Natural Phyto-Bioactive Compounds for the Treatment of Type 2 Diabetes: Inflammation as a Target
Abstract
:1. Introduction
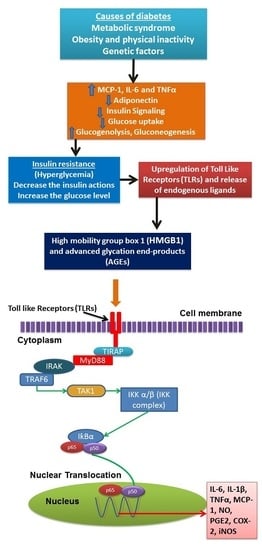
2. Inflammation Status in Diabetes Mellitus
3. Anti-Inflammatory Based Therapeutics for Diabetes
4. Natural Phyto Bioactive Compounds Role in Anti-Diabetics
5. Extraction, Identification and Characterization of Bioactive Compounds from Natural Products
5.1. Extraction
5.2. Novel Technologies Used in Extraction of Phyto Based Antidiabetic Compounds
5.3. Ultra Sound Extraction of Phyto Compounds from Anti-Diabetic Plants
5.4. Microwave Assisted Extraction of Phyto Compounds from Antidiabetic Plants
5.5. Supercritical Fluid Extraction of Bioactive Compounds from Antidiabetic Plants
5.6. Purification of Bioactive Compounds
5.7. Structural Characterization of Purified Compounds
6. Natural Anti-Diabetic/Inflammatory Bioactive Compounds and Their Mechanisms of Action
7. Phytochemicals Bioavailability and Their Effect on Human Metabolism
7.1. Digestibility of Phytochemicals
7.2. Absorption of Phytochemicals
7.3. Bioavailability of Phytochemicals
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wu, Y.; Ding, Y.; Tanaka, Y.; Zhang, W. Risk Factors Contributing to Type 2 Diabetes and Recent Advances in the Treatment and Prevention. Int. J. Med. Sci. 2014, 11, 1185–1200. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.; Choi, M.-S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Mukhopadhyay, S. The evil axis of obesity, inflammation and type-2 diabetes. Endocr. Metab. Immune Disord. Drug Targets 2011, 11, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.; Yaqub, A.; Nicholas, A.; Arastus, W.; Muhammad, M.; Abdullahi, S. Review on diabetes, synthetic drugs and glycemic effects of medicinal plants. J. Med. Plants Res. 2013, 7, 2628–2637. [Google Scholar]
- Patel, D.; Prasad, S.; Kumar, R.; Hemalatha, S. An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pac. J. Trop. Biomed. 2012, 2, 320–330. [Google Scholar] [CrossRef]
- Arulselvan, P.; Ghofar, H.A.A.; Karthivashan, G.; Halim, M.F.A.; Ghafar, M.S.A.; Fakurazi, S. Antidiabetic therapeutics from natural source: A systematic review. Biomed. Prev. Nutr. 2014, 4, 607–617. [Google Scholar] [CrossRef]
- Wang, X.; Bao, W.; Liu, J.; Ouyang, Y.Y.; Wang, D.; Rong, S.; Xiao, X.; Shan, Z.L.; Zhang, Y.; Yao, P.; et al. Inflammatory markers and risk of type 2 diabetes: A systematic review and meta-analysis. Diabetes Care 2013, 36, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Goldfine, A.B.; Fonseca, V.; Jablonski, K.A.; Pyle, L.; Staten, M.A.; Shoelson, S.E. The effects of salsalate on glycemic control in patients with type 2 diabetes: A randomized trial. Ann. Intern. Med. 2010, 152, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Spranger, J.; Kroke, A.; Möhlig, M.; Hoffmann, K.; Bergmann, M.M.; Ristow, M.; Boeing, H.; Pfeiffer, A.F. Inflammatory cytokines and the risk to develop type 2 diabetes results of the prospective population-based european prospective investigation into cancer and nutrition (epic)-potsdam study. Diabetes 2003, 52, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.M.; Cooper, M.E. Mechanisms of diabetic complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef] [PubMed]
- Chiquette, E.; Chilton, R. Cardiovascular disease: Much more aggressive in patients with type 2 diabetes. Curr. Atheroscler. Rep. 2002, 4, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Karamifar, H.; Habibian, N.; Amirhakimi, G.; Karamizadeh, Z.; Alipour, A. Adiponectin is a good marker for metabolic state among type 1 diabetes mellitus patients. Iran. J. Pediatr. 2013, 23, 295–301. [Google Scholar] [PubMed]
- Mogensen, T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef] [PubMed]
- Piccinini, A.; Midwood, K. Dampening inflammation by modulating TLR signalling. Mediat. Inflamm. 2010, 2010. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Sohn, E.; Kim, C.-S.; Jo, K.; Kim, J.S. The role of high-mobility group box-1 protein in the development of diabetic nephropathy. Am. J. Nephrol. 2011, 33, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Qiao, F.; Zhao, Y.; Wang, Y.; Liu, G. HMGB1 is activated in type 2 diabetes mellitus patients and in mesangial cells in response to high glucose. Int. J. Clin. Exp. Pathol. 2015, 8, 6683. [Google Scholar] [PubMed]
- Vlassara, H.; Uribarri, J. Advanced glycation end products (AGE) and diabetes: Cause, effect, or both? Curr. Diabetes Rep. 2014, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chawla, A.; Nguyen, K.D.; Goh, Y.P. Macrophage-mediated inflammation in metabolic disease. Nat. Rev. Immunol. 2011, 11, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, R.; Wang, H.; Liang, F. Mechanisms linking inflammation to insulin resistance. Int. J. Endocrinol. 2015, 2015, 508409. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.G.; Hayden, M.S.; Ghosh, S. Nf-κb, inflammation, and metabolic disease. Cell Metab. 2011, 13, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Chinetti-Gbaguidi, G.; Staels, B. Macrophage polarization in metabolic disorders: Functions and regulation. Curr. Opin. Lipidol. 2011, 22, 365–372. [Google Scholar] [CrossRef] [PubMed]
