Effects of Resveratrol on Cognitive Performance, Mood and Cerebrovascular Function in Post-Menopausal Women; A 14-Week Randomised Placebo-Controlled Intervention Trial
Abstract
:1. Introduction
2. Subjects and Methods
2.1. Study Design
2.2. Study Population
2.3. Investigational Product and Allocation
2.4. Screening and Baseline Assessments
2.5. Intervention
2.6. Statistical Analysis
3. Results
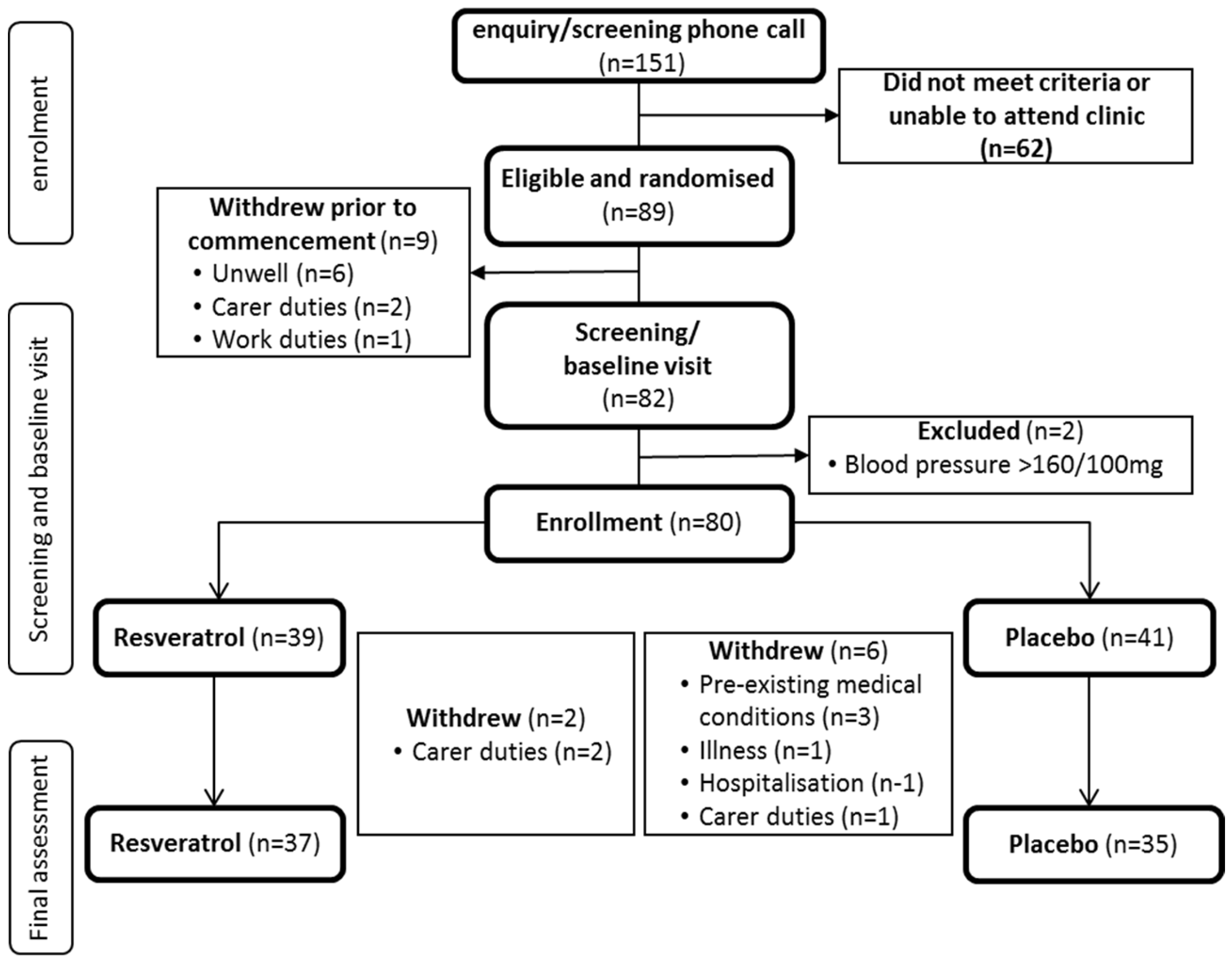
3.1. Participant Disposition
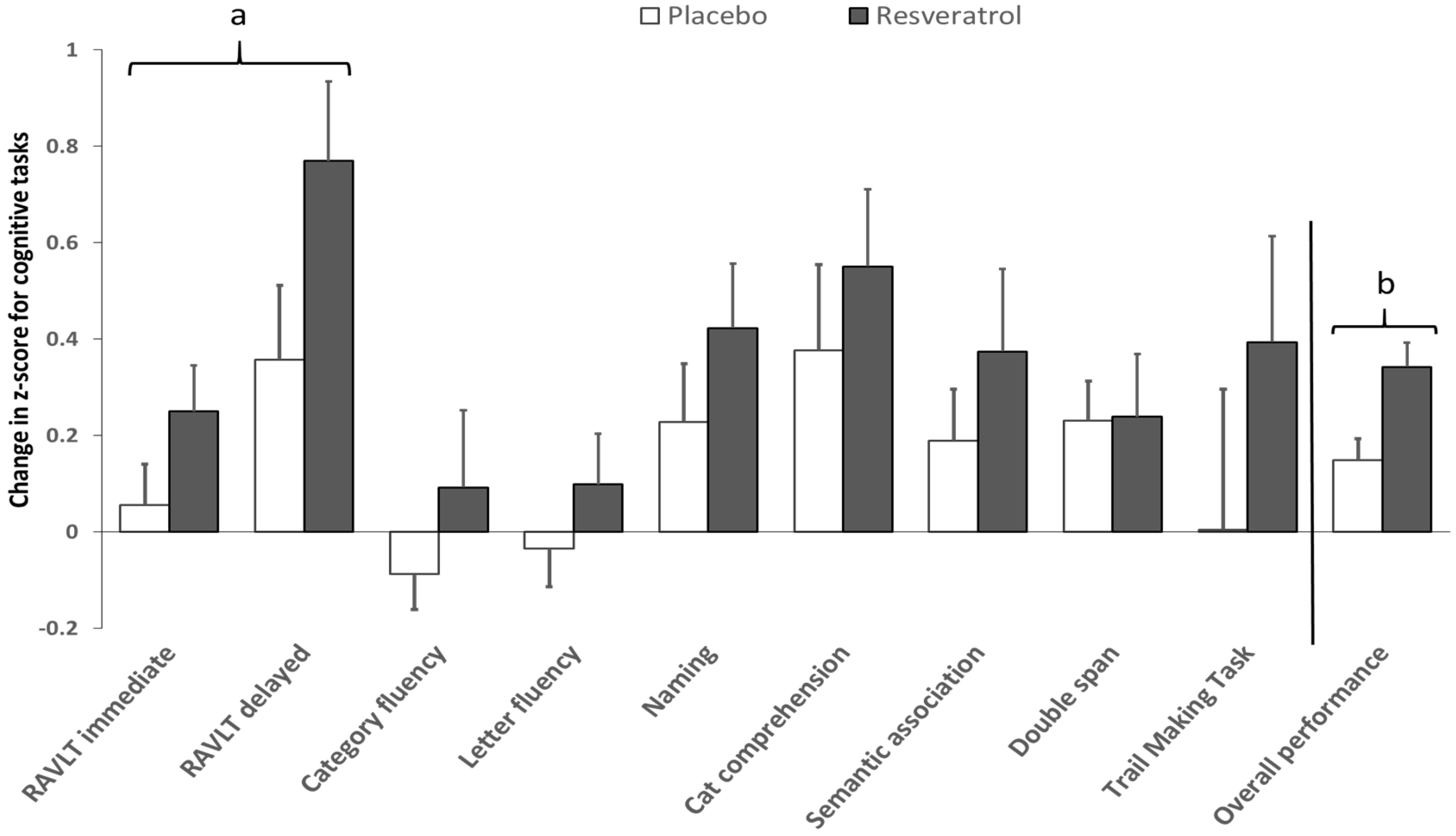
3.2. Cognitive Performance
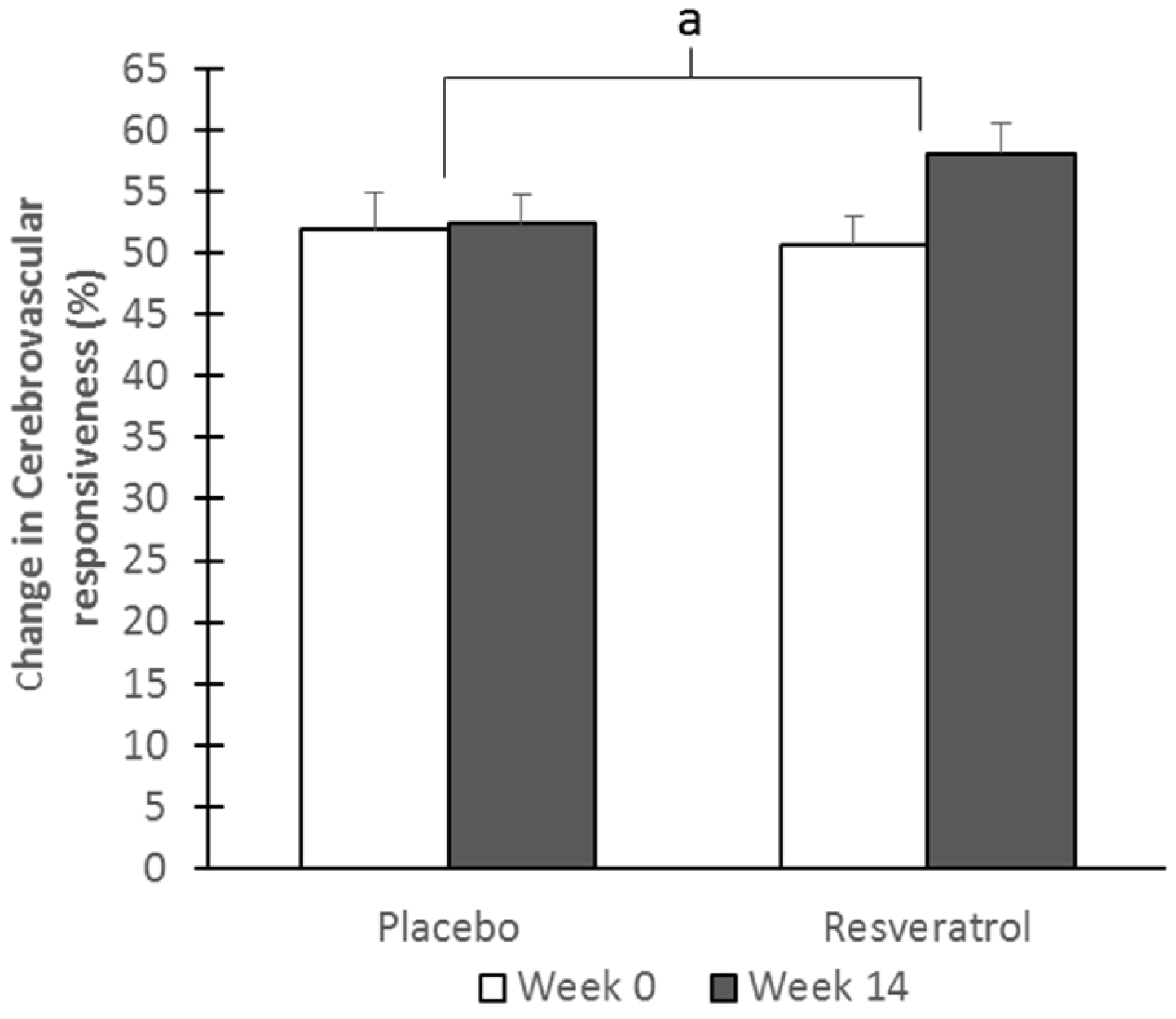
3.3. Cerebrovascular Function
3.4. Mood
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CBF | Cerebral blood flow |
| CVR | Cerebrovascular responsiveness |
| ER | Estrogen receptors |
| ANZCTR | Australian and New Zealand Clinical Trial Registry |
