Metabolic Equivalent in Adolescents, Active Adults and Pregnant Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Anthropometric Data
2.3. Resting Energy Expenditure
2.4. Total and Activity-Related Energy Expenditure
2.5. Statistical Analyses
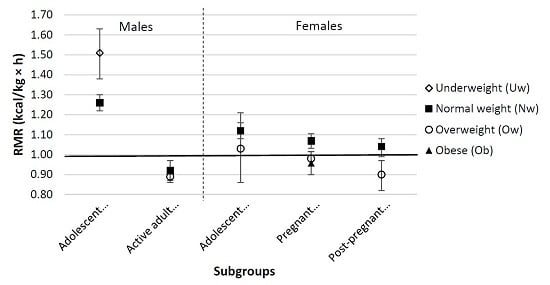
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| MET | Metabolic equivalent |
| RMR | Resting metabolic rate |
| PAL | Physical activity level |
| TEE | Total energy expenditure |
| BMI | Body mass index |
| V’O2 | Oxygen consumption |
| V’CO2 | Carbon dioxide production |
| AEE | Activity energy expenditure |
| DLW | Doubly labelled water |
| CI | Confidence Intervals |
References
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R., Jr.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 Compendium of Physical Activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Compher, C.; Frankenfield, D.; Keim, N.; Roth-Yousey, L. Best practice methods to apply to measurement of resting metabolic rate in adults: A systematic review. J. Am. Diet. Assoc. 2006, 106, 881–903. [Google Scholar] [CrossRef] [PubMed]
- Frankenfield, D.; Roth-Yousey, L.; Compher, C. Comparison of predictive equations for resting metabolic rate in healthy nonobese and obese adults: A systematic review. J. Am. Diet. Assoc. 2005, 105, 775–789. [Google Scholar] [CrossRef] [PubMed]
- Tompuri, T. Metabolic equivalents of task are confounded by adiposity, which disturbs ojective measurement of physical activity. Front. Physiol. 2015, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Warburton, D.E.; Nicol, C.W.; Bredin, S.S. Prescribing exercise as preventive therapy. CMAJ 2006, 174, 961–974. [Google Scholar] [CrossRef] [PubMed]
- Byrne, N.M.; Hills, A.P.; Hunter, G.R.; Weinsier, R.L.; Schutz, Y. Metabolic equivalent: One size does not fit all. J. Appl. Physiol. 2005, 99, 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- McMurray, R.G.; Soares, J.; Caspersen, C.J.; McCurdy, T. Examining variations of resting metabolic rate of adults: A public health perspective. Med. Sci. Sports Exerc. 2014, 46, 1352–1358. [Google Scholar] [CrossRef] [PubMed]
- Melzer, K.; Schutz, Y.; Boulvain, M.; Kayser, B. Pregnancy-related changes in activity energy expenditure and resting metabolic rate in Switzeand. Eur. J. Clin. Nutr. 2009, 63, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Melzer, K.; Renaud, A.; Zurbuchen, S.; Tschopp, C.; Lehmann, J.; Malatesta, D.; Ruch, N.; Schutz, Y.; Kayser, B.; Mader, U. Alterations in energy balance from an exercise intervention with ad libitum food intake. J. Nutr. Sci. 2016, 5, e7. [Google Scholar] [CrossRef] [PubMed]
- WHO. Obesity: Preventing and Managing the Global Epidemic; Report of a WHO Consultation; WHO Technical Report Series 894; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Institute of Medicine (US) Committee on Nutritional Status During Pregnancy and Lactation. Nutrition During Pregnancy, Weight Gain and Nutrient Supplements; National Academies Press: Waschington, DC, USA, 1990. [Google Scholar]
- Kromeyer-Hauschild, K.; Wabitsch, M.; Kunze, D.; Geller, F.; Geiss, H.; Hesse, V.; von Hippel, A.; Jaeger, U.; Johnsen, D.; Korte, W.; et al. Perzentile für den Body-Mass-Index für das Kindes- und Jugendalter unter Heranziehung verschiedener deutscher Stichproben. Monatsschr. Kinderheilkd. 2001, 149, 807–818. [Google Scholar] [CrossRef]
- Braegger, C.J.O.; Konrad, D.; Molinari, L. Neue Wachstumskurven für die Schweiz. Paediatrica 2011, 22, 9–11. [Google Scholar]