- McArdle, M.A.; Finucane, O.M.; Connaughton, R.M.; McMorrow, A.M.; Roche, H.M. Mechanisms of obesity-induced inflammation and insulin resistance: Insights into the emerging role of nutritional strategies. Front. Endocrinol. 2013, 4, 52. [Google Scholar] [CrossRef] [PubMed]
- Cancello, R.; Henegar, C.; Viguerie, N.; Taleb, S.; Poitou, C.; Rouault, C.; Coupaye, M.; Pelloux, V.; Hugol, D.; Bouillot, J.L.; et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes 2005, 54, 2277–2286. [Google Scholar] [CrossRef] [PubMed]
- Badawi, A.; Klip, A.; Haddad, P.; Cole, D.E.; Bailo, B.G.; El-Sohemy, A.; Karmali, M. Type 2 diabetes mellitus and inflammation: Prospects for biomarkers of risk and nutritional intervention. Diabetes Metab. Syndr. Obes. 2010, 3, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Rendell, M. The role of sulphonylureas in the management of type 2 diabetes mellitus. Drugs 2004, 64, 1339–1358. [Google Scholar] [CrossRef] [PubMed]
- Krentz, A.J.; Bailey, C.J. Oral antidiabetic agents: Current role in type 2 diabetes mellitus. Drugs 2005, 65, 385–411. [Google Scholar] [CrossRef] [PubMed]
- Shoelson, S.E.; Lee, J.; Yuan, M. Inflammation and the ikk beta/i kappa b/nf-kappa b axis in obesity- and diet-induced insulin resistance. Int. J. Obes. Relat. Metab. Disord. 2003, 27 (Suppl. S3), S49–S52. [Google Scholar] [CrossRef] [PubMed]
- Hundal, R.S.; Petersen, K.F.; Mayerson, A.B.; Randhawa, P.S.; Inzucchi, S.; Shoelson, S.E.; Shulman, G.I. Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J. Clin. Investig. 2002, 109, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, G. Insulin and insulin resistance. Clin. Biochem. Rev. 2005, 26, 19. [Google Scholar] [PubMed]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.L.; Lin, Y.; Bartolome, A.P.; Chen, Y.-C.; Chiu, S.-C.; Yang, W.-C. Herbal therapies for type 2 diabetes mellitus: Chemistry, biology, and potential application of selected plants and compounds. Evid. Based Complement. Alternat. Med. 2013, 2013, 378657. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, B.; Vaidya, A.D.; Chorghade, M. Ayurveda and natural products drug discovery. Curr. Sci. 2004, 86, 789–799. [Google Scholar]
- Coman, C.; Rugina, O.D.; Socaciu, C. Plants and natural compounds with antidiabetic action. Not. Bot. Horti Agrobot. Cluj-Napoca 2012, 40, 314. [Google Scholar]
- Zhang, Z.; Luo, A.; Zhong, K.; Huang, Y.; Gao, Y.; Zhang, J.; Gao, H.; Xu, Z.; Gao, X. A-glucosidase inhibitory activity by the flower buds of Lonicera japonica Thunb. J. Funct. Foods 2013, 5, 1253–1259. [Google Scholar] [CrossRef]
- Takahashi, T.; Miyazawa, M. Potent α-glucosidase inhibitors from safflower (Carthamus tinctorius L.) seed. Phytother. Res. 2012, 26, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Egan, J.M. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol. Rev. 2008, 60, 470–512. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jwa, H.; Yanagawa, Y.; Park, T. Extract from Dioscorea batatas ameliorates insulin resistance in mice fed a high-fat diet. J. Med. Food 2012, 15, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Grace, M.H.; Ribnicky, D.M.; Kuhn, P.; Poulev, A.; Logendra, S.; Yousef, G.G.; Raskin, I.; Lila, M.A. Hypoglycemic activity of a novel anthocyanin-rich formulation from lowbush blueberry, vaccinium angustifolium aiton. Phytomedicine 2009, 16, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, D.S.; Kang, S. Gastrodia elata blume water extracts improve insulin resistance by decreasing body fat in diet-induced obese rats: Vanillin and 4-hydroxybenzaldehyde are the bioactive candidates. Eur. J. Nutr. 2011, 50, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Kho, M.C.; Lee, Y.J.; Cha, J.D.; Choi, K.M.; Kang, D.G.; Lee, H.S. Gastrodia elata ameliorates high-fructose diet-induced lipid metabolism and endothelial dysfunction. Evid. Based Complement. Alternat. Med. 2014, 2014, 101624. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Chen, S.; Feng, B.; Wu, Y.; Lui, E.; Chakrabarti, S. Preventive effects of north American ginseng (Panax quinquefolium) on diabetic nephropathy. Phytomedicine 2012, 19, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Choi, H. Dietary red chilli (Capsicum frutescens L.) is insulinotropic rather than hypoglycemic in type 2 diabetes model of rats. Phytother. Res. 2008, 22, 1025–1029. [Google Scholar] [CrossRef] [PubMed]
- Madkor, H.R.; Mansour, S.W.; Ramadan, G. Modulatory effects of garlic, ginger, turmeric and their mixture on hyperglycaemia, dyslipidaemia and oxidative stress in streptozotocin–nicotinamide diabetic rats. Br. J. Nutr. 2011, 105, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.S.; Shetty, A.; Sambaiah, K.; Salimath, P. Antidiabetic property of fenugreek seed mucilage and spent turmeric in streptozotocin-induced diabetic rats. Nutr. Res. 2005, 25, 1021–1028. [Google Scholar] [CrossRef]
- Haidari, F.; Omidian, K.; Rafiei, H.; Zarei, M.; Mohamad Shahi, M. Green tea (Camellia sinensis) supplementation to diabetic rats improves serum and hepatic oxidative stress markers. Iran. J. Pharm. Res. 2013, 12, 109–114. [Google Scholar] [PubMed]
