4 (0.3–0.6) |
| p trend | <0.001 | 0.11 | 0.11 | 0.004 | <0.001 |
| Use of supplements (vs. non-user) | |||||
| User | 0.2 (0.2–0.2) | 0.8 (0.6–1.0) | 0.5 (0.4–0.6) | 0.6 (0.5–0.8) | 0.3 (0.3–0.4) |
| p trend | <0.001 | 0.03 | <0.001 | <0.001 | <0.001 |
| Neopterin (vs. ≤6.20 µmol/L, Quartile 1) | |||||
| 6.21–7.17 | 1.1 (0.8–1.4) | 1.1 (0.8–1.5) | 1.1 (0.8–1.5) | 0.9 (0.7–1.2) | 1.0 (0.8–1.3) |
| 7.18–8.40 | 1.3 (1.0–1.8) | 1.0 (0.8–1.4) | 1.1 (0.8–1.4) | 0.9 (0.7–1.1) | 1.1 (0.9–1.4) |
| ≥8.41 | 1.2 (0.9–1.6) | 1.2 (0.9–1.6) | 1.7 (1.3–2.2) | 0.9 (0.7–1.2) | 1.2 (1.0–1.6) |
| p trend | 0.11 | 0.24 | <0.001 | 0.38 | 0.05 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bjørke-Monsen, A.-L.; Ulvik, A.; Nilsen, R.M.; Midttun, Ø.; Roth, C.; Magnus, P.; Stoltenberg, C.; Vollset, S.E.; Reichborn-Kjennerud, T.; Ueland, P.M. Impact of Pre-Pregnancy BMI on B Vitamin and Inflammatory Status in Early Pregnancy: An Observational Cohort Study. Nutrients 2016, 8, 776. https://doi.org/10.3390/nu8120776
Bjørke-Monsen A-L, Ulvik A, Nilsen RM, Midttun Ø, Roth C, Magnus P, Stoltenberg C, Vollset SE, Reichborn-Kjennerud T, Ueland PM. Impact of Pre-Pregnancy BMI on B Vitamin and Inflammatory Status in Early Pregnancy: An Observational Cohort Study. Nutrients. 2016; 8(12):776. https://doi.org/10.3390/nu8120776
Chicago/Turabian StyleBjørke-Monsen, Anne-Lise, Arve Ulvik, Roy M. Nilsen, Øivind Midttun, Christine Roth, Per Magnus, Camilla Stoltenberg, Stein Emil Vollset, Ted Reichborn-Kjennerud, and Per Magne Ueland. 2016. "Impact of Pre-Pregnancy BMI on B Vitamin and Inflammatory Status in Early Pregnancy: An Observational Cohort Study" Nutrients 8, no. 12: 776. https://doi.org/10.3390/nu8120776




