Influence of Bovine Whey Protein Concentrate and Hydrolysate Preparation Methods on Motility in the Isolated Rat Distal Colon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Preparation and Composition of Whey Protein
2.2.1. Whey Protein Concentrate
2.2.2. Whey Protein Hydrolysate
2.3. Modulators
2.4. Distal Colon Motility
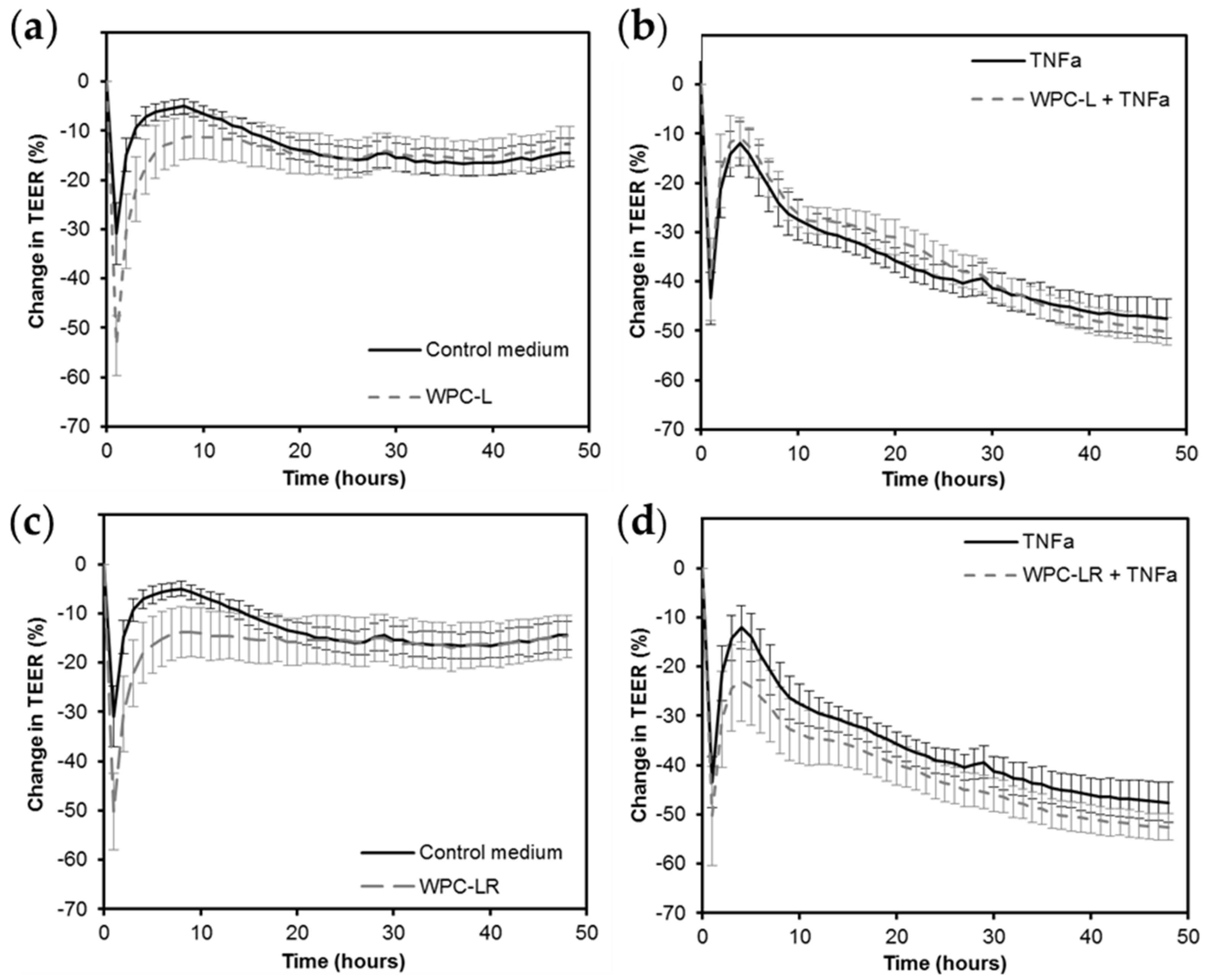
2.5. Intestinal Barrier Integrity
2.6. Cytokine Expression
2.7. Statistical Analysis
2.7.1. Colonic Motility
2.7.2. Intestinal Barrier Integrity
2.7.3. Cytokine Expression
3. Results
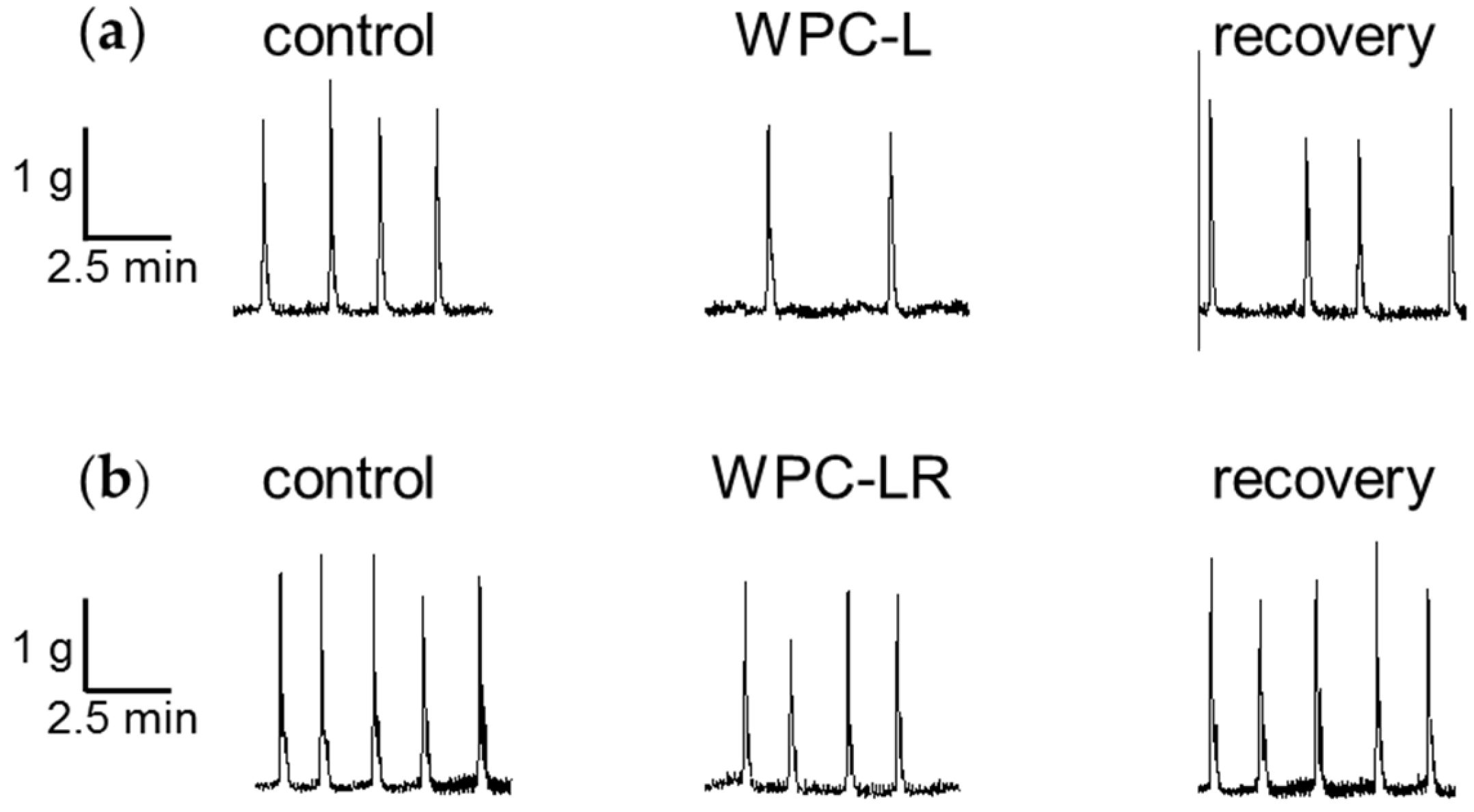
3.1. Whey Protein Concentrate
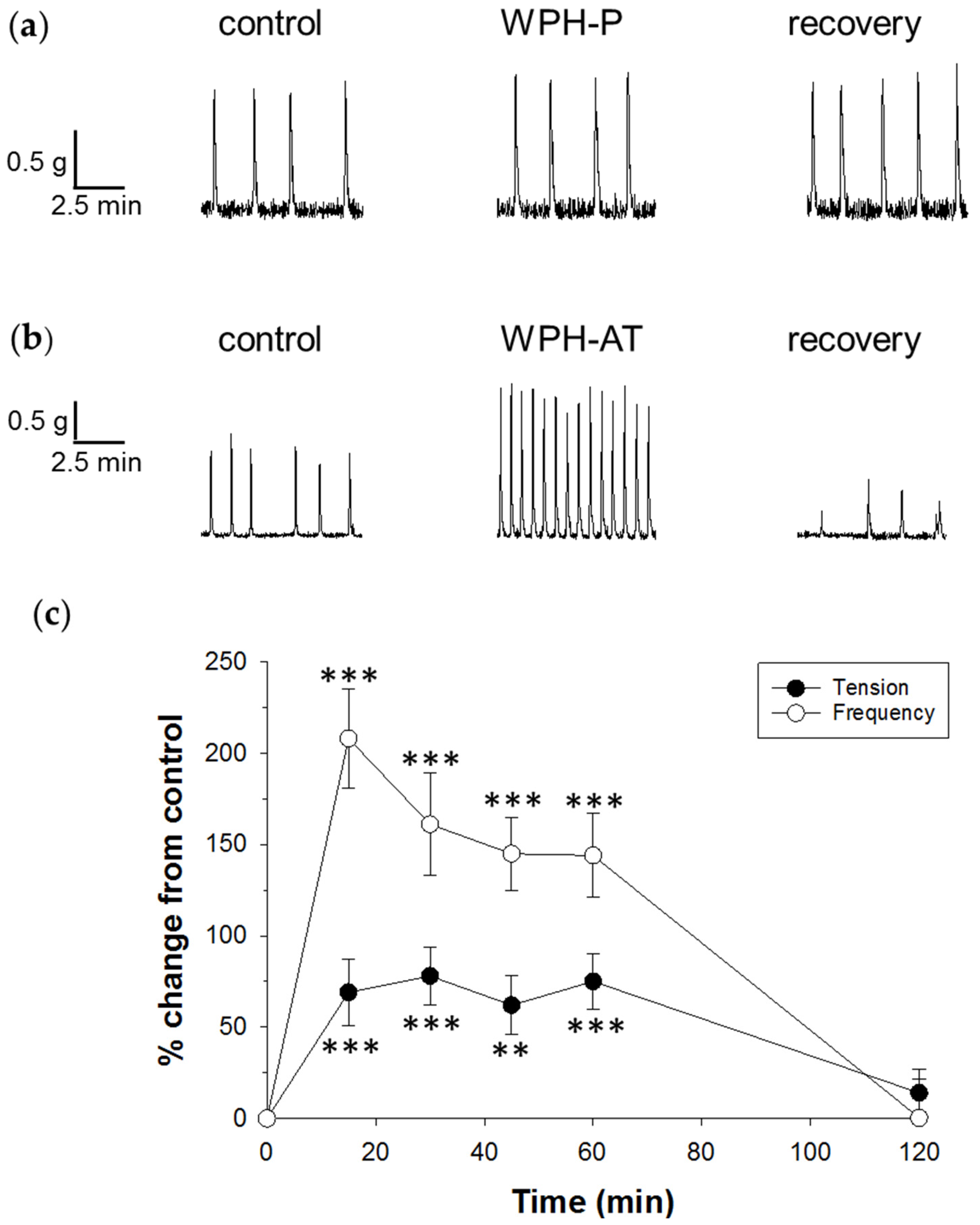
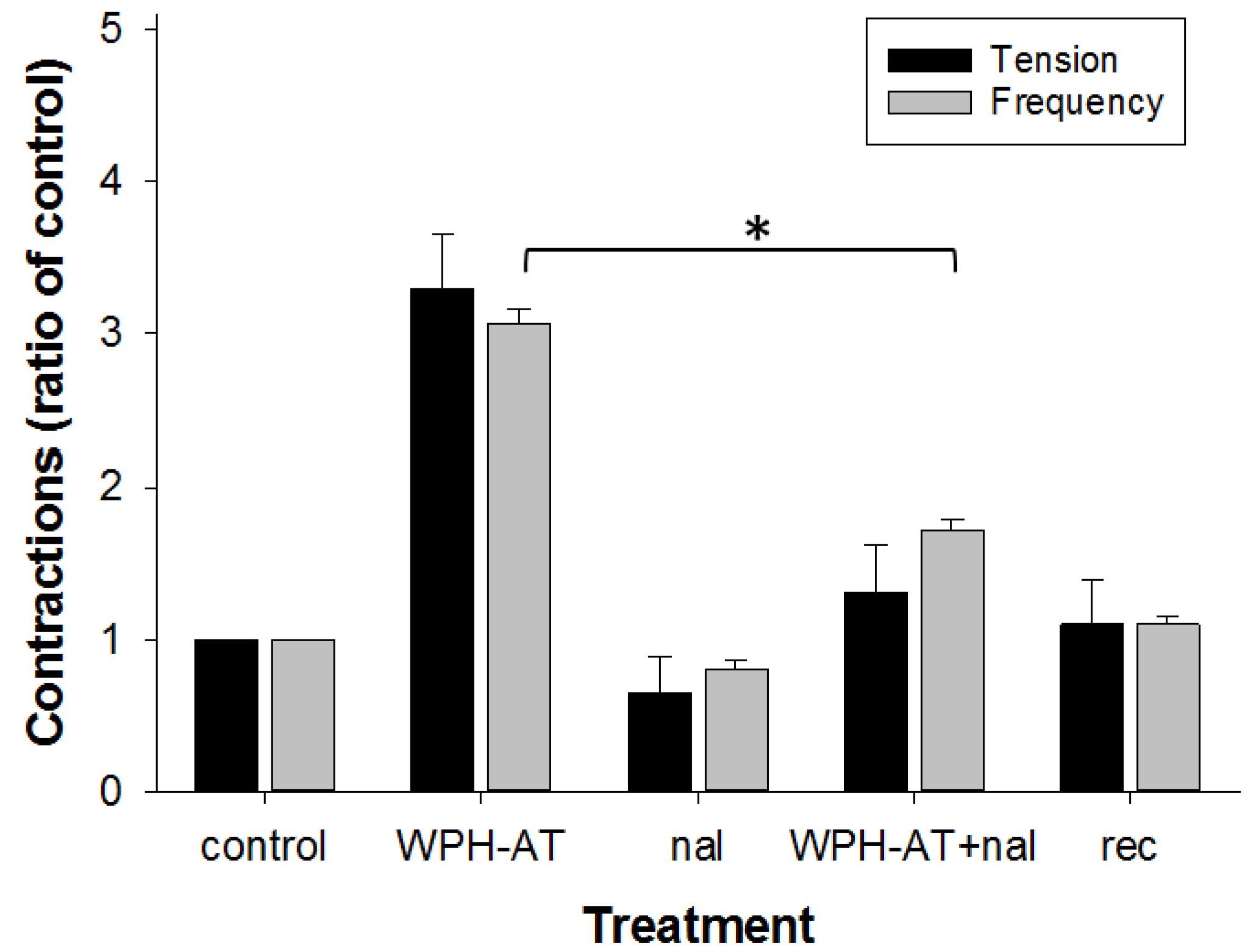
3.2. Whey Protein Hydrolysate
4. Discussion
4.1. Whey Protein Concentrate
4.2. Whey Protein Hydrolysate
4.3. In Vivo Implications
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Patel, S. Functional food relevance of whey protein: A review of recent findings and scopes ahead. J. Funct. Foods 2015, 19, 308–319. [Google Scholar] [CrossRef]
- Abrahão, V. Nourishing the dysfunctional gut and whey protein. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, J.; Dupont, C.; Lemieux, P. Whey proteins and peptides: Beneficial effects on immune health. Therapy 2006, 3, 69–78. [Google Scholar] [CrossRef]