- Aybar, M.J.; Riera, A.N.S.; Grau, A.; Sanchez, S.S. Hypoglycemic effect of the water extract of Smallantus sonchifolius (yacon) leaves in normal and diabetic rats. J. Ethnopharmacol. 2001, 74, 125–132. [Google Scholar] [CrossRef]
- Abascal, K.; Ganora, L.; Yarnell, E. The effect of freeze-drying and its implications for botanical medicine: A review. Phytother. Res. 2005, 19, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Asami, D.K.; Hong, Y.-J.; Barrett, D.M.; Mitchell, A.E. Comparison of the total phenolic and ascorbic acid content of freeze-dried and air-dried marionberry, strawberry, and corn grown using conventional, organic, and sustainable agricultural practices. J. Agric. Food Chem. 2003, 51, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Chang, S. A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J. Food Sci. 2007, 72, S159–S166. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Joana Gil-Chávez, G.; Villa, J.A.; Fernando Ayala-Zavala, J.; Basilio Heredia, J.; Sepulveda, D.; Yahia, E.M.; González-Aguilar, G.A. Technologies for extraction and production of bioactive compounds to be used as nutraceuticals and food ingredients: An overview. Compr. Rev. Food Sci. Food Saf. 2013, 12, 5–23. [Google Scholar] [CrossRef]
- Robards, K. Strategies for the determination of bioactive phenols in plants, fruit and vegetables. J. Chromatogr. A 2003, 1000, 657–691. [Google Scholar] [CrossRef]
- Liu, C.-W.; Wang, Y.-C.; Lu, H.-C.; Chiang, W.-D. Optimization of ultrasound-assisted extraction conditions for total phenols with anti-hyperglycemic activity from Psidium guajava leaves. Process Biochem. 2014, 49, 1601–1605. [Google Scholar] [CrossRef]
- Ştefănuţ, M.N.; Căta, A.; Pop, R.; Tănasie, C.; Boc, D.; Ienaşcu, I.; Ordodi, V. Anti-hyperglycemic effect of bilberry, blackberry and mulberry ultrasonic extracts on diabetic rats. Plant Foods Hum. Nutr. 2013, 68, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; You, L.-J.; Abbasi, A.M.; Fu, X.; Liu, R.H. Optimization for ultrasound extraction of polysaccharides from mulberry fruits with antioxidant and hyperglycemic activity in vitro. Carbohydr. Polym. 2015, 130, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Devgan, M.; Nanda, A.; Ansari, S.H. Comparative evaluation of the anti-diabetic activity of Pterocarpus marsupium Roxb. Heartwood in alloxan induced diabetic rats using extracts obtained by optimized conventional and non conventional extraction methods. Pak. J. Pharm. Sci. 2013, 26, 973–976. [Google Scholar] [PubMed]
- Spigno, G.; De Faveri, D. Microwave-assisted extraction of tea phenols: A phenomenological study. J. Food Eng. 2009, 93, 210–217. [Google Scholar] [CrossRef]
- Rojas, R.; Contreras-Esquivel, J.C.; Orozco-Esquivel, M.T.; Muñoz, C.; Aguirre-Joya, J.A.; Aguilar, C.N. Mango peel as source of antioxidants and pectin: Microwave assisted extraction. Waste Biomass Valorization 2015, 6, 1095–1102. [Google Scholar] [CrossRef]
- Rahath Kubra, I.; Kumar, D.; Rao, L.J.M. Effect of microwave-assisted extraction on the release of polyphenols from ginger (Zingiber officinale). Int. J. Food Sci. Technol. 2013, 48, 1828–1833. [Google Scholar] [CrossRef]
- Policegoudra, R.; Aradhya, S.; Singh, L. Mango ginger (Curcuma amada Roxb.)—A promising spice for phytochemicals and biological activities. J. Biosci. 2011, 36, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Maran, J.P.; Swathi, K.; Jeevitha, P.; Jayalakshmi, J.; Ashvini, G. Microwave-assisted extraction of pectic polysaccharide from waste mango peel. Carbohydr. Polym. 2015, 123, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Pandit, S.G.; Vijayanand, P.; Kulkarni, S. Pectic principles of mango peel from mango processing waste as influenced by microwave energy. LWT Food Sci. Technol. 2015, 64, 1010–1014. [Google Scholar] [CrossRef]
- Yunus, S.; Zaki, M.; Asyikin, N.; Ku Hamid, K.H. Microwave Drying Characteristics and Antidiabetic Properties of Aquilaria subintegra and Aquilaria malaccensis Leaves. Adv. Mater. Res. 2015, 1113, 352–357. [Google Scholar] [CrossRef]
- Zou, T.; Wu, H.; Li, H.; Jia, Q.; Song, G. Comparison of microwave-assisted and conventional extraction of mangiferin from mango (Mangifera indica L.) leaves. J. Sep. Sci. 2013, 36, 3457–3462. [Google Scholar] [PubMed]
- Salomon, S.; Sevilla, I.; Betancourt, R.; Romero, A.; Nuevas-Paz, L.; Acosta-Esquijarosa, J. Extraction of mangiferin from Mangifera indica L. Leaves using microwave-assisted technique. Emir. J. Food Agric. 2014, 26, 616. [Google Scholar] [CrossRef]
- Veggi, P.C.; Cavalcanti, R.N.; Meireles, M.A.A. Production of phenolic-rich extracts from Brazilian plants using supercritical and subcritical fluid extraction: Experimental data and economic evaluation. J. Food Eng. 2014, 131, 96–109. [Google Scholar] [CrossRef]
- Herrero, M.; del Pilar Sánchez-Camargo, A.; Cifuentes, A.; Ibáñez, E. Plants, seaweeds, microalgae and food by-products as natural sources of functional ingredients obtained using pressurized liquid extraction and supercritical fluid extraction. TrAC Trends Anal. Chem. 2015, 71, 26–38. [Google Scholar] [CrossRef]
