Preventive Effects of Fermented Brown Rice and Rice Bran against Prostate Carcinogenesis in TRAP Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Assessment of Prostate Neoplastic Lesion Development
2.3. Immunohistochemistry
2.4. Western Blot Analysis
2.5. Statistical Analysis
3. Results
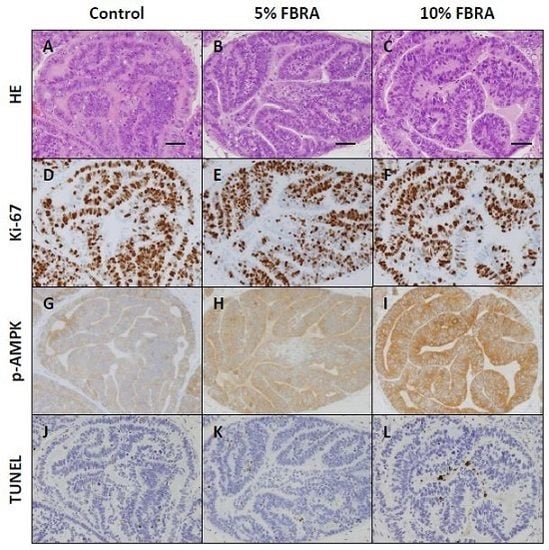
3.1. FBRA Suppressed Progression of Prostate Carcinogenesis without Toxicity
3.2. Effects of FBRA on Cell Proliferation and Apoptosis
3.3. Effect of FBRA on Activation of AMPK
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| VP | ventral prostate |
| LP | lateral prostate |
| LG-PIN | low grade intraepithelial neoplasia |
| HG-PIN | high grade intraepithelial neoplasia |
| AMPK | AMP-activated protein kinase |
| FASN | fatty acid synthase |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, R.A.; Chambers, S.K.; Williams, S.G.; Yaxley, J.; Samaratunga, H.; Frydenberg, M. Prostate cancer—Part one: Detection. In Endotext; De Groot, L.J., Beck-Peccoz, P., Chrousos, G., Dungan, K., Grossman, A., Hershman, J.M., Koch, C., McLachlan, R., New, M., Rebar, R., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Rebbeck, T.R.; Haas, G.P. Temporal trends and racial disparities in global prostate cancer prevalence. Can. J. Urol. 2014, 21, 7496–7506. [Google Scholar] [PubMed]
- Lin, P.H.; Aronson, W.; Freedland, S.J. Nutrition, dietary interventions and prostate cancer: The latest evidence. BMC Med. 2015, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Goufo, P.; Trindade, H. Rice antioxidants: Phenolic acids, flavonoids, anthocyanins, proanthocyanidins, tocopherols, tocotrienols, gamma-oryzanol, and phytic acid. Food Sci. Nutr. 2014, 2, 75–104. [Google Scholar] [CrossRef] [PubMed]
- Henderson, A.J.; Ollila, C.A.; Kumar, A.; Borresen, E.C.; Raina, K.; Agarwal, R.; Ryan, E.P. Chemopreventive properties of dietary rice bran: Current status and future prospects. Adv. Nutr. 2012, 3, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Somintara, S.; Leardkamolkarn, V.; Suttiarporn, P.; Mahatheeranont, S. Anti-tumor and immune enhancing activities of rice bran gramisterol on acute myelogenous leukemia. PLoS ONE 2016, 11, e0146869. [Google Scholar] [CrossRef] [PubMed]
- Kuno, T.; Hirose, Y.; Hata, K.; Kato, K.; Qiang, S.H.; Kitaori, N.; Hara, A.; Iwasaki, T.; Yoshimura, T.; Wada, K.; et al. Preventive effect of fermented brown rice and rice bran on N-nitrosomethylbenzylamine-induced esophageal tumorigenesis in rats. Int. J. Oncol. 2004, 25, 1809–1815. [Google Scholar] [CrossRef] [PubMed]
- Tomita, H.; Kuno, T.; Yamada, Y.; Oyama, T.; Asano, N.; Miyazaki, Y.; Baba, S.; Taguchi, A.; Hara, A.; Iwasaki, T.; et al. Preventive effect of fermented brown rice and rice bran on N-methyl-N′-nitro-N-nitrosoguanidine-induced gastric carcinogenesis in rats. Oncol. Rep. 2008, 19, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Katyama, M.; Yoshimi, N.; Yamada, Y.; Sakata, K.; Kuno, T.; Yoshida, K.; Qiao, Z.; Vihn, P.Q.; Iwasaki, T.; Kobayashi, H.; et