- Krissansen, G.W. Emerging health properties of whey proteins and their clinical implications. J. Am. Coll. Nutr. 2007, 26, 713S–723S. [Google Scholar] [CrossRef] [PubMed]
- Madureira, A.R.; Tavares, T.; Gomes, A.M.P.; Pintado, M.E.; Malcata, F.X. Invited review: Physiological properties of bioactive peptides obtained from whey proteins. J. Dairy Sci. 2010, 93, 437–455. [Google Scholar] [CrossRef] [PubMed]
- Ha, E.; Zemel, M.B. Functional properties of whey, whey components, and essential amino acids: Mechanisms underlying health benefits for active people (review). J. Nutr. Biochem. 2003, 14, 251–258. [Google Scholar] [CrossRef]
- Smithers, G.W. Whey and whey proteins-from “gutter-to-gold”. Int. Dairy J. 2008, 18, 695–704. [Google Scholar] [CrossRef]
- Thorne, R. Whey protein. Altern. Med. Rev. 2008, 13, 341–347. [Google Scholar]
- Vandenplas, Y.; Cruchet, S.; Faure, C.; Lee, H.C.; di Lorenzo, C.; Staiano, A.; Chundi, X.; Aw, M.M.; Gutiérrez-Castrellõn, P.; Asery, A.; et al. When should we use partially hydrolysed formulae for frequent gastrointestinal symptoms and allergy prevention? Acta Paediatr. Int. J. Paediatr. 2014, 103, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Bongers, M.E.J.; de Lorijn, F.; Reitsma, J.B.; Groeneweg, M.; Taminiau, J.A.J.M.; Benninga, M.A. The clinical effect of a new infant formula in term infants with constipation: A double-blind, randomized cross-over trial. Nutr. J. 