- Liau, B.-C.; Shen, C.-T.; Liang, F.-P.; Hong, S.-E.; Hsu, S.-L.; Jong, T.-T.; Chang, C.-M.J. Supercritical fluids extraction and anti-solvent purification of carotenoids from microalgae and associated bioactivity. J. Supercrit. Fluids 2010, 55, 169–175. [Google Scholar] [CrossRef]
- Hsieh, T.-J.; Tsai, Y.-H.; Liao, M.-C.; Du, Y.-C.; Lien, P.-J.; Sun, C.-C.; Chang, F.-R.; Wu, Y.-C. Anti-diabetic properties of non-polar Toona sinensis roem extract prepared by supercritical-co 2 fluid. Food Chem. Toxicol. 2012, 50, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.; Gamarra, F.; Oliveira, A.; Cabral, F. Extraction of bixin from annatto seeds using supercritical carbon dioxide. Braz. J. Chem. Eng. 2008, 25, 419–426. [Google Scholar] [CrossRef] [Green Version]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.; Latha, L.Y. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, E.F.; Ioset, J.R.; Ndjoko, K.; Guntern, A.; Foggin, C.M.; Hostettmann, K. On-line identification of the bioactive compounds from Blumea gariepina by HPLC-UV-MS and HPLC-UV-NMR, combined with HPLC-micro-fractionation. Phytochem. Anal. 2005, 16, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Ek, S.; Kartimo, H.; Mattila, S.; Tolonen, A. Characterization of phenolic compounds from Lingonberry (Vaccinium vitis-idaea). J. Agric. Food Chem. 2006, 54, 9834–9842. [Google Scholar] [CrossRef] [PubMed]
- Kingston, D.G. Modern natural products drug discovery and its relevance to biodiversity conservation. J. Nat. Prod. 2010, 74, 496–511. [Google Scholar] [CrossRef] [PubMed]
- Bucar, F.; Wube, A.; Schmid, M. Natural product isolation—How to get from biological material to pure compounds. Nat. Prod. Rep. 2013, 30, 525–545. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R.; Deng, Z. Separation procedures for naturally occurring antioxidant phytochemicals. J. Chromatogr. B 2004, 812, 85–99. [Google Scholar] [CrossRef]
- Cai, Z.; Lee, F.S.; Wang, X.R.; Yu, W.J. A capsule review of recent studies on the application of mass spectrometry in the analysis of Chinese medicinal herbs. J. Mass Spectrom. 2002, 37, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Daffre, S.; Bulet, P.; Spisni, A.; Ehret-Sabatier, L.; Rodrigues, E.G.; Travassos, L.R. Bioactive natural peptides. Stud. Nat. Prod. Chem. 2008, 35, 597–691. [Google Scholar]
- Bobzin, S.C.; Yang, S.; Kasten, T.P. Application of liquid chromatography–nuclear magnetic resonance spectroscopy to the identification of natural products. J. Chromatogr. B 2000, 748, 259–267. [Google Scholar] [CrossRef]
- Koehn, F.E.; Carter, G.T. The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov. 2005, 4, 206–220. [Google Scholar] [CrossRef] [PubMed]
- Thitilertdecha, N.; Teerawutgulrag, A.; Kilburn, J.D.; Rakariyatham, N. Identification of major phenolic compounds from Nephelium lappaceum L. And their antioxidant activities. Molecules 2010, 15, 1453–1465. [Google Scholar] [PubMed]
- Bouallagui, Z.; Bouaziz, M.; Han, J.; Boukhris, M.; Rigane, G.; Friha, I.; Jemai, H.; Ghorbel, H.; Isoda, H.; Sayadi, S. Valorization of olive processing by-products-characterization, investigation of chemico-biological activities and identification on active compounds. J. Arid Land Stud. 2012, 22–21. [Google Scholar]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach; John Wiley & Sons: New York, NY, USA, 2002. [Google Scholar]
- Roglic, G.; Unwin, N.; Bennett, P.H.; Mathers, C.; Tuomilehto, J.; Nag, S.; Connolly, V.; King, H. The burden of mortality attributable to diabetes realistic estimates for the year 2000. Diabetes Care 2005, 28, 2130–2135. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [PubMed]
- Parr, A.J.; Bolwell, G.P. Phenols in the plant and in man. The potential for possible nutritional enhancement of the diet by modifying the phenols content or profile. J. Sci. Food Agric. 2000, 80, 985–1012. [Google Scholar] [CrossRef]
- Wink, M. Compartmentation of secondary metabolites and xenobiotics in plant vacuoles. Adv. Bot. Res. 1997, 25, 141–170. [Google Scholar]
- Clifford, M.; Scalbert, A. Ellagitannins, occurrence in food, bioavailability and cancer prevention. J. Food Sci. Agric. 2000, 80, 1118–1125. [Google Scholar] [CrossRef]
- Vitrac, X.; Monti, J.-P.; Vercauteren, J.; Deffieux, G.; Mérillon, J.-M. Direct liquid chromatographic analysis of resveratrol derivatives and flavanonols in wines with absorbance and fluorescence detection. Anal. Chim. Acta 2002, 458, 103–110. [Google Scholar] [CrossRef]
- Adlercreutz, H.; Mazur, W. Phyto-oestrogens and western diseases. Ann. Med. 1997, 29, 95–120. [Google Scholar] [CrossRef] [PubMed]
- Ohno, M.; Shibata, C.; Kishikawa, T.; Yoshikawa, T.; Takata, A.; Kojima, K.; Akanuma, M.; Kang, Y.J.; Yoshida, H.; Otsuka, M. The flavonoid apigenin improves glucose tolerance through inhibition of microRNA maturation in miRNA103 transgenic mice. Sci. Rep. 2013. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, G.; Gurley, E.C.; Zhou, H. Flavonoid apigenin inhibits lipopolysaccharide-induced inflammatory response through multiple mechanisms in macrophages. PLoS ONE 2014, 9, e107072. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Bansal, M.K.; Dalvi, R.; Upganlawar, A.; Somani, R. Protective effect of diosmin against diabetic neuropathy in experimental rats. J. Integr. Med. 2014, 12, 35–41. [Google Scholar] [CrossRef]