al. Preventive effect of fermented brown rice and rice bran against colon carcinogenesis in male F344 rats. Oncol. Rep. 2002, 9, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Katayama, M.; Sugie, S.; Yoshimi, N.; Yamada, Y.; Sakata, K.; Qiao, Z.; Iwasaki, T.; Kobayashi, H.; Mori, H. Preventive effect of fermented brown rice and rice bran on diethylnitrosoamine and phenobarbital-induced hepatocarcinogenesis in male F344 rats. Oncol. Rep. 2003, 10, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Phutthaphadoong, S.; Yamada, Y.; Hirata, A.; Tomita, H.; Taguchi, A.; Hara, A.; Limtrakul, P.N.; Iwasaki, T.; Kobayashi, H.; Mori, H. Chemopreventive effects of fermented brown rice and rice bran against 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis in female A/J mice. Oncol. Rep. 2009, 21, 321–327. [Google Scholar] [PubMed]
- Kuno, T.; Takahashi, S.; Tomita, H.; Hisamatsu, K.; Hara, A.; Hirata, A.; Kobayashi, H.; Mori, H. Preventive effects of fermented brown rice and rice bran against N-nitrosobis (2-oxopropyl) amine-induced pancreatic tumorigenesis in male hamsters. Oncol. Lett. 2015, 10, 3377–3384. [Google Scholar] [CrossRef] [PubMed]
- Kuno, T.; Hirose, Y.; Yamada, Y.; Hata, K.; Qiang, S.H.; Asano, N.; Oyama, T.; Zhi, H.; Iwasaki, T.; Kobayashi, H.; Mori, H. Chemoprevention of mouse urinary bladder carcinogenesis by fermented brown rice and rice bran. Oncol. Rep. 2006, 15, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Asamoto, M.; Hokaiwado, N.; Cho, Y.M.; Takahashi, S.; Ikeda, Y.; Imaida, K.; Shirai, T. Prostate carcinomas developing in transgenic rats with SV40 T antigen expression under probasin promoter control are strictly androgen dependent. Cancer Res. 2001, 61, 4693–4700. [Google Scholar] [PubMed]
- Cho, Y.M.; Takahashi, S.; Asamoto, M.; Suzuki, S.; Inaguma, S.; Hokaiwado, N.; Shirai, T. Age-dependent histopathological findings in the prostate of probasin/SV40 T antigen transgenic rats: Lack of influence of carcinogen or testosterone treatment. Cancer Sci. 2003, 94, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Naiki-Ito, A.; Chewonarin, T.; Tang, M.; Pitchakarn, P.; Kuno, T.; Ogawa, K.; Asamoto, M.; Shirai, T.; Takahashi, S. Ellagic acid, a component of pomegranate fruit juice, suppresses androgen-dependent prostate carcinogenesis via induction of apoptosis. Prostate 2015, 75, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Shiraga, K.; Sato, S.; Punfa, W.; Naiki-Ito, A.; Yamashita, Y.; Shirai, T.; Takahashi, S. Apocynin, an NADPH oxidase inhibitor, suppresses rat prostate carcinogenesis. Cancer Sci. 2013, 104, 1711–1717. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Ogawa, K.; Asamoto, M.; Hokaiwado, N.; Seeni, A.; Suzuki, S.; Takahashi, S.; Tanaka, T.; Ichikawa, K.; Shirai, T. Protective effects of citrus nobiletin and auraptene in transgenic rats developing adenocarcinoma of the prostate (TRAP) and human prostate carcinoma cells. Cancer Sci. 2007, 98, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Seeni, A.; Takahashi, S.; Takeshita, K.; Tang, M.; Sugiura, S.; Sato, S.Y.; Shirai, T. Suppression of prostate cancer growth by resveratrol in the transgenic rat for adenocarcinoma of prostate (TRAP) model. Asian Pac. J. Cancer Prev. 2008, 9, 7–14. [Google Scholar] [PubMed]