- Marshall, W.A.; Tanner, J.M. Variations in pattern of pubertal changes in girls. Arch. Dis. Child. 1969, 44, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Marshall, W.A.; Tanner, J.M. Variations in the pattern of pubertal changes in boys. Arch. Dis. Child. 1970, 45, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.C.; Crockett, L.; Richards, M.; Boxer, A. A self-report measure of pubertal status: Reliability, validity, and initial norms. J. Youth Adolesc. 1988, 17, 117–133. [Google Scholar] [CrossRef] [PubMed]
- Melzer, K.; Schutz, Y.; Soehnchen, N.; Othenin-Girard, V.; Martinez de Tejada, B.; Irion, O.; Boulvain, M.; Kayser, B. Effects of recommended levels of physical activity on pregnancy outcomes. Am. J. Obstet. Gynecol. 2010, 202, 266. [Google Scholar] [CrossRef] [PubMed]
- Rosdahl, H.; Lindberg, T.; Edin, F.; Nilsson, J. The Moxus Modular metabolic system evaluated with two sensors for ventilation against the Douglas bag method. Eur. J. Appl. Physiol. 2013, 113, 1353–1367. [Google Scholar] [CrossRef] [PubMed]
- Weir, J.B. New methods for calculating metabolic rate with special reference to protein metabolism. 1949. Nutrition 1990, 6, 213–221. [Google Scholar] [PubMed]
- Brage, S.; Brage, N.; Ekelund, U.; Luan, J.; Franks, P.W.; Froberg, K.; Wareham, N.J. Effect of combined movement and heart rate monitor placement on physical activity estimates during treadmill locomotion and free-living. Eur. J. Appl. Physiol. 2006, 96, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Brage, S.; Brage, N.; Franks, P.W.; Ekelund, U.; Wareham, N.J. Reliability and validity of the combined heart rate and movement sensor Actiheart. Eur. J. Clin. Nutr. 2005, 59, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Brage, S.; Ekelund, U.; Brage, N.; Hennings, M.A.; Froberg, K.; Franks, P.W.; Wareham, N.J. Hierarchy of individual calibration levels for heart rate and accelerometry to measure physical activity. J. Appl. Physiol. 2007, 103, 682–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melzer, K.; Lazzeri, M.; Armand, S.; Boulvain, M.; Kayser, B. Validation of the Actiheart for estimating physical activity related energy expenditure in pregnancy. Clin. Nutr. ESPEN 2012, 7, e5–e10. [Google Scholar] [CrossRef]
- Thompson, D.; Batterham, A.M.; Bock, S.; Robson, C.; Stokes, K. Assessment of low-to-moderate intensity physical activity thermogenesis in young adults using synchronized heart rate and accelerometry with branched-equation modeling. J. Nutr. 2006, 136, 1037–1042. [Google Scholar] [PubMed]
- Corder, K.; Brage, S.; Wareham, N.J.; Ekelund, U. Comparison of PAEE from combined and separate heart rate and movement models in children. Med. Sci. Sports Exerc. 2005, 37, 1761–1767. [Google Scholar] [CrossRef] [PubMed]
- Crouter, S.E.; Churilla, J.R.; Bassett, D.R., Jr. Accuracy of the Actiheart for the assessment of energy expenditure in adults. Eur. J. Clin. Nutr. 2008, 62, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Villars, C.; Bergouignan, A.; Dugas, J.; Antoun, E.; Schoeller, D.A.; Roth, H.; Maingon, A.C.; Lefai, E.; Blanc, S.; Simon, C. Validity of combining heart rate and uniaxial acceleration to measure free-living physical activity energy expenditure in young men. J. Appl. Physiol. 2012, 113, 1763–1771. [Google Scholar] [CrossRef] [PubMed]
- Holliday, M.A.; Potter, D.; Jarrah, A.; Bearg, S. The relation of metabolic rate to body weight and organ size. Pediatr. Res. 1967, 1, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, M.; Payne, P.R.; Stunkard, A.J.; Rivers, J.P.; Cox, M. Metabolic rate and physical development in children at risk of obesity. Lancet 1990, 336, 76–78. [Google Scholar] [CrossRef]