- Mahmoud, M.F.; Hassan, N.A.; El Bassossy, H.M.; Fahmy, A. Quercetin protects against diabetes-induced exaggerated vasoconstriction in rats: Effect on low grade inflammation. PLoS ONE 2013, 8, e63784. [Google Scholar]
- Nair, M.P.; Mahajan, S.; Reynolds, J.L.; Aalinkeel, R.; Nair, H.; Schwartz, S.A.; Kandaswami, C. The flavonoid quercetin inhibits proinflammatory cytokine (tumor necrosis factor α) gene expression in normal peripheral blood mononuclear cells via modulation of the NF-κβ system. Clin. Vaccine Immunol. 2006, 13, 319–328. [Google Scholar] [PubMed]
- Abo-Salem, O.M. Kaempferol attenuates the development of diabetic neuropathic pain in mice: Possible anti-inflammatory and anti-oxidant mechanisms. Maced. J. Med. Sci. 2014, 7, 424–430. [Google Scholar] [CrossRef]
- Zang, Y.; Zhang, L.; Igarashi, K.; Yu, C. The anti-obesity and anti-diabetic effects of kaempferol glycosides from unripe soybean leaves in high-fat-diet mice. Food Funct. 2015, 6, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-Y.; Lee, J.-J.; Kim, Y.; Kim, I.-S.; Han, J.-H.; Lee, S.-G.; Ahn, M.-J.; Jung, S.-H.; Myung, C.-S. Effect of eriodictyol on glucose uptake and insulin resistance in vitro. J. Agric. Food Chem. 2012, 60, 7652–7658. [Google Scholar] [CrossRef] [PubMed]
- Bucolo, C.; Leggio, G.M.; Drago, F.; Salomone, S. Eriodictyol prevents early retinal and plasma abnormalities in streptozotocin-induced diabetic rats. Biochem. Pharmacol. 2012, 84, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.-J.; Huang, C.-S.; Mong, M.-C.; Kam, W.-Y.; Huang, H.-Y.; Yin, M.-C. Anti-inflammatory and antifibrotic effects of naringenin in diabetic mice. J. Agric. Food Chem. 2011, 60, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Yokozawa, T.; Oura, H. Improvement of hyperglycemia and hyperlipemia in streptozotocin-diabetic rats by a methanolic extract of Prunus davidiana stems and its main component, prunin. Planta Med. 1991, 57, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Gupta, S.K.; Srinivasan, B.; Nag, T.C.; Srivastava, S.; Saxena, R.; Jha, K.A. Hesperetin rescues retinal oxidative stress, neuroinflammation and apoptosis in diabetic rats. Microvasc. Res. 2013, 87, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, S.; Katsumata, S.; Suzuki, K.; Ishimi, Y.; Wu, J.; Uehara, M. Dietary hesperidin exerts hypoglycemic and hypolipidemic effects in streptozotocin-induced marginal type 1 diabetic rats. J. Clin. Biochem. Nutr. 2010, 46, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.-P.; Sun, H.-L.; Wu, L.-M.; Guo, X.-J.; Dou, H.-L.; Tso, M.O.; Zhao, L.; Li, S.-M. Baicalein reduces inflammatory process in a rodent model of diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2319–2327. [Google Scholar] [CrossRef] [PubMed]
- Ahad, A.; Mujeeb, M.; Ahsan, H.; Siddiqui, W.A. Prophylactic effect of baicalein against renal dysfunction in type 2 diabetic rats. Biochimie 2014, 106, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Pu, P.; Wang, X.-A.; Salim, M.; Zhu, L.-H.; Wang, L.; Xiao, J.-F.; Deng, W.; Shi, H.-W.; Jiang, H.; Li, H.-L. Baicalein, a natural product, selectively activating AMPKα 2 and ameliorates metabolic disorder in diet-induced mice. Mol. Cell. Endocrinol. 2012, 362, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Ahad, A.; Ganai, A.A.; Mujeeb, M.; Siddiqui, W.A. Chrysin, an anti-inflammatory molecule, abrogates renal dysfunction in type 2 diabetic rats. Toxicol. Appl. Pharmacol. 2014, 279, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, U.E.-S. Effect of green tea and green tea rich with catechin on blood glucose levels, serum lipid profile and liver and kidney functions in diabetic rats. Biological 2014, 7, 7–12. [Google Scholar] [CrossRef]
- Stofkova, A. Leptin and adiponectin: From energy and metabolic dysbalance to inflammation and autoimmunity. Endocr. Regul. 2009, 43, 157–168. [Google Scholar] [PubMed]
- Kapoor, R.; Kakkar, P. Protective role of morin, a flavonoid, against high glucose induced oxidative stress mediated apoptosis in primary rat hepatocytes. PLoS ONE 2012, 7, e41663. [Google Scholar] [CrossRef] [PubMed]
- Abuohashish, H.M.; Al-Rejaie, S.S.; Al-Hosaini, K.A.; Parmar, M.Y.; Ahmed, M.M. Alleviating effects of morin against experimentally-induced diabetic osteopenia. Diabetol. Metab. Syndr. 2013, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Vanitha, P.; Uma, C.; Suganya, N.; Bhakkiyalakshmi, E.; Suriyanarayanan, S.; Gunasekaran, P.; Sivasubramanian, S.; Ramkumar, K. Modulatory effects of morin on hyperglycemia by attenuating the hepatic key enzymes of carbohydrate metabolism and β-cell function in streptozotocin-induced diabetic rats. Environ. Toxicol. Pharmacol. 2014, 37, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Dongare, S.; Mathur, R.; Mohanty, I.R.; Srivastava, S.; Mathur, S.; Nag, T.C. Genistein ameliorates cardiac inflammation and oxidative stress in streptozotocin-induced diabetic cardiomyopathy in rats. Mol. Cell. Biochem. 2015, 408, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Young, N.A.; Bruss, M.S.; Gardner, M.; Willis, W.L.; Mo, X.; Valiente, G.R.; Cao, Y.; Liu, Z.; Jarjour, W.N.; Wu, L.-C. Oral administration of nano-emulsion curcumin in mice suppresses inflammatory-induced NF-κB signaling and macrophage migration. PLoS ONE 2014, 9, e111559. [Google Scholar] [CrossRef] [PubMed]