- Raina, K.; Ravichandran, K.; Rajamanickam, S.; Huber, K.M.; Serkova, N.J.; Agarwal, R. Inositol hexaphosphate inhibits tumor growth, vascularity, and metabolism in TRAMP mice: A multiparametric magnetic resonance study. Cancer Prev. Res. 2013, 6, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Itoh, M.; Nishibori, N.; Sagara, T.; Horie, Y.; Motojima, A.; Morita, K. Extract of fermented brown rice induces apoptosis of human colorectal tumor cells by activating mitochondrial pathway. Phytother. Res. 2012, 26, 1661–1666. [Google Scholar] [CrossRef] [PubMed]
- Horie, Y.; Nemoto, H.; Itoh, M.; Kosaka, H.; Morita, K. Fermented brown rice extract causes apoptotic death of human acute lymphoblastic leukemia cells via death receptor pathway. Appl. Biochem. Biotechnol. 2016, 178, 1599–1611. [Google Scholar] [CrossRef] [PubMed]
- Margel, D.; Urbach, D.R.; Lipscombe, L.L.; Bell, C.M.; Kulkarni, G.; Austin, P.C.; Fleshner, N. Metformin use and all-cause and prostate cancer-specific mortality among men with diabetes. J. Clin. Oncol. 2013, 31, 3069–3075. [Google Scholar] [CrossRef] [PubMed]
- Zadra, G.; Photopoulos, C.; Tyekucheva, S.; Heidari, P.; Weng, Q.P.; Fedele, G.; Liu, H.; Scaglia, N.; Priolo, C.; Sicinska, E.; et al. A novel direct activator of AMPK inhibits prostate cancer growth by blocking lipogenesis. EMBO Mol. Med. 2014, 6, 519–538. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.X.; Yao, X.J.; Xu, S.W.; Wong, V.K.; He, J.X.; Ding, J.; Xue, W.W.; Mujtaba, T.; Michelangeli, F.; Huang, M.; et al. (Z)3,4,5,4′-trans-tetramethoxystilbene, a new analogue of resveratrol, inhibits gefitinb-resistant non-small cell lung cancer via selectively elevating intracellular calcium level. Sci. Rep. 2015, 5, 16348. [Google Scholar] [CrossRef] [PubMed]
- Ryan, E.P.; Heuberger, A.L.; Weir, T.L.; Barnett, B.; Broeckling, C.D.; Prenni, J.E. Rice bran fermented with saccharomyces boulardii generates novel metabolite profiles with bioactivity. J. Agric. Food Chem. 2011, 59, 1862–1870. [Google Scholar] [CrossRef] [PubMed]
- Onuma, K.; Kanda, Y.; Ikeda, S.S.; Sakaki, R.; Nonomura, T.; Kobayashi, M.; Osaki, M.; Shikanai, M.; Kobayashi, H.; Okada, F. Fermented brown rice and rice bran with aspergillus oryzae (FBRA) prevents inflammation-related carcinogenesis in mice, through inhibition of inflammatory cell infiltration. Nutrients 2015, 7, 10237–10250. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Takeshita, K.; Seeni, A.; Sugiura, S.; Tang, M.; Sato, S.Y.; Kuriyama, H.; Nakadate, M.; Abe, K.; Maeno, Y.; et al. Suppression of prostate cancer in a transgenic rat model via gamma-tocopherol activation of caspase signaling. Prostate 2009, 69, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Eitsuka, T.; Tatewaki, N.; Nishida, H.; Kurata, T.; Nakagawa, K.; Miyazawa, T. Synergistic inhibition of cancer cell proliferation with a combination of delta-tocotrienol and ferulic acid. Biochem. Biophys. Res. Commun. 