Oral Administration of Fermented Soymilk Products Protects the Skin of Hairless Mice against Ultraviolet Damage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preliminary UVB Irradiation Experiment
2.2. Main UVB Irradiation Experiments
2.2.1. Preparation of FSM
2.2.2. Mice
2.2.3. UV Irradiation
2.2.4. Erythema and Skin Thickness
2.2.5. Enzyme-Linked Immunosorbent Assay for Mouse IL-6
2.2.6. Preparation of Skin Samples
2.2.7. Thymine Dimer Analysis
2.2.8. Lipid Peroxide and Thiobarbituric Acid-Reactive Substance Analysis
2.2.9. Measurement of Isoflavone Concentrations
2.2.10. Statistical Analysis
3. Results
3.1. Preliminary UVB Irradiation Experiment
Effects of Ovariectomy on Erythema
3.2. Main UVB Irradiation Experiments
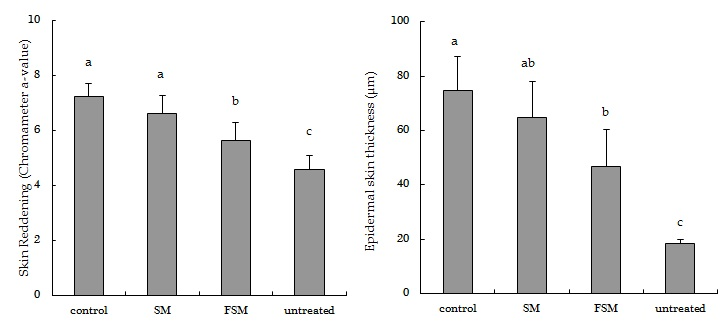
3.2.1. Effects of Soymilk Products on Photodamage
3.2.2. Bioavailability of Isoflavones
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| SM | Soymilk |
| FSM | Fermented soymilk |
| OVX | Ovariectomized |
| TBARS | Thiobarbituric acid-reactive substances |
| UVB | Ultraviolet B |
| O-DMA | O-Desmethylangolensin |
| LPO | Lipid peroxide |
| CPD | Cyclobutane pyrimidine dimer |
| ROS | Reactive oxygen species |
| 6-4PPs | Pyrimidine-pyrimidone photoproducts |
| DHD | Dihydrodaidzein |
References
- Davies, R.E.; Forbes, P.D. Effect of UV radiation on survival of non-haired mice. Photochem. Photobiol. 1986, 43, 267–274. [Google Scholar] [CrossRef] [PubMed]
- De Gruijl, F.R.; Sterenborg, H.J.; Forbes, P.D.; Davies, R.E.; Cole, C.; Kelfkens, G.; van Weelden, H.; Slaper, H.; van der Leun, J.C. Wavelength dependence of skin cancer induction by ultraviolet irradiation of albino hairless mice. Cancer Res. 1993, 53, 53–60. [Google Scholar] [PubMed]
- Lambert, M.W. Ozone depletion: The biologic consequences. J. Am. Acad. Dermatol. 1992, 27, 783–785. [Google Scholar] [CrossRef]
- O'Shaughnessy, J.A.; Kelloff, G.J.; Gordon, G.B.; Dannenberg, A.J.; Hong, W.K.; Fabian, C.J.; Sigman, C.C.; Bertagnolli, M.M.; Stratton, S.P.; Lam, S.; et al. Treatment and prevention of intraepithelial neoplasia: An important target for accelerated new agent development. Clin. Cancer Res. 2002, 8, 314–346. [Google Scholar] [PubMed]
- Stern, R.S. Prevalence of a history of skin cancer in 2007: Results of an incidence-based model. Arch. Dermatol. 2010, 146, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Ratner, D. Cutaneous squamous-cell carcinoma. N. Engl. J. Med. 2001, 344, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, U.; Moore, C.E.; de Spirt, S.; Tronnier, H.; Stahl, W. Green tea polyphenols provide photoprotection, increase microcirculation, and modulate skin properties of women. J. Nutr. 2011, 141, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Stahl, W. Nutritional protection against skin damage from sunlight. Annu. Rev. Nutr. 2004, 24, 173–200. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Heinrich, U.; Jungmann, H.; Sies, H.; Tronnier, H. Carotenoids and carotenoids plus vitamin E protect against ultraviolet light-induced erythema in humans. Am. J. Clin. Nutr. 2000, 7, 795–798. [Google Scholar]