2007, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Savage, K.; Kritas, S.; Schwarzer, A.; Davidson, G.; Omari, T. Whey-vs casein-based enteral formula and gastrointestinal function in children with cerebral palsy. J. Parenter. Enter. Nutr. 2012, 36, 118S–123S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mihatsch, W.A.; Högel, J.; Pohlandt, F. Hydrolysed protein accelerates the gastrointestinal transport of formula in preterm infants. Acta Paediatr. Int. J. Paediatr. 2001, 90, 196–198. [Google Scholar] [CrossRef]
- Brighton, P.J.; Wise, A.; Dass, N.B.; Willars, G.B. Paradoxical behavior of neuromedin u in isolated smooth muscle cells and intact tissue. J. Pharmacol. Exp. Ther. 2008, 325, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Nijkamp, F.P.; van Ree, J.M. Effects of endorphins on different parts of the gastrointestinal tract of rat and guinea-pig in vitro. Br. J. Pharmacol. 1980, 68, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, H.; Yomota, E.; Nogi, K.; Onoda, Y.; Ikezawa, K. The effect of nociceptin, an endogenous ligand for the orl1 receptor, on rat colonic contraction and transit. Eur. J. Pharmacol. 1998, 353, 265–271. [Google Scholar] [CrossRef]
- Dalziel, J.E.; Dunstan, K.E.; Finch, S.C. Combined effects of fungal alkaloids on intestinal motility in an in vitro rat model. J. Anim. Sci. 2013, 91, 5177–5182. [Google Scholar] [CrossRef] [PubMed]