- Wongeakin, N.; Bhattarakosol, P.; Patumraj, S. Molecular mechanisms of curcumin on diabetes-induced endothelial dysfunctions: Txnip, ICAM-1, and NOX2 expressions. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Lee, S.H.; Kim, D.K.; Jin, R.; Jung, D.-S.; Kwak, S.-J.; Kim, S.H.; Han, S.H.; Lee, J.E.; Moon, S.J. Colchicine attenuates inflammatory cell infiltration and extracellular matrix accumulation in diabetic nephropathy. Am. J. Physiol. Ren. Physiol. 2009, 297, F200–F209. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Liu, B.; Wang, K.; Zhou, S.; Li, W.; Xu, Y. Resveratrol ameliorates diabetic vascular inflammation and macrophage infiltration in db/db mice by inhibiting the NF-κB pathway. Diabete Vasc. Dis. Res. 2014, 11, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, O. Cerebroprotective effect of resveratrol through antioxidant and anti-inflammatory effects in diabetic rats. Naunyn Schmiedebergs Arch. Pharmacol. 2013, 386, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, R.; Lv, P.; Yang, J.; Deng, Y.; Xu, J.; Zhu, R.; Zhang, D.; Yang, Y. Emodin upregulates glucose metabolism, decreases lipolysis, and attenuates inflammation in vitro. J. Diabetes 2015, 7, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Ku, S.K.; Lee, D.; Lee, T.; Bae, J.S. Emodin-6-o-β-d-glucoside inhibits high-glucose-induced vascular inflammation. Inflammation 2014, 37, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Salim, S. Oxidative stress and psychological disorders. Curr. Neuropharmacol. 2014, 12, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Zatalia, S.R.; Sanusi, H. The role of antioxidants in the pathophysiology, complications, and management of diabetes mellitus. Acta Med. Indones. 2013, 45, 141–147. [Google Scholar] [PubMed]
- Arts, I.C.; Hollman, P.C. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005, 81, 317S–325S. [Google Scholar] [PubMed]
- Michalak, A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol. J. Environ. Stud. 2006, 15, 523. [Google Scholar]
- Medeiros, K.; Figueiredo, C.; Figueredo, T.; Freire, K.; Santos, F.; Alcantara-Neves, N.; Silva, T.; Piuvezam, M. Anti-allergic effect of bee pollen phenolic extract and myricetin in ovalbumin-sensitized mice. J. Ethnopharmacol. 2008, 119, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Chavez, J.H.; Leal, P.C.; Yunes, R.A.; Nunes, R.J.; Barardi, C.R.; Pinto, A.R.; Simoes, C.M.; Zanetti, C.R. Evaluation of antiviral activity of phenolic compounds and derivatives against rabies virus. Vet. Microbiol. 2006, 116, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Nohynek, L.J.; Alakomi, H.-L.; Kähkönen, M.P.; Heinonen, M.; Helander, I.M.; Oksman-Caldentey, K.-M.; Puupponen-Pimiä, R.H. Berry phenolics: Antimicrobial properties and mechanisms of action against severe human pathogens. Nutr. Cancer 2006, 54, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Hur, H.J.; Lee, H.J.; Lee, C.Y. Antiproliferative effects of dietary phenolic substances and hydrogen peroxide. J. Agric. Food Chem. 2005, 53, 1990–1995. [Google Scholar] [CrossRef] [PubMed]
- Wahle, K.W.; Brown, I.; Rotondo, D.; Heys, S.D. Plant phenolics in the prevention and treatment of cancer. In Bio-Farms for Nutraceuticals; Springer: Aberdeen, UK, 2010; pp. 36–51. [Google Scholar]
- Prakash, D.; Singh, B.N.; Upadhyay, G. Antioxidant and free radical scavenging activities of phenols from onion (Allium cepa). Food Chem. 2007, 102, 1389–1393. [Google Scholar] [CrossRef]
- Catanzaro, D.; Vianello, C.; Ragazzi, E.; Caparrotta, L.; Montopoli, M. Cell cycle control by natural phenols in cisplatin-resistant cell lines. Nat. Prod. Commun. 2014, 9, 1465–1468. [Google Scholar] [PubMed]
- Maheshwari, R.; Dubey, R. Nickel-induced oxidative stress and the role of antioxidant defence in rice seedlings. Plant Growth Regul. 2009, 59, 37–49. [Google Scholar] [CrossRef]
- Montane, J.; Cadavez, L.; Novials, A. Stress and the inflammatory process: A major cause of pancreatic cell death in type 2 diabetes. Diabetes Metab. Syndr. Obes. 2014, 7, 25–34. [Google Scholar] [PubMed]
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Campanero, M.A.; Escolar, M.; Perez, G.; Garcia-Quetglas, E.; Sadaba, B.; Azanza, J.R. Simultaneous determination of diosmin and diosmetin in human plasma by ion trap liquid chromatography-atmospheric pressure chemical ionization tandem mass spectrometry: Application to a clinical pharmacokinetic study. J. Pharm. Biomed. Anal. 2010, 51, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Hertog, M.; Bueno-de-Mesquita, H.B.; Fehily, A.M.; Sweetnam, P.M.; Elwood, P.C.; Kromhout, D. Fruit and vegetable consumption and cancer mortality in the caerphilly study. Cancer Epidemiol. Biomark. Prev. 1996, 5, 673–677. [Google Scholar]