- Kitagawa, S.; Inoue, K.; Teraoka, R.; Morita, S.Y. Enhanced skin delivery of genistein and other two isoflavones by microemulsion and prevention against UV irradiation-induced erythema formation. Chem. Pharm. Bull. (Tokyo) 2010, 58, 398–401. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Saladi, R.; Lu, Y.; Wang, Y.; Palep, S.R.; Moore, J.; Phelps, R.; Shyong, E.; Lebwohl, M.G. Isoflavone genistein: Photoprotection and clinical implications in dermatology. J. Nutr. 2003, 133, 3811S–3819S. [Google Scholar] [PubMed]
- Widyarini, S. Protective effect of the isoflavone equol against DNA damage induced by ultraviolet radiation to hairless mouse skin. J. Vet. Sci. 2006, 7, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Widyarini, S.; Allanson, M.; Gallagher, N.L.; Pedley, J.; Boyle, G.M.; Parsons, P.G.; Whiteman, D.C.; Walker, C.; Reeve, V.E. Isoflavonoid photoprotection in mouse and human skin is dependent on metallothionein. J. Invest. Dermatol. 2006, 126, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.Y.; Qin, L.Q. Soy isoflavones consumption and risk of breast cancer incidence or recurrence: A meta-analysis of prospective studies. Breast Cancer Res. Treat. 2011, 125, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Husain, D.; Khanna, K.; Puri, S.; Haghighizadeh, M. Supplementation of soy isoflavones improved sex hormones, blood pressure, and postmenopausal symptoms. J. Am. Coll. Nutr. 2015, 34, 42–48. [Google Scholar] [PubMed]
- Ishimi, Y. Bone and Nutrition. Effect of isoflavones on bone health. Clin. Calcium. 2015, 25, 999–1005. [Google Scholar] [PubMed]
- Messina, M. Soy foods, isoflavones, and the health of postmenopausal women. Am. J. Clin. Nutr. 2014, 100, 423S–430S. [Google Scholar] [CrossRef] [PubMed]
- Nagata, Y.; Sonoda, T.; Mori, M.; Miyanaga, N.; Okumura, K.; Goto, K.; Naito, S.; Fujimoto, K.; Hirao, Y.; Takahashi, A.; et al. Dietary isoflavones may protect against prostate cancer in Japanese men. J. Nutr. 2007, 137, 1974–1979. [Google Scholar] [PubMed]
- Setchell, K.D.; Cassidy, A. Dietary isoflavones: Biological effects and relevance to human health. J. Nutr. 1999, 129, 758S–767S. [Google Scholar] [PubMed]
- Wang, H.; Murphy, P.A. Isoflavone content in commercial soybean foods. J. Agric. Food Chem. 1994, 42, 1666–1673. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [PubMed]
- Atkinson, C.; Frankenfeld, C.L.; Lampe, J.W. Gut bacterial metabolism of the soy isoflavone daidzein: Exploring the relevance to human health. Exp. Biol. Med. 2005, 230, 155–170. [Google Scholar]
- Tsuji, H.; Moriyama, K.; Nomoto, K.; Miyanaga, N.; Akaza, H. Isolation and characterization of the equol-producing bacterium Slackia sp. strain NATTS. Arch. Microbiol. 2010, 192, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Kano, M.; Takayanagi, T.; Harada, K.; Sawada, S.; Ishikawa, F. Bioavailability of isoflavones after ingestion of soy beverages in healthy adults. J. Nutr. 2006, 136, 2291–2296. [Google Scholar] [PubMed]