2014, 453, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Chiang, E.P.; Tsai, S.Y.; Kuo, Y.H.; Pai, M.H.; Chiu, H.L.; Rodriguez, R.L.; Tang, F.Y. Caffeic acid derivatives inhibit the growth of colon cancer: Involvement of the PI3-K/Akt and AMPK signaling pathways. PLoS ONE 2014, 9, e99631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Lau, E.Y.; Chen, J.; Yong, J.; Tang, K.D.; Lo, J.; Ng, I.O.; Lee, T.K.; Ling, M.T. Polysaccharopeptide enhanced the anti-cancer effect of gamma-tocotrienol through activation of AMPK. BMC Complement. Altern. Med. 2014, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, S.; Suzuki, S.; Naiki-Ito, A.; Komiya, M.; Ne, L.; Kato, H.; Sagawa, H.; Yamashita, Y.; Shirai, T.; Takahashi, S. Establishment of an invasive prostate cancer model in transgenic rats by intermittent testosterone administration. J. Toxicol. Pathol. 2014, 27, 43–49. [Google Scholar] [CrossRef] [PubMed]








| Treatment (No. of Rats) | Body Weight (g) | Liver Weight (g) | Kidney Weight (g) | Ventral Prostate Weight (g) |
|---|---|---|---|---|
| Control (12) | 679.3 ± 47.6 | 20.2 ± 2.1 | 3.3 ± 0.3 | 0.45 ± 0.40 |
| 5% FBRA (12) | 699.1 ± 45.6 | 21.8 ± 1.6 | 3.6 ± 0.3 * | 0.34 ± 0.06 |
| 10% FBRA (12) | 703.4 ± 63.3 | 21.2 ± 1.7 | 3.5 ± 0.3 | 0.38 ± 0.09 |
| Treatment (No. of Rats) | Ventral Prostate | Lateral Prostate | ||||||
|---|---|---|---|---|---|---|---|---|
| Incidence of Carcinoma (%) | % of Lesions in Prostate | Incidence of Carcinoma (%) | % of Lesions in Prostate | |||||
| LG-PIN | HG-PIN | Adenoca. | LG-PIN | HG-PIN | Adenoca. | |||
| Control (12) | 12 (100) | 8.6 ± 3.5 | 75.7 ± 8.1 | 15.7 ± 9.1 | 12 (100) | 13.6 ± 9.0 | 77.0 ± 9.4 | 9.4 ± 5.0 |
| 5% FBRA (12) | 12 (100) | 8.6 ± 3.2 | 79.1 ± 3.2 | 12.3 ± 4.1 | 12 (100) | 12.2 ± 4.7 | 80.7 ± 4.6 | 7.1 ± 3.1 |
| 10% FBRA (12) | 12 (100) | 12.9 ± 3.9 ** | 75.0 ± 7.6 | 12.1 ± 7.1 | 9 (75) | 21.4 ± 14.8 | 74.2 ± 14.1 | 4.4 ± 5.3 * |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuno, T.; Nagano, A.; Mori, Y.; Kato, H.; Nagayasu, Y.; Naiki-Ito, A.; Suzuki, S.; Mori, H.; Takahashi, S. Preventive Effects of Fermented Brown Rice and Rice Bran against Prostate Carcinogenesis in TRAP Rats. Nutrients 2016, 8, 421. https://doi.org/10.3390/nu8070421
Kuno T, Nagano A, Mori Y, Kato H, Nagayasu Y, Naiki-Ito A, Suzuki S, Mori H, Takahashi S. Preventive Effects of Fermented Brown Rice and Rice Bran against Prostate Carcinogenesis in TRAP Rats. Nutrients. 2016; 8(7):421. https://doi.org/10.3390/nu8070421
Chicago/Turabian StyleKuno, Toshiya, Aya Nagano, Yukiko Mori, Hiroyuki Kato, Yuko Nagayasu, Aya Naiki-Ito, Shugo Suzuki, Hideki Mori, and Satoru Takahashi. 2016. "Preventive Effects of Fermented Brown Rice and Rice Bran against Prostate Carcinogenesis in TRAP Rats" Nutrients 8, no. 7: 421. https://doi.org/10.3390/nu8070421