- Navarro-González, J.F.; Mora-Fernández, C.; de Fuentes, M.M.; García-Pérez, J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat. Rev. Nephrol. 2011, 7, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Tan, K. Dyslipidaemia, Inflammation and Endothelial Dysfunction in Diabetes Mellitus. Int. Congr. Ser. 2004, 1262, 511–514. [Google Scholar] [CrossRef]
- Häkkinen, S.H.; Kärenlampi, S.O.; Heinonen, I.M.; Mykkänen, H.M.; Törrönen, A.R. Content of the flavonols quercetin, myricetin, and kaempferol in 25 edible berries. J. Agric. Food Chem. 1999, 47, 2274–2279. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, L.J.; Borradaile, N.M.; Huff, M.W. Antiatherogenic properties of naringenin, a citrus flavonoid. Cardiovasc. Drug Rev. 1999, 17, 160–178. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Erbay, E. Nutrient sensing and inflammation in metabolic diseases. Nat. Rev. Immunol. 2008, 8, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Emim, J.A.D.S.; Oliveira, A.B.; Lapa, A.J. Pharmacological evaluation of the anti-inflammatory activity of a citrus bioflavonoid, hesperidin, and the isoflavonoids, duartin and claussequinone, in rats and mice. Pharm. Pharmacol. 1994, 46, 118–122. [Google Scholar] [CrossRef]
- Kim, Y.O.; Leem, K.; Park, J.; Lee, P.; Ahn, D.-K.; Lee, B.C.; Park, H.K.; Suk, K.; Kim, S.Y.; Kim, H. Cytoprotective effect of Scutellaria baicalensis in CA1 hippocampal neurons of rats after global cerebral ischemia. J. Ethnopharmacol. 2001, 77, 183–188. [Google Scholar] [CrossRef]
- Dhawan, K.; Kumar, S.; Sharma, A. Beneficial effects of chrysin and benzoflavone on virility in 2-year-old male rats. J. Med. Food 2002, 5, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.T.; Ryu, G.-M.; Kwon, B.-M.; Lee, W.-H.; Suk, K. Anti-inflammatory effects of catechols in lipopolysaccharide-stimulated microglia cells: Inhibition of microglial neurotoxicity. Eur. J. Pharmacol. 2008, 588, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Hummasti, S.; Hotamisligil, G.S. Endoplasmic reticulum stress and inflammation in obesity and diabetes. Circ. Res. 2010, 107, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhao, Y.; Suo, S.; Liu, Y.; Zhao, B. Green tea catechins ameliorate adipose insulin resistance by improving oxidative stress. Free Radic. Biol. Med. 2012, 52, 1648–1657. [Google Scholar] [CrossRef] [PubMed]
- Tanti, J.-F.; Ceppo, F.; Jager, J.; Berthou, F. Implication of inflammatory signaling pathways in obesity-induced insulin resistance. Front. Endocrinol. 2013, 3, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Ricardo, K.F.S.; Oliveira, T.T.D.; Nagem, T.J.; Pinto, A.D.S.; Oliveira, M.G.A.; Soares, J.F. Effect of flavonoids morin; quercetin and nicotinic acid on lipid metabolism of rats experimentally fed with triton. Braz. Arch. Boil. Technol. 2001, 44, 263–267. [Google Scholar] [CrossRef]
- Messina, M.; Messina, V. The role of soy in vegetarian diets. Nutrients 2010, 2, 855–888. [Google Scholar] [CrossRef] [PubMed]
- Vinayagam, R.; Xu, B. Antidiabetic properties of dietary flavonoids: A cellular mechanism review. Nutr. Metab. 2015, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhen, W.; Yang, Z.; Carter, J.D.; Si, H.; Reynolds, K.A. Genistein acutely stimulates insulin secretion in pancreatic β-cells through a CAMP-dependent protein kinase pathway. Diabetes 2006, 55, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Yamawaki, H.; Pan, S.; Lee, R.T.; Berk, B.C. Fluid shear stress inhibits vascular inflammation by decreasing thioredoxin-interacting protein in endothelial cells. J. Clin. Investig. 2005, 115, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Kannel, W.B.; McGee, D.L. Diabetes and cardiovascular disease: The Framingham study. JAMA 1979, 241, 2035–2038. [Google Scholar] [CrossRef] [PubMed]
- Fürst, R.; Zündorf, I. Plant-derived anti-inflammatory compounds: Hopes and disappointments regarding the translation of preclinical knowledge into clinical progress. Mediat. Inflamm. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Chow, F.; Ozols, E.; Nikolic-Paterson, D.J.; Atkins, R.C.; Tesch, G.H. Macrophages in mouse type 2 diabetic nephropathy: Correlation with diabetic state and progressive renal injury. Kidney Int. 2004, 65, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.L.; Vincent, A.M.; Cheng, H.T.; Feldman, E.L. Diabetic neuropathy: Mechanisms to management. Pharmacol. Ther. 2008, 120, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Rock, R.B.; Peterson, P.K. Microglia as a pharmacological target in infectious and inflammatory diseases of the brain. J. Neuroimmune Pharmacol. 2006, 1, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, S.; Atif, F.; Ahmad, M.; Hoda, N.; Ishrat, T.; Khan, B.; Islam, F. Resveratrol exerts its neuroprotective effect by modulating mitochondrial dysfunctions and associated cell death during cerebral ischemia. Brain Res. 2009, 1250, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Prasad, S.; Reuter, S.; Kannappan, R.; Yadev, V.R.; Park, B.; Kim, J.H.; Gupta, S.C.; Phromnoi, K.; Sundaram, C.; et al. Identification of novel anti-inflammatory agents from ayurvedic medicine for prevention of chronic diseases: “Reverse pharmacology” and “bedside to bench” approach. Curr. Drug Targets 2011, 12, 1595–1653. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T.; McDougall, G.J.; Alegría, A.; Alminger, M.; Arrigoni, E.; Aura, A.M.; Brito, C.; Cilla, A.; El, S.N.; Karakaya, S. Mind the gap—Deficits in our knowledge of aspects impacting the bioavailability of phytochemicals and their metabolites—A position paper focusing on carotenoids and polyphenols. Mol. Nutr. Food Res. 2015, 59, 1307–1323. [Google Scholar] [CrossRef] [PubMed]
