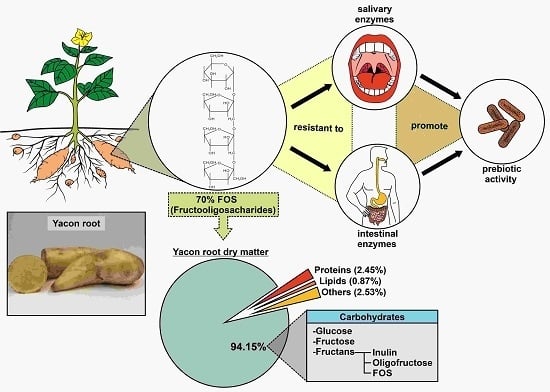
Yacon (Smallanthus sonchifolius) as a Food Supplement: Health-Promoting Benefits of Fructooligosaccharides
Abstract
:1. Introduction
2. Fructooligosacharides: Bioactivity and Potential Health Benefits
3. FOS Effects on Colorectal Cancer
4. FOS Effects on Diabetes
5. FOS Effects on Obesity
6. Yacon Consumption Adverse Effects
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BW | body weight |
| CRC | colorectal cancer |
| CCK | cholecystokinin |
| DMH | 1,2-dimethylhydrazine |
| GLP-1 | glucagon-like peptide 1 |
| SCFA | short chain fatty acids |
| FFA | free fatty acids |
| FFAR | free fatty acid receptor |
| FOS | fructooligosacharides |
| HDL | high density lipoprotein |
| LDL | low density lipoprotein |
| PAMPs | pathogen-associated molecular patterns |
| PYY | peptide YY |
| TAG | tryacylglycerol |
| TLR | toll-like receptors |
| VLDL | very low density lipoprotein |
References
- Lachman, J.; Fernández, E.C.; Orsák, M. Yacon (Smallanthus sonchifolia (Poepp. et Endl.) H. Robinson) chemical composition and use-a review. Plant Soil Environ. 2003, 49, 283–290. [Google Scholar]
- Zardini, E. Ethnobotanical notes of yacon, Polymnia sonchifolia (Asteraceae). Econ Bot. 1991, 45, 72–85. [Google Scholar] [CrossRef]
- Grau, A.; Rea, J. Yacon. Smallanthus sonchifolius (Poepp. & Endl.) H. Robinson. In Andean Roots and Tuberous Roots: Ahipa, Arracacha, Maca and Yacon; Gatersleben/IPGRI: Rome, Italy, 1997; pp. 199–256. [Google Scholar]
- Ojansivua, I.; Ferreira, C.L.; Salminena, S. Yacon, a new source of prebiotic oligosaccharides with a history of safe use. Trends Food Sci. Tech. 2011, 22, 40–46. [Google Scholar] [CrossRef]
- Campos, D.; Betalleluz-Pallardel, I.; Chirinos, R.; Aguilar-Galvez, A.; Noratto, G. Prebiotic effects of yacon (Smallanthus sonchifolius Poepp. & Endl), a source of fructooligosaccharides and phenolic compounds with antioxidant activity. Food Chem. 2012, 135, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.; Céspedes, G.; Carballo, S.; Bergenståhl, B.; Tornberg, E. Dietary fiber, fructooligosaccharides, and physicochemical properties of homogenized aqueous suspensions of yacon (Smallanthus sonchifolius). Food Res. Int. 2013, 50, 392–400. [Google Scholar] [CrossRef]
- Jiménez, M.E.; Sammán, N. Chemical characterization and quantification of fructooligosaccharides, phenolic compounds and antiradical activity of Andean roots and tubers grown in Northwest of Argentina. Arch. Latinoam Nutr. 2014, 64, 131–138. [Google Scholar] [PubMed]
- Pereira, J.A.R.; Barcelos, M.F.P.; Pereira, M.C.A.; Ferreira, E.B. Studies of chemical and enzymatic characteristics of Yacon (Smallanthus sonchifolius) and its flour. Food Sci. Technol. 2013, 33. [Google Scholar] [CrossRef]
- Delgado, G.T.; Tamashiro, W.M.; Maróstica-Junior, M.R.; Pastore, G.M. Yacon (Smallanthus sonchifolius): A functional food. Plant Foods Hum. Nutr. 2013, 68, 222–228. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, P.H.A.; Abranches, M.V.; de Luces Fortes Ferreira, C.L. Yacon (Smallanthus sonchifolius): A food with multiple functions. Crit. Rev. Food Sci. Nutr. 2015, 55, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Apolinário, A.C.; de Lima Damasceno, B.P.; de Macêdo Beltrão, N.E; Pessoa, A.; Converti, A.; da Silva, J.A. Inulin-type fructans: A review on different aspects of biochemical and pharmaceutical technology. Carbohydr Polym. 2014, 30, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, M.; Furuyama, K.; Ishihiro, Y.; Onodera, S.; Fukushi, E.; Benkeblia, N.; Shiomi, N. Isolation and Structural Analysis In Vivo of Newly Synthesized Fructooligosaccharides in Onion Bulbs Tissues (Allium cepa L.) during storage. Int. J. Carbohydr. 2009. [Google Scholar] [CrossRef]