- Nagino, T.; Kano, M.; Masuoka, N.; Kaga, C.; Anbe, M.; Miyzaki, K.; Kamachi, K.; Isozaki, M.; Suzuki, C.; Kasuga, C.; et al. Intake of a fermented soymilk beverage containing moderate levels of isoflavone aglycones enhances bioavailability of isoflavones in healthy premenopausal Japanese women: A double-blind, placebo-controlled, single-dose, crossover trial. Biosci. Microbiota Food Health 2016, 35, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [PubMed]
- Ishikawa, F. Probiotic foods expected to prevent life-style derived diseases. Healthist 2002, 150, 69–76. (In Japanese) [Google Scholar]
- Takahashi, Y.; Moriwaki, S.; Sugiyama, Y.; Endo, Y.; Yamazaki, K.; Mori, T.; Takigawa, M.; Inoue, S. Decreased gene expression responsible for post-ultraviolet DNA repair synthesis in aging: A possible mechanism of age-related reduction in DNA repair capacity. J. Invest. Dermatol. 2005, 12, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Kikugawa, K.; Kojima, T.; Yamaki, S.; Kosugi, H. Interpretation of the thiobarbituric acid reactivity of rat liver and brain homogenates in the presence of ferric ion and ethylenediaminetetraacetic acid. Anal. Biochem. 1992, 201, 249–255. [Google Scholar] [CrossRef]
- Busby, M.G.; Jeffcoat, A.R.; Bloedon, L.T.; Koch, M.A.; Black, T.; Dix, K.J.; Heizer, W.D.; Thomas, B.F.; Hill, J.M.; Crowell, J.A.; et al. Clinical characteristics and pharmacokinetics of purified soy isoflavones: Single-dose administration to healthy men. Am. J. Clin. Nutr. 2002, 75, 126–136. [Google Scholar] [PubMed]
- Setchell, K.D. Phytoestrogens: The biochemistry, physiology, and implications for human health of soy isoflavones. Am. J. Clin. Nutr. 1998, 68, 1333S–1346S. [Google Scholar] [PubMed]
- Kim, S.; Kim, S.J.; Lee, J.Y.; Kim, W.G.; Park, W.S.; Sim, Y.C.; Lee, S.J. Protective effects of dietary soy isoflavones against UV-induced skin-aging in hairless mouse model. J. Am. Coll. Nutr. 2004, 23, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Shimoi, K.; Saka, N.; Nozawa, R.; Sato, M.; Amano, I.; Nakayama, T.; Kinae, N. Deglucuronidation of a flavonoid, luteolin monoglucuronide, during inflammation. Drug Metab. Dispos. 2001, 29, 1521–1524. [Google Scholar] [PubMed]
- Shimoi, K.; Nakayama, T. Glucuronidase deconjugation in inflammation. Methods Enzymol. 2005, 400, 263–272. [Google Scholar] [PubMed]
- Cadet, J.; Douki, T.; Pouget, J.P.; Ravanat, J.L. Singlet oxygen DNA damage products: Formation and measurement. Methods Enzymol. 2000, 319, 143–153. [Google Scholar] [PubMed]
- Kulms, D.; Zeise, E.; Pöppelmann, B.; Schwarz, T. DNA damage, death receptor activation and reactive oxygen species contribute to ultraviolet radiation-induced apoptosis in an essential and independent way. Oncogene 2002, 21, 5844–5851. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of photoaging and chronological skin aging. Arch. Dermatol. 2002, 138, 1461–1470. [Google Scholar] [CrossRef]