- Dalziel, J.E.; Mohan, V.; Peters, J.; Anderson, R.C.; Gopal, P.K.; Roy, N.C. The probiotic Escherichia coli nissle 1917 inhibits propagating colonic contractions in the rat isolated large intestine. Food Funct. 2015, 6, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Dalziel, J.E.; Spencer, N.J.; Dunstan, K.E.; Lynch, A.T.; Haggarty, N.W.; Gopal, P.K.; Roy, N.C. An in vitro rat model of colonic motility to determine the effect of β-casomorphin-5 on propagating contractions. Food Funct. 2014, 5, 2768–2774. [Google Scholar] [CrossRef] [PubMed]
- Frister, H.; Meisel, H.; Schlimme, E. Opa method modified by use of n,n-dimethyl-2-mercaptoethylammonium chloride as thiol component. Z. Anal. Chem. 1988, 330, 631–633. [Google Scholar] [CrossRef]
- Le Ferrec, E.; Chesne, C.; Artusson, P.; Brayden, D.; Fabre, G.; Gires, P.; Guillou, F.; Rousset, M.; Rubas, W.; Scarino, M.L. In vitro models of the intestinal barrier: The report and recommendations of ecvam workshop 46 1,2. ATLA Altern. Lab. Anim. 2001, 29, 649–668. [Google Scholar] [PubMed]
- Shah, P.; Jogani, V.; Bagchi, T.; Misra, A. Role of caco-2 cell monolayers in prediction of intestinal drug absorption. Biotechnol. Prog. 2006, 22, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.A.; Bassett, S.; Haggarty, N.; Gopal, P.; Armstrong, K.; Roy, N.C. Early-lactation, but not mid-lactation, bovine lactoferrin preparations increases epithelial barrier integrity of caco-2 cell layers possibly due to co-elution of angiogenin. J. Dairy Sci. 2016, in press. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.N.; Sangild, P.T.; Li, Y.; Bering, S.B.; Chatterton, D.E.W. Processing of whey modulates proliferative and immune functions in intestinal epithelial cells. J. Dairy Sci. 2016, 99, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Pihlanto-Leppälä, A. Bioactive peptides derived from bovine whey proteins: Opioid and ace-inhibitory peptides. Trends Food Sci. Technol. 2000, 11, 347–356. [Google Scholar] [CrossRef]
- Dallas, D.C.; Underwood, M.A.; Zivkovic, A.M.; German, J.B. Digestion of protein in premature and term infants. J. Nutr. Disord. Ther. 2012, 2, 112. [Google Scholar] [CrossRef] [PubMed]

| Nutrients (w/w%) | WPC-L | WPC-LR | WPC-MA | WPH-P 1 | WPH-AT 1 |
|---|---|---|---|---|---|
| Total protein | 80 | 80 | 80 | 80 | 77 |
| % protein | |||||
| α-lactoglubulin | 16 | 13 | 16 | ND | ND |
| β-lactoglubulin | 58 | 43 | 52 | ND | ND |
| BSA | 2 | 2 | 2 | ND | ND |
| IgG | 4 | 4 | 6 | ND | ND |
| GMP | 0 | 21 | 0 | ND | ND |
| Other | 20 | 17 | 24 | ND | ND |
| Lactose | 4–6 | 4–6 | 4–6 | 1 | 4 |
| Fat | 4 | 5 | 5 | 4 | 6 |
| Ash | 3–9 | 3–9 | 3–9 | 4 | 9 |
| Calcium (mg/100 g) | 240 | 400 | 230 | 43 | 1 |
| Moisture | 3 | 4 | 4 | 4 | ND |
| Mass Range (Daltons) | WPH-P % Protein | WPH-AT % Protein |
|---|---|---|
| >20,000 | 44 | 13 |