- Santana, I.; Cardoso, M.H. Yacon tuberous root (Smallanthus sonchifolius): Cultivation potentialities, technological and nutritional aspects. Ciência Rural 2008, 38, 898–905. [Google Scholar] [CrossRef]
- Sabater-Molina, M.; Larqué, E.; Torrella, F.; Zamora, S. Dietary fructooligosaccharides and potential benefits on health. J. Physiol. Biochem. 2009, 65, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Sivieri, K.; Morales, M.V.; Saad, S.M.I.; Adorno, M.A.; Sakamoto, I.K.; Rossi, E.A. Prebiotic effect of fructooligosaccharide in the Simulator of the Human Intestinal Microbial Ecosystem (SHIME Model). J. Med. Food. 2014, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bibas Bonet, M.E.; Meson, O.; de Moreno de LeBlanc, A.; Dogib, C.A.; Chaves, S.; Kortsarz, A.; Grau, A.; Perdigón, G. Prebiotic effect of yacon (Smallanthus sonchifolius) on intestinal mucosa using a mouse model. In Food Agric. Immunol.; 2010. [Google Scholar] [CrossRef]
- Gibson, G.R. Dietary modulation of the human gut microflora using prebiotics. Br. J. Nutr. 1998, 4, 209–212. [Google Scholar]
- Whelan, K. Mechanisms and effectiveness of prebiotics in modifying the gastrointestinal microbiota for the management of digestive disorders. Proc. Nutr. Soc. 2013, 72, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; O’Riordan, M.X.D. Regulation of Bacterial Pathogenesis by Intestinal Short-Chain Fatty Acids. Adv. Appl. Microbiol. 2013, 85, 93–118. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [PubMed]
- Roberfroid, M. Prebiotics: The concept revisited. J. Nutr. 2007, 137, 830S–837S. [Google Scholar] [PubMed]
- Pineiro, M.; Asp, N.G.; Reid, G.; Macfarlane, S.; Morelli, L.; Brunser, O.; Tuohy, K. FAO Technical meeting on prebiotics. J. Clin. Gastroenterol. 2008. [Google Scholar] [CrossRef] [PubMed]
- Valentová, K.; Ulrichová, J. Smallanthus sonchifolius and Lepidium meyenii—Prospective andean crops for the prevention of chronic diseases. Biomed. Papers 2003, 147, 119–130. [Google Scholar] [CrossRef]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 22, 1417–1435. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66. [Google Scholar] [CrossRef] [PubMed]
- Jasperson, K.W.; Tuohy, T.M.; Neklason, D.W.; Burt, R.W. Hereditary and familial colon cancer. Gastroenterology 2010, 138, 2044–2058. [Google Scholar] [CrossRef] [PubMed]
- Baena, R.; Show, S. Diet and colorectal cancer. Maturitas 2015, 80, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Steck, S.E.; Guinter, M.; Zheng, J.; Thomson, C.A. Index-based dietary patterns and colorectal cancer risk: A systematic review. Adv. Nutr. 2015, 6, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Peeters, P.J.; Bazelier, M.T.; Leufkens, H.G.; de Vries, F.; De Bruin, M.L. The risk of colorectal cancer in patients with type 2 diabetes: Associations with treatment stage and obesity. Diabetes Care 2015, 38, 495–502. [Google Scholar] [CrossRef] [PubMed]
- De Moura, N.A.; Caetano, B.F.R.; Sivieri, K.; Urbano, L.H.; Cabello, C.; Rodrigues, M.A.; Barbisan, L.F. Protective effects of yacon (Smallanthus sonchifolius) intake on experimental colon carcinogenesis. Food Chem. Toxicol. 2012, 50, 2902–2910. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.P.S.; Avia, C.M.; Barbisan, L.F.; de Moura, N.A.; Caetano, B.F.R.; Romualdo, G.R.; Sivieri, K. Yacon (Smallanthus sonchifolius) and Lactobacillus acidophilus CRL 1014 reduce the early phases of colon carcinogenesis in male Wistar rats. Food Res. Int. 2015, 74, 48–54. [Google Scholar] [CrossRef]