- Heck, D.E.; Gerecke, D.R.; Vetrano, A.M.; Laskin, J.D. Solar ultraviolet radiation as a trigger of cell signal transduction. Toxicol. Appl. Pharmacol. 2004, 195, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Berlett, B.S.; Stadtman, E.R. Protein oxidation in aging, disease, and oxidative stress. J. Biol. Chem. 1997, 272, 20313–20316. [Google Scholar] [CrossRef] [PubMed]
- De Gruijl, F.R. Photocarcinogenesis: UVA vs. UVB radiation. Skin Pharmacol. Appl. Skin Physiol. 2002, 15, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Thornton, M.J. Estrogens and aging skin. Dermatoendocrinology 2013, 1, 264–270. [Google Scholar] [CrossRef] [PubMed]







| Isoflavones | Free Isoflavone | Total Isoflavone | Ratio of Free Isoflavone | |||
|---|---|---|---|---|---|---|
| SM Group | FSM Group | SM Group | FSM Group | SM Group | FSM Group | |
| nM | nM | % (Free/Total) | ||||
| Genistein | 28.2 ± 17.5 * | 73.8 ± 14.5 | 343.1 ± 187.2 * | 1175.7 ± 333.7 | 8.7 ± 3.6 | 6.5 ± 1.4 |
| Daidzein | 31.3 ± 31.1 * | 99.6 ± 27.0 | 238.2 ± 189.1 * | 619.7 ± 171.6 | 11.0 ± 5.4 | 16.5 ± 4.7 |
| Glycitein | 3.4 ± 2.6 | 4.0 ± 1.0 | 76.1 ± 54.6 | 125.8 ± 39.9 | 6.3 ± 4.2 | 3.2 ± 0.6 |
| DHD | nd | nd | <50 | <50 | ||
| Equol | nd | nd | 1100.4 ± 531.7 | 1429.4 ± 278.3 | ||
| O-DMA | nd | nd | 394.9 ± 377.3 | 255.6 ± 90.9 | ||
| Isoflavones | Free Isoflavone | Total Isoflavone | Ratio of Free Isoflavone | |||
|---|---|---|---|---|---|---|
| SM Group | FSM Group | SM Group | FSM Group | SM Group | FSM Group | |
| pmol/g Skin | pmol/g Skin | % (Free/Total) | ||||
| Genistein | 121.5 ± 28.1 * | 204.7 ± 66.2 | 336.7 ± 170.2 * | 691.1 ± 335.5 | 39.5 ± 8.8 | 31.1 ± 4.3 |
| Daidzein | 38.9 ± 8.8 * | 158.5 ± 84.1 | 110.0 ± 27.7 * | 509.0 ± 381.3 | 35.8 ± 3.2 | 34.3 ± 7.7 |
| Glycitein | 14.3 ± 4.1 * | 46.1 ± 18.5 | 43.3 ± 11.3 | 196.4 ± 159.8 | 33.0 ± 2.3 | 29.8 ± 14.9 |
| DHD | nd | nd | <50 | <50 | ||
| Equol | nd | nd | 313.3 ± 49.6 | 398.5 ± 111.2 | ||
| O-DMA | nd | nd | 106.4 ± 63.0 | 244.2 ± 133.6 | ||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kano, M.; Kubota, N.; Masuoka, N.; Hori, T.; Miyazaki, K.; Ishikawa, F. Oral Administration of Fermented Soymilk Products Protects the Skin of Hairless Mice against Ultraviolet Damage. Nutrients 2016, 8, 514. https://doi.org/10.3390/nu8080514
Kano M, Kubota N, Masuoka N, Hori T, Miyazaki K, Ishikawa F. Oral Administration of Fermented Soymilk Products Protects the Skin of Hairless Mice against Ultraviolet Damage. Nutrients. 2016; 8(8):514. https://doi.org/10.3390/nu8080514
Chicago/Turabian StyleKano, Mitsuyoshi, Norihiro Kubota, Norie Masuoka, Tetsuji Hori, Kouji Miyazaki, and Fumiyasu Ishikawa. 2016. "Oral Administration of Fermented Soymilk Products Protects the Skin of Hairless Mice against Ultraviolet Damage" Nutrients 8, no. 8: 514. https://doi.org/10.3390/nu8080514
APA StyleKano, M., Kubota, N., Masuoka, N., Hori, T., Miyazaki, K., & Ishikawa, F. (2016). Oral Administration of Fermented Soymilk Products Protects the Skin of Hairless Mice against Ultraviolet Damage. Nutrients, 8(8), 514. https://doi.org/10.3390/nu8080514