- FAO. Human Energy Requirements; Report of a Joint FAO/WHO/UNU Expert Consultation; Food and Nutrition Technical Report Series; FAO: Rome, Italy, 2004. [Google Scholar]
- Schofield, W.N. Predicting basal metabolic rate, new standards and review of previous work. Hum. Nutr. Clin. Nutr. 1985, 39, 5–41. [Google Scholar] [PubMed]
- Bandini, L.G.; Schoeller, D.A.; Dietz, W.H. Energy expenditure in obese and nonobese adolescents. Pediatr. Res. 1990, 27, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Bitar, A.; Fellmann, N.; Vernet, J.; Coudert, J.; Vermorel, M. Variations and determinants of energy expenditure as measured by whole-body indirect calorimetry during puberty and adolescence. Am. J. Clin. Nutr. 1999, 69, 1209–1216. [Google Scholar] [PubMed]
- Bratteby, L.E.; Sandhagen, B.; Fan, H.; Enghardt, H.; Samuelson, G. Total energy expenditure and physical activity as assessed by the doubly labeled water method in Swedish adolescents in whom energy intake was underestimated by 7-d diet records. Am. J. Clin. Nutr. 1998, 67, 905–911. [Google Scholar]
- De Lorenzo, A.; Bertini, I.; Puijia, A.; Testolin, G.; Testolin, C. Comparison between measured and predicted resting metabolic rate in moderately active adolescents. Acta Diabetol. 1999, 36, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Elliot, D.L.; Goldberg, L.; Kuehl, K.S.; Hanna, C. Metabolic evaluation of obese and nonobese siblings. J. Pediatr. 1989, 114, 957–962. [Google Scholar] [CrossRef]
- Henry, C.J.; Dyer, S.; Ghusain-Choueiri, A. New equations to estimate basal metabolic rate in children aged 10–15 years. Eur. J. Clin. Nutr. 1999, 53, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Lazzer, S.; Boirie, Y.; Bitar, A.; Montaurier, C.; Vernet, J.; Meyer, M.; Vermorel, M. Assessment of energy expenditure associated with physical activities in free-living obese and nonobese adolescents. Am. J. Clin. Nutr. 2003, 78, 471–479. [Google Scholar] [PubMed]
- Livingstone, M.B.; Coward, W.A.; Prentice, A.M.; Davies, P.S.; Strain, J.J.; McKenna, P.G.; Mahoney, C.A.; White, J.A.; Stewart, C.M.; Kerr, M.J. Daily energy expenditure in free-living children: Comparison of heart-rate monitoring with the doubly labeled water (2H2(18)O) method. Am. J. Clin. Nutr. 1992, 56, 343–352. [Google Scholar] [PubMed]
- Molnar, D.; Jeges, S.; Erhardt, E.; Schutz, Y. Measured and predicted resting metabolic rate in obese and nonobese adolescents. J. Pediatr. 1995, 127, 571–577. [Google Scholar] [CrossRef]
- Molnar, D.; Schutz, Y. The effect of obesity, age, puberty and gender on resting metabolic rate in children and adolescents. Eur. J. Pediatr. 1997, 156, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Ribeyre, J.; Fellmann, N.; Montaurier, C.; Delaitre, M.; Vernet, J.; Coudert, J.; Vermorel, M. Daily energy expenditure and its main components as measured by whole-body indirect calorimetry in athletic and non-athletic adolescents. Br. J. Nutr. 2000, 83, 355–362. [Google Scholar] [PubMed]