- Vipperla, K.; O’Keefe, S. Diet, microbiota, and dysbiosis: A “recipe” for colorectal cancer. Food Funct. 2016, 20, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Hold, G.L.; Flint, H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2009, 12, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Mondot, S.; Lepage, P. The human gut microbiome and its dysfunctions through the meta-omics prism. Ann NY Acad. Sci. 2016. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.; DeCoffe, D.; Molcan, E.; Gibson, D.L. Diet-Induced Dysbiosis of the Intestinal Microbiota and the Effects on Immunity and Disease. Nutrients 2012, 4, 1095–1119. [Google Scholar] [CrossRef] [PubMed]
- Rolim, P.M. Development of prebiotic food products and health benefits. Food Sci. Technol. 2015, 35. [Google Scholar] [CrossRef]
- Respondek, F.; Gerard, P.; Bossis, M.; Boschat, L.; Bruneau, A.; Rabot, S.; Wagner, A.; Martin, J.C. Short-chain fructo-oligosaccharides modulate intestinal microbiota and metabolic parameters of humanized gnotobiotic diet induced obesity mice. PLoS ONE 2013, 8, e71026. [Google Scholar] [CrossRef] [PubMed]
- Raman, M.; Ambalam, P.; Kondepudi, K.K; Pithva, S.; Kothari, C.; Patel, A.T.; Purama, R.K.; Dave, J.M.; Vyas, B.R. Potential of probiotics, prebiotics and synbiotics for management of colorectal cancer. Gut. Microbes 2013, 4, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.; de Souza, R.; Kendall, C.W.; Emam, A.; Jenkins, D. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Chen, Y.; Jiang, H.; Nie, D. The role of short-chain fatty acids in orchestrating two types of programmed cell death in colon cancer. Autophagy 2011, 7, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.C.; Park, J.; Kim, M. Gut Microbiota-Derived Short-Chain Fatty Acids, T Cells, and Inflammation. Immune Netw. 2014, 14, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Vaz-Tostes, M.; Viana, M.L.; Grancieri, M.; Luz, T.C.; Paula, H.; Pedrosa, R.G.; Costa, N.M. Yacon effects in immune response and nutritional status of iron and zinc in preschool children. Nutrition 2014, 30, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Peshev, D.; Van den Ende, W. Fructans: Prebiotics and immunomodulators. J. Funct. Foods. 2014, 8, 348–357. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, J.; Zhou, H. TLRs as pharmacological targets for plant-derived compounds in infectious and inflammatory diseases. Int. Immunopharmacol. 2011, 10, 1451–1456. [Google Scholar] [CrossRef] [PubMed]
- Vogt, L.; Ramasamy, U.; Meyer, D.; Pullens, G.; Venema, K.; Faas, M.M.; Schols, H.A.; de Vos, P. Immune modulation by different types of β2→1-fructans is toll-like receptor dependent. PLoS ONE 2013, 5, e68367. [Google Scholar] [CrossRef] [PubMed]
- Delgado, G.T.; Thomé, R.; Gabriel, D.L.; Tamashiro, W.M.; Pastore, G.M. Yacon (Smallanthus sonchifolius)-derived fructooligosaccharides improves the immune parameters in the mouse. Nutr. Res. 2012, 32, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Velez, E.; Castillo, N.; Mesón, O.; Grau, A.; Bonet, M.E.B.; Perdigón, G. Study of the effect exerted by fructo-oligosaccharides from yacon (Smallanthus sonchifolius) root flour in an intestinal infection model with Salmonella Typhimurium. Br. J. Nutr. 2013, 109, 1971–1979. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Nosaka, S.; Suzuki, M.; Nagafuchi, S.; Takahashi, T.; Yajima, T.; Takenouchi-Ohkubo, N.; Iwase, T.; Moro, I. Dietary fructooligosaccharides up-regulate immunoglobulin A res-ponse and polymeric immunoglobulin receptor expression in intestines of infant mice. Clin. Exp. Immunol. 2004, 137, 52–58. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO)—Diabetes Fact Sheet. Available online: http://www.who.int/mediacentre/factsheets/fs312/en/ (accessed on 9 May 2016).