- Svelander, C.A.; Lopez-Sanchez, P.; Pudney, P.D.; Schumm, S.; Alminger, M.A. High pressure homogenization increases the in vitro bioaccessibility of α-and β-carotene in carrot emulsions but not of lycopene in tomato emulsions. J. Food Sci. 2011, 76, H215–H225. [Google Scholar] [CrossRef] [PubMed]
- Epriliati, M.I. Nutriomic Analysis of Fresh and Processed Fruits through the Development of an In Vitro Model of Human Digestive System. Ph.D. Thesis, The University of Queensland, St Lucia, Australia, February 2008. [Google Scholar]
- Williams, A.W.; Boileau, T.W.; Erdman, J.W. Factors influencing the uptake and absorption of carotenoids. Exp. Biol. Med. 1998, 218, 106–108. [Google Scholar] [CrossRef]
- Panozzo, A.; Lemmens, L.; Van Loey, A.; Manzocco, L.; Nicoli, M.C.; Hendrickx, M. Microstructure and bioaccessibility of different carotenoid species as affected by high pressure homogenisation: A case study on differently coloured tomatoes. Food Chem. 2013, 141, 4094–4100. [Google Scholar] [CrossRef] [PubMed]
- Manners, G.D.; Jacob, R.A.; Andrew, P., III; Schoch, T.K.; Hasegawa, S. Bioavailability of citrus limonoids in humans. J. Agric. Food Chem. 2003, 51, 4156–4161. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.J. Human studies on bioavailability and plasma response of lycopene. Exp. Biol. Med. 1998, 218, 115–120. [Google Scholar] [CrossRef]
















| Serial No. | Extraction Methods | Phyto Bioactive Compounds | Plant Parts | Antidiabetic Activities | Reference |
|---|---|---|---|---|---|
| 1 | Ultrasound assisted extraction | Polysaccharides | Mulberry fruits | α-glucosidase inhibition | [62] |
| Polyphenols | Guava leaves | Anti-hyperglycemic | [60] | ||
| Anthocyanins | Berry fruits | Anti-hyperglycemic | [61] | ||
| Crude extract | Heart woods | Anti-diabetic | [63] | ||
| 2 | Microwave assisted extraction | Crude extracts | Night shade leaves | Anti-diabetic | [74] |
| Dried leaves extracts | Aquilaria leaves | Anti-diabetic | [70] | ||
| 3 | Supercritical fluid extraction | Phytol | Toona sinensis leaves | Antidiabetic | [75] |
| Bixin | Annatoo seeds | Anti-hyperglycemic | [76] |
| Class | Compounds | Plant Sources | Mechanism of Actions | Reference |
|---|---|---|---|---|
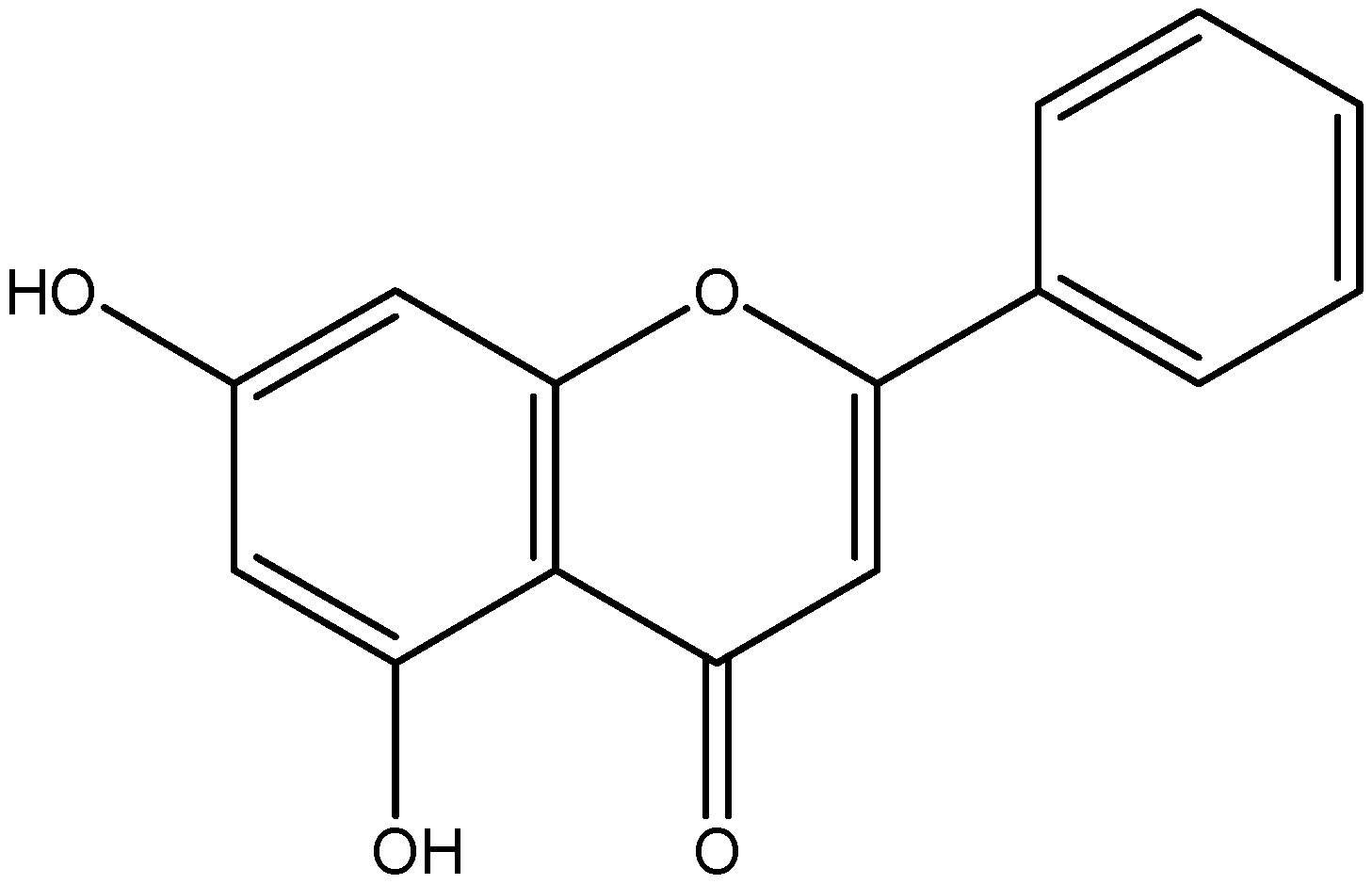
| Flavone | Apigenin | Parsley |
| [96,97] |
| Celery | ||||
| Rosemary | ||||
| Oregano | ||||
| Thyme | ||||
| Basil | ||||
| Coriander | ||||
| Chamomile | ||||
| Cloves | ||||
| Diosmin | Lemon |
| [98] | |
| Orange | ||||
| Buddha fingers | ||||
| Flavonol | Quercetin | Capers |
| [99,100] |
| Onions | ||||
| Cranberries | ||||
| Blueberrie | ||||
| Chokeberris | ||||
| Kaempferol | Tomatoes |
| [101,102] | |
| Green Tea | ||||
| Potatoes | ||||
| Broccoli | ||||
| Brussels | ||||
| Sprouts | ||||
| Squash | ||||
| Eriodictyol | Lemons |
| [103,104] | |
| Mountain balm | ||||
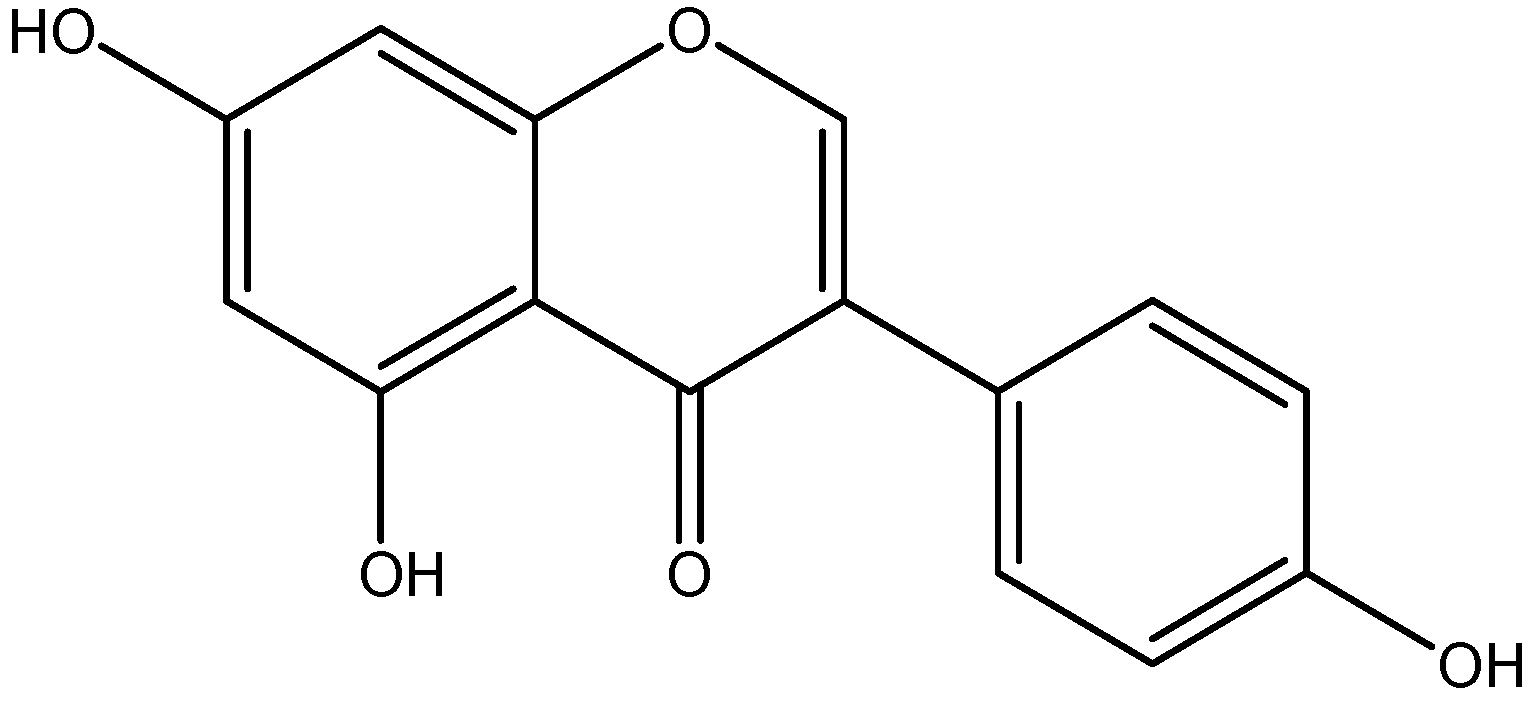
| Flavanone | Naringenin | Grapefruit |
| [105,106] |
| oranges | ||||
| tomatoes | ||||
| Hesperetin | LemonOrange |
| [107,108] | |
| Peppermint | ||||
| Tangerine | ||||
| Baicalein | ParsleyCellery |
| [109,110,111] | |
| Capsicum | ||||
| Pepper | ||||
| Chrysin | Skullcap |
| [112] | |
| Honey | ||||
| Flavanol | Catechin | Green tea |
| [113,114] |
| Chocholate | ||||
| Beans | ||||
| Cherry | ||||
| Morin | Indian guava |
| [115,116,117] | |
| Green tea extract | ||||
| Almond | ||||
| Isoflavonoid | Genistein 4′,5,7-OH | Soy flour |
| [118] |
| Soy milk | ||||
| Soy beans | ||||
| Phenolic acid | Curcumin | Turmeric |
| [119,120] |
| Curry powder | ||||
| Mango Ginger | ||||
| Colchicine | Saffron |
| [121] | |
| Colchicum | ||||
| Stilbene | Resveratrol | Grapes |
| [122,123] |
| Wine | ||||
| Grape | ||||
| Peanuts | ||||
| Cocoa | ||||
| Berries | ||||
| Emodin | Japanese knotweed |
| [124,125] | |
| Rhubarb | ||||
| Buckthorn |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gothai, S.; Ganesan, P.; Park, S.-Y.; Fakurazi, S.; Choi, D.-K.; Arulselvan, P. Natural Phyto-Bioactive Compounds for the Treatment of Type 2 Diabetes: Inflammation as a Target. Nutrients 2016, 8, 461. https://doi.org/10.3390/nu8080461
Gothai S, Ganesan P, Park S-Y, Fakurazi S, Choi D-K, Arulselvan P. Natural Phyto-Bioactive Compounds for the Treatment of Type 2 Diabetes: Inflammation as a Target. Nutrients. 2016; 8(8):461. https://doi.org/10.3390/nu8080461
Chicago/Turabian StyleGothai, Sivapragasam, Palanivel Ganesan, Shin-Young Park, Sharida Fakurazi, Dong-Kug Choi, and Palanisamy Arulselvan. 2016. "Natural Phyto-Bioactive Compounds for the Treatment of Type 2 Diabetes: Inflammation as a Target" Nutrients 8, no. 8: 461. https://doi.org/10.3390/nu8080461