- Rieper, H.; Karst, H.; Noack, R.; Johnsen, D. Intra- and inter-individual variations in energy expenditure of 14–15-year-old schoolgirls as determined by indirect calorimetry. Br. J. Nutr. 1993, 69, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, G.; Moreno, L.A.; Sarria, A.; Fleta, J.; Bueno, M. Resting energy expenditure in children and adolescents: Agreement between calorimetry and prediction equations. Clin. Nutr. 2002, 21, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Van Mil, E.G.; Westerterp, K.R.; Kester, A.D.; Saris, W.H. Energy metabolism in relation to body composition and gender in adolescents. Arch. Dis. Child. 2001, 85, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Hytten, F.; Chamberlain, G. Clinical Physiology in Obstetrics; Blackwell Scientific Publications: Oxford, UK, 1980. [Google Scholar]
- Butte, N.F.; Wong, W.W.; Treuth, M.S.; Ellis, K.J.; O’Brian Smith, E. Energy requirements during pregnancy based on total energy expenditure and energy deposition. Am. J. Clin. Nutr. 2004, 79, 1078–1087. [Google Scholar] [PubMed]
- Forsum, E.; Kabir, N.; Sadurskis, A.; Westerterp, K. Total energy expenditure of healthy Swedish women during pregnancy and lactation. Am. J. Clin. Nutr. 1992, 56, 334–342. [Google Scholar] [PubMed]
- Goldberg, G.R.; Prentice, A.M.; Coward, W.A.; Davies, H.L.; Murgatroyd, P.R.; Wensing, C.; Black, A.E.; Harding, M.; Sawyer, M. Longitudinal assessment of energy expenditure in pregnancy by the doubly labeled water method. Am. J. Clin. Nutr. 1993, 57, 494–505. [Google Scholar] [PubMed]
- Kopp-Hoolihan, L.E.; van Loan, M.D.; Wong, W.W.; King, J.C. Longitudinal assessment of energy balance in well-nourished, pregnant women. Am. J. Clin. Nutr. 1999, 69, 697–704. [Google Scholar] [PubMed]
- Lof, M.; Olausson, H.; Bostrom, K.; Janerot-Sjoberg, B.; Sohlstrom, A.; Forsum, E. Changes in basal metabolic rate during pregnancy in relation to changes in body weight and composition, cardiac output, insulin-like growth factor I, and thyroid hormones and in relation to fetal growth. Am. J. Clin. Nutr. 2005, 81, 678–685. [Google Scholar] [PubMed]
- Prentice, A.M.; Spaaij, C.J.; Goldberg, G.R.; Poppitt, S.D.; van Raaij, J.M.; Totton, M.; Swann, D.; Black, A.E. Energy requirements of pregnant and lactating women. Eur. J. Clin. Nutr. 1996, 50, S82–S110. [Google Scholar] [PubMed]
- Poppitt, S.D.; Prentice, A.M.; Jequier, E.; Schutz, Y.; Whitehead, R.G. Evidence of energy sparing in Gambian women during pregnancy: A longitudinal study using whole-body calorimetry. Am. J. Clin. Nutr. 1993, 57, 353–364. [Google Scholar] [PubMed]
- Hytten, F.; Leitch, I. The Physiology of Human Pregnancy; Blackwell: Oxford, UK, 1971. [Google Scholar]
- Nelson, K.M.; Weinsier, R.L.; Long, C.L.; Schutz, Y. Prediction of resting energy expenditure from fat-free mass and fat mass. Am. J. Clin. Nutr. 1992, 56, 848–856. [Google Scholar] [PubMed]
- Van Raaij, J.M.; Schonk, C.M.; Vermaat-Miedema, S.H.; Peek, M.E.; Hautvast, J.G. Energy cost of lactation, and energy balances of well-nourished Dutch lactating women: Reappraisal of the extra energy requirements of lactation. Am. J. Clin. Nutr. 1991, 53, 612–619. [Google Scholar] [PubMed]
- Butte, N.F.; Wong, W.W.; Hopkinson, J.M. Energy requirements of lactating women derived from doubly labeled water and milk energy output. J. Nutr. 2001, 131, 53–58. [Google Scholar] [PubMed]
- Goldberg, G.R.; Prentice, A.M.; Coward, W.A.; Davies, H.L.; Murgatroyd, P.R.; Sawyer, M.B.; Ashford, J.; Black, A.E. Longitudinal assessment of the components of energy balance in well-nourished lactating women. Am. J. Clin. Nutr. 1991, 54, 788–798. [Google Scholar] [PubMed]
- Sadurskis, A.; Kabir, N.; Wager, J.; Forsum, E. Energy metabolism, body composition, and milk production in healthy Swedish women during lactation. Am. J. Clin. Nutr. 1988, 48, 44–49. [Google Scholar] [PubMed]