- Qin, L.; Mirjam, J.; Knol, S.; Corpeleijn, E.; Ronald, P. Does physical activity modify the risk of obesity for type 2 diabetes: A review of epidemiological data. Eur. J. Epidemiol. 2010, 25, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Tunaiji, H.A.; Davis, J.C.; Mackey, D.C.; Khan, K.M. Population attributable fraction of type 2 diabetes due to physical inactivity in adults: A systematic review. BMC Public Health 2014, 14, 469. [Google Scholar] [CrossRef] [PubMed]
- Franz, M.J.; Boucher, J.L.; Evert, A.B. Evidence-based diabetes nutrition therapy recommendations are effective: The key is individualization. Diabetes Metab. Syndr. Obes. 2014, 7, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Russo, D.; Valentão, P.; Andrade, P.B.; Fernandez, E.C.; Milellal, L. Evaluation of Antioxidant, Antidiabetic and Anticholinesterase Activities of Smallanthus sonchifolius Landraces and Correlation with Their Phytochemical Profiles. Int. J. Mol. Sci. 2015, 16, 17696–17718. [Google Scholar] [CrossRef] [PubMed]
- Habib, N.C.; Honoré, S.M.; Genta, S.B.; Sánchez, S.S. Hypolipidemic effect of Smallanthus sonchifolius (yacon) roots on diabetic rats: Biochemical approach. Chem. Biol. Interact. 2011, 194, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Satoh, H.; Nguyen, M.T.A.; Kudoh, A.; Watanabe, T. Yacon diet (Smallanthus sonchifolius, Asteraceae) improves hepatic insulin resistance via reducing Trb3 expression in Zucker fa/fa rats. Nutr. Diabetes. 2013. [Google Scholar] [CrossRef] [PubMed]
- Scheid, M.M.; Genaro, P.S.; Moreno, Y.M.; Pastore, G.M. Freeze-dried powdered yacon: Effects of FOS on serum glucose, lipids and intestinal transit in the elderly. Eur. J. Nutr. 2014, 53, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Genta, S.; Cabrera, W.; Habib, N.; Pons, J.; Carillo, I.M.; Grau, A.; Sara, S. Yacon syrup: Beneficial effects on obesity and insulin resistance in humans. Clin. Nutr. 2009, 28, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Triplitt, C.L. Examining the mechanisms of glucose regulation. Am. J. Manag. Care 2012, 18, S4–10. [Google Scholar] [PubMed]
- López, V.L.; Medina, J.A.L.; Gutiérrez, M.V.; Soto, M.L.F. Carbohydrate: Current role in diabetes mellitus and metabolic disease. Nutr. Hosp. 2014, 30, 1020–1031. [Google Scholar] [CrossRef]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2014, 11, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.; Vogt, J.; Wolever, T.M. Intravenous acetate elicits a greater free fatty acid rebound in normal than hyperinsulinaemic humans. Eur. J. Clin. Nutr. 2012, 66, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Boillot, J.; Alamowitch, C.; Berger, A.M.; Luo, J.; Bruzzo, F.; Bornet, F.R.; Slama, G. Effects of dietary propionate on hepatic glucose production, whole-body glucose utilization, carbohydrate and lipid metabolism in normal rats. Br. J. Nutr. 1995, 73, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Psichas, A.; Sleeth, M.L.; Murphy, K.G.; Brooks, L.; Bewick, G.A.; Hanyaloglu, A.C.; Ghatei, M.A.; Bloom, S.R.; Frost, G. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int. J. Obes. 2015, 39, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Ahima, R.S.; Lazar, M.A. The Health Risk of Obesity—Better Metrics Imperative. Science 2013, 341, 6148. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO)—Obesity and overweight Fact Sheet. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/ (accessed on 9 May 2016).