| 5000–20,000 | 17 | 20 |
| 1500–5000 | 16 | 23 |
| 1000–1500 | 6 | 8 |
| 500–1000 | 8 | 12 |
| <500 | 9 | 23 |
| Whey | Tension | Frequency | n |
|---|---|---|---|
| WPC-L | ↑ 5 ± 6 | ↓ 48 ± 12 *** | 13 |
| WPC-LR | ↑ 8 ± 5 | ↓ 17 ± 11 * | 15 |
| WPC-MA | ↓ 29 ± 7 *** | ↓ 36 ± 7 *** | 12 |
| WPH-P | ↑ 19 ± 8 | ↑ 24 ± 12 | 12 |
| WPH-AT | ↑ 65 ± 15 *** | ↑ 131 ± 28 *** | 12 |
| AT-Active | ↓ 11 ± 9 | ↓ 4 ± 12 | 10 |
| AT-Inactive | ↑ 6 ± 13 | ↓ 21 ± 11 | 9 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalziel, J.E.; Anderson, R.C.; Bassett, S.A.; Lloyd-West, C.M.; Haggarty, N.W.; Roy, N.C. Influence of Bovine Whey Protein Concentrate and Hydrolysate Preparation Methods on Motility in the Isolated Rat Distal Colon. Nutrients 2016, 8, 809. https://doi.org/10.3390/nu8120809
Dalziel JE, Anderson RC, Bassett SA, Lloyd-West CM, Haggarty NW, Roy NC. Influence of Bovine Whey Protein Concentrate and Hydrolysate Preparation Methods on Motility in the Isolated Rat Distal Colon. Nutrients. 2016; 8(12):809. https://doi.org/10.3390/nu8120809
Chicago/Turabian StyleDalziel, Julie E., Rachel C. Anderson, Shalome A. Bassett, Catherine M. Lloyd-West, Neill W. Haggarty, and Nicole C. Roy. 2016. "Influence of Bovine Whey Protein Concentrate and Hydrolysate Preparation Methods on Motility in the Isolated Rat Distal Colon" Nutrients 8, no. 12: 809. https://doi.org/10.3390/nu8120809