- Spaaij, C.J.; van Raaij, J.M.; de Groot, L.C.; van der Heijden, L.J.; Boekholt, H.A.; Hautvast, J.G. Effect of lactation on resting metabolic rate and on diet- and work-induced thermogenesis. Am. J. Clin. Nutr. 1994, 59, 42–47. [Google Scholar] [PubMed]
- Butte, N.F.; King, J.C. Energy requirements during pregnancy and lactation. Public Health Nutr. 2005, 8, 1010–1027. [Google Scholar] [CrossRef] [PubMed]
- Kyle, U.G.; Melzer, K.; Kayser, B.; Picard-Kossovsky, M.; Gremion, G.; Pichard, C. Eight-year longitudinal changes in body composition in healthy Swiss adults. J. Am. Coll. Nutr. 2006, 25, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Bosy-Westphal, A.; Eichhorn, C.; Kutzner, D.; Illner, K.; Heller, M.; Muller, M.J. The age-related decline in resting energy expenditure in humans is due to the loss of fat-free mass and to alterations in its metabolically active components. J. Nutr. 2003, 133, 2356–2362. [Google Scholar] [PubMed]
- Piers, L.S.; Soares, M.J.; McCormack, L.M.; O’Dea, K. Is there evidence for an age-related reduction in metabolic rate? J. Appl. Physiol. 1998, 85, 2196–2204. [Google Scholar] [PubMed]
- Wang, Z.; Heshka, S.; Heymsfield, S.B.; Shen, W.; Gallagher, D. A cellular-level approach to predicting resting energy expenditure across the adult years. Am. J. Clin. Nutr. 2005, 81, 799–806. [Google Scholar] [PubMed]
- Henry, C.J. Mechanisms of changes in basal metabolism during ageing. Eur. J. Clin. Nutr. 2000, 54, S77–S91. [Google Scholar] [CrossRef] [PubMed]
- Arciero, P.J.; Goran, M.I.; Poehlman, E.T. Resting metabolic rate is lower in women than in men. J. Appl. Physiol. 1993, 75, 2514–2520. [Google Scholar] [PubMed]
- Ferraro, R.; Lillioja, S.; Fontvieille, A.M.; Rising, R.; Bogardus, C.; Ravussin, E. Lower sedentary metabolic rate in women compared with men. J. Clin. Investig. 1992, 90, 780–784. [Google Scholar] [CrossRef] [PubMed]
- Speakman, J.R.; Selman, C. Physical activity and resting metabolic rate. Proc. Nutr. Soc. 2003, 62, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Almeras, N.; Mimeault, N.; Serresse, O.; Boulay, M.R.; Tremblay, A. Non-exercise daily energy expenditure and physical activity pattern in male endurance athletes. Eur. J. Appl. Physiol. Occup. Physiol. 1991, 63, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Broeder, C.E.; Burrhus, K.A.; Svanevik, L.S.; Wilmore, J.H. The effects of aerobic fitness on resting metabolic rate. Am. J. Clin. Nutr. 1992, 55, 795–801. [Google Scholar] [PubMed]
- Lee, M.G.; Sedlock, D.A.; Flynn, M.G.; Kamimori, G.H. Resting metabolic rate after endurance exercise training. Med. Sci. Sports Exerc. 2009, 41, 1444–1451. [Google Scholar] [CrossRef] [PubMed]
- Wilmore, J.H.; Stanforth, P.R.; Hudspeth, L.A.; Gagnon, J.; Daw, E.W.; Leon, A.S.; Rao, D.C.; Skinner, J.S.; Bouchard, C. Alterations in resting metabolic rate as a consequence of 20 wk of endurance training: The HERITAGE Family Study. Am. J. Clin. Nutr. 1998, 68, 66–71. [Google Scholar] [PubMed]
- Zanuso, S.; Bergamin, M.; Jimenez, A.; Pugliese, G.; D’Errico, V.; Nicolucci, A.; Ermolao, A.; Balducci, S. Determination of metabolic equivalents during low- and high-intensity resistance exercise in healthy young subjects and patients with type 2 diabetes. Biol. Sport 2016, 33, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Dolezal, B.A.; Potteiger, J.A. Concurrent resistance and endurance training influence basal metabolic rate in nondieting individuals. J. Appl. Physiol. 1998, 85, 695–700. [Google Scholar]
- Bielinski, R.; Schutz, Y.; Jequier, E. Energy metabolism during the postexercise recovery in man. Am. J. Clin. Nutr. 1985, 42, 69–82. [Google Scholar] [PubMed]