- Lehnert, T.; Sonntag, D.; Konnopka, A.; Riedel-Heller, S.; König, H.H. Economic costs of overweight and obesity. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Lifshitz, F.; Lifshitz, J.Z. Globesity: The root causes of the obesity epidemic in the USA and now worldwide. Pediatr. Endocrinol. Rev. 2014, 12, 17–34. [Google Scholar] [PubMed]
- Kaur, J. A Comprehensive Review on Metabolic Syndrome. Cardiol. Res. Pract. 2014. [Google Scholar] [CrossRef] [PubMed]
- Leão, L.S.; de Moraes, M.M.; de Carvalho, G.X.; Koifman, R.J. Nutritional interventions in metabolic syndrome: A systematic review. Arq. Bras. Cardiol. 2011, 97, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S. Functional foods against metabolic syndrome (obesity, diabetes, hypertension and dyslipidemia) and cardio vascular disease. Trends Food Sci. Technol. 2014, 35, 114–128. [Google Scholar] [CrossRef]
- Genta, S.B.; Cabrera, W.M.; Grau, A.; Sánchez, S.S. Subchronic 4-month oral toxicity study of dried Smallanthus sonchifolius (yacon) roots as a diet supplement in rats. Food Chem. Toxicol. 2005, 43, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Roselino, M.N.; Pauly-Silveira, N.D.; Cavallini, D.C.; Celiberto, L.S.; Pinto, R.A.; Vendramini, R.C.; Rossi, E.A. A potential synbiotic product improves the lipid profile of diabetic rats. Lipids Health Dis. 2012. [Google Scholar] [CrossRef] [PubMed]
- Geyer, M.; Manrique, I.; Degen, L.; Beglinger, C. Effect of yacon (Smallanthus sonchifolius) on colonic transit time in healthy volunteers. Digestion 2008, 78, 30–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mora, S.; Fullerton, R. Effects of Short Chain Fatty Acids on Glucose and Lipid metabolism in Adipocytes. FASEB J. 2015, 29, 627–625. [Google Scholar]
- Hara, H.; Haga, S.; Aoyama, Y.; Kiriyama, S. Short-chain fatty acids suppress cholesterol synthesis in rat liver and intestine. J. Nutr. 1999, 129, 942–948. [Google Scholar] [PubMed]
- Byrne, S.; Chambers, E.S.; Morrison, D.J.; Frost, G. The role of short chain fatty acids in appetite regulation and energy homeostasis. Int. J. Obes. 2015, 39, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- D’Alessio, D. Intestinal hormones and regulation of satiety: The case for CCK, GLP-1, PYY, and Apo A-IV. JPEN J. Parenter Enteral Nutr. 2008, 32, 567–568. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, A.; Hasegawa, S.; Kasubuchi, M.; Kimura, I. Free fatty acid receptors as therapeutic targets for the treatment of diabetes. Front Pharmacol. 2014, 5, 236. [Google Scholar] [CrossRef] [PubMed]
- Isken, F.; Klaus, S.; Osterhoff, M.; Pfeiffer, A.F.; Weickert, M.O. Effects of long-term soluble vs. insoluble dietary fiber intake on high-fat diet-induced obesity in C57BL/6J mice. J. Nutr. Biochem. 2010, 21, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Eswaran, S.; Muir, J.; Chey, W.D. Fiber and functional gastrointestinal disorders. Am. J. Gastroenterol. 2013, 108, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Yun, E.Y.; Kim, H.S.; Kim, Y.E.; Kang, M.K.; Ma, J.E.; Lee, G.D.; Cho, Y.J.; Kim, H.C.; Lee, J.D.; Hwang, Y.S.; et al. A case of anaphylaxis after the ingestion of yacon. Allergy Asthma. Immunol. Res. 2010, 2, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Graefea, S.; Hermann, M.; Manrique, I.; Golombeka, S.; Buerkerta, A. Effects of post-harvest treatments on the carbohydrate composition of yacon roots in the Peruvian Andes. F. Cr. Res. 2004, 86, 157–165. [Google Scholar] [CrossRef]
- Di Bartolomeo, F.; Van den Ende, W. Fructose and Fructans: Opposite Effects on Health. Plant Foods Hum. Nutr. 2015, 70, 227–237. [Google Scholar] [CrossRef] [PubMed]


| Yacon Source | Research, Subject Randomized, Dose and Duration | Health Properties | References |
|---|---|---|---|
| Dried extract of yacon root | Mouse (BALB/c) Dose: 340 mg/kg day in diet, for 75 days | Growth of Bifidobacteria and Lactobacilli | Bonet et al. [16] |
| Dried extract of yacon root | Rats (Wistar) Dose: 0.5%, 1.0% (20.4% FOS) in diet for 13 weeks | Reduce tumor multiplicity, preneoplastic lesions and cell proliferation | De Moura et al. [27] |
| Aqueous extract of yacon root | Rats (Wistar) Dose: 2.2 mL (1% FOS) for 8 months | Reduce DNA damage and cell proliferation | Almeida et al. [28] |
| Dried extract of yacon root | Mouse (BALB/c) Dose: 3.0%, 5% FOS in diet for 30 days | Improves the immune parameters | Delgado et al. [46] |
| Yacon Source | Research, Subject Randomized, Dose and Duration | Health Properties | References |
|---|---|---|---|
| Yacon flour | Rats (Wistar) | Increase insulin-positive pancreatic cell | Habib et al. [54] |
| Dose: Yacon flour (340 mg FOS/kg/day) for 90 days | |||
| Dried extract of yacon root | Rats (Zucker fa/fa) | Improve insulin sensitivity in the insulin-resistant state | Satoh et al. [55] |
| Dose: 6.5% yacon for 5 weeks | |||
| Dried extract of yacon root | Elderly man and woman | Decrease in serum glucose levels | Scheid et al. [56] |
| Dose: Yacon powder (7.4 g of FOS) for 9 weeks | |||
| Yacon syrup | Obese and slightly dyslipidemic pre-menopausal women | Improve insulin-resistance state | Genta et al. [57] |
| Dose: Yacon syrup (0.29 g and 0.14 g FOS/kg/day), for 120 days |
| Yacon Source | Research, Subject Randomized, Dose and Duration | Health properties | References |
|---|---|---|---|
| Yacon flour | Rats (Wistar) Dose: Yacon flour (340 mg FOS/kg/day) for 90 days | Hypolipidemic effect | Habib et al. [54] |
| Yacon syrup | Obese and slightly dyslipidemic pre-menopausal women Dose: Yacon syrup (0.29/g and 0.14/g FOS/kg/day) for 120 days. | Increased defecation frequency and satiety sensation | Genta et al. [57] |
| Dried extract of yacon root | Rats (wistar) Dose: Dried yacon root (340 mg and 6800 mg FOS/bw) for 4 months | Reduced post-prandial serum TAG levels | Genta et al. [73] |
| Aqueous extract of yacon root | Rats (wistar) Dose: 1 mL/kg body weight/day, 4.30 g/100 g of frutans, for 7 weeks | Positive effect on TAG and HDL | Roselino et al. [74] |
| Yacon syrup | Healthy individuals Dose: 6.4 g FOS/day | Accelerates the colonic transit | Geyer et al. [75] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caetano, B.F.R.; De Moura, N.A.; Almeida, A.P.S.; Dias, M.C.; Sivieri, K.; Barbisan, L.F. Yacon (Smallanthus sonchifolius) as a Food Supplement: Health-Promoting Benefits of Fructooligosaccharides. Nutrients 2016, 8, 436. https://doi.org/10.3390/nu8070436
Caetano BFR, De Moura NA, Almeida APS, Dias MC, Sivieri K, Barbisan LF. Yacon (Smallanthus sonchifolius) as a Food Supplement: Health-Promoting Benefits of Fructooligosaccharides. Nutrients. 2016; 8(7):436. https://doi.org/10.3390/nu8070436
Chicago/Turabian StyleCaetano, Brunno F. R., Nelci A. De Moura, Ana P. S. Almeida, Marcos C. Dias, Kátia Sivieri, and Luís F. Barbisan. 2016. "Yacon (Smallanthus sonchifolius) as a Food Supplement: Health-Promoting Benefits of Fructooligosaccharides" Nutrients 8, no. 7: 436. https://doi.org/10.3390/nu8070436