- Tremblay, A.; Nadeau, A.; Fournier, G.; Bouchard, C. Effect of a three-day interruption of exercise-training on resting metabolic rate and glucose-induced thermogenesis in training individuals. Int. J. Obes. 1988, 12, 163–168. [Google Scholar] [PubMed]
- Fullmer, S.; Benson-Davies, S.; Earthman, C.P.; Frankenfield, D.C.; Gradwell, E.; Lee, P.S.; Piemonte, T.; Trabulsi, J. Evidence analysis library review of best practices for performing indirect calorimetry in healthy and non-critically ill individuals. J. Acad. Nutr. Diet. 2015, 115, 1417–1446. [Google Scholar] [CrossRef] [PubMed]
- Pontzer, H. Constrained total energy expenditure and the evolutionary biology of energy balance. Exerc. Sport. Sci. Rev. 2015, 43, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Melzer, K.; Schutz, Y.; Kayser, B. Normalization of basal metabolic rate for differences in body weight in pregnant women. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 159, 480–481. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, W.H.; Poehlman, E.T.; O’Connell, M.; Goran, M.I. Influence of body composition and resting metabolic rate on variation in total energy expenditure: A meta-analysis. Am. J. Clin. Nutr. 1995, 61, 4–10. [Google Scholar] [PubMed]
- Allison, D.B.; Paultre, F.; Goran, M.I.; Poehlman, E.T.; Heymsfield, S.B. Statistical considerations regarding the use of ratios to adjust data. Int. J. Obes. Relat. Metab. Disord. 1995, 19, 644–652. [Google Scholar] [PubMed]
- Weinsier, R.L.; Schutz, Y.; Bracco, D. Reexamination of the relationship of resting metabolic rate to fat-free mass and to the metabolically active components of fat-free mass in humans. Am. J. Clin. Nutr. 1992, 55, 790–794. [Google Scholar] [PubMed]
- Nevill, A.M.; Ramsbottom, R.; Williams, C. Scaling physiological measurements for individuals of different body size. Eur. J. Appl. Physiol. Occup. Physiol. 1992, 65, 110–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Subjects | Males | Females | |||
|---|---|---|---|---|---|
| Adolescent (n = 50) | Active Adult (n = 30) | Adolescent (n = 50) | Pregnant (n = 46) | Post-Pregnant (n = 27) | |
| Age (years) | 14.7 ± 1.7 # | 29.7 ± 7.2 | 14.8 ± 1.6 # | 31.3 ± 5.5 | 31.8 ± 5.3 |
| Height (cm) | 169 ± 11 | 180 ± 6.9 * | 164 ± 6 ° | 166 ± 6 | 166 ± 7 |
| Weight (kg) | 56.4 ± 11.3 §,& | 81.0 ± 7.3 | 57.3 ± 8.4 §,& | 77.2 ± 12.6 | 62.3 ± 10.6 §,& |
| BMI (kg/m2) | 19.6 ± 2.3 # | 25.5 ± 2.3 | 21.2 ± 2.7 §,& | 28.1 ± 4.1 | 22.5 ± 3.0 §,& |
| PAL | 1.6 ± 0.2 | 1.9 ± 0.2 * | 1.6 ± 0.1 | 1.5 ± 0.2 ° | 1.7 ± 0.3 § |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melzer, K.; Heydenreich, J.; Schutz, Y.; Renaud, A.; Kayser, B.; Mäder, U. Metabolic Equivalent in Adolescents, Active Adults and Pregnant Women. Nutrients 2016, 8, 438. https://doi.org/10.3390/nu8070438
Melzer K, Heydenreich J, Schutz Y, Renaud A, Kayser B, Mäder U. Metabolic Equivalent in Adolescents, Active Adults and Pregnant Women. Nutrients. 2016; 8(7):438. https://doi.org/10.3390/nu8070438
Chicago/Turabian StyleMelzer, Katarina, Juliane Heydenreich, Yves Schutz, Anne Renaud, Bengt Kayser, and Urs Mäder. 2016. "Metabolic Equivalent in Adolescents, Active Adults and Pregnant Women" Nutrients 8, no. 7: 438. https://doi.org/10.3390/nu8070438
APA StyleMelzer, K., Heydenreich, J., Schutz, Y., Renaud, A., Kayser, B., & Mäder, U. (2016). Metabolic Equivalent in Adolescents, Active Adults and Pregnant Women. Nutrients, 8(7), 438. https://doi.org/10.3390/nu8070438