| 3MS | Mini Modified Mental State examination |
| TCD | Transcranial Doppler |
| MCA | Middle cerebral artery |
| RAVLT | Rey Auditory Verbal Learning Test |
| TMT | Trail Making Task |
| POMS | Profile of Mood States |
| CES-D | Centre for Epidemiologic Studies Depression scale |
| ANCOVA | Analysis of covariance |
References
- Prince, M.; Wimo, A.; Geurchet, M.; Ali, G.; Wu, Y.; Prina, M. The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends; Alzhiemer’s Disease International: London, UK, 2015. [Google Scholar]
- Li, R.; Cui, J.; Shen, Y. Brain sex matters: Estrogen in cognition and Alzheimer’s disease. Mol. Cell. Endocrinol. 2014, 389, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.; Stanczyk, F.Z.; Dennerstein, L.; Mack, W.J.; Clark, M.S.; Szoeke, C.; Henderson, V.W. Executive functions in recently post-menopausal women: Absence of strong association with serum gonadal steroids. Brain Res. 2011, 1379, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Craig, M.C.; Brammer, M.; Maki, P.M.; Fletcher, P.C.; Daly, E.M.; Rymer, J.; Giampietro, V.; Picchioni, M.; Stahl, D.; Murphy, D.G.M. The interactive effect of acute ovarian suppression and the cholinergic system on visuospatial working memory in young women. Psychoneuroendocrinology 2010, 35, 987–1000. [Google Scholar] [CrossRef] [PubMed]
- Silvestrini, M.; Pasqualetti, P.; Baruffaldi, R.; Bartolini, M.; Handouk, Y.; Matteis, M.; Moffa, F.; Provinciali, L.; Vernieri, F. Cerebrovascular reactivity and cognitive decline in patients with Alzheimer disease. Stroke 2006, 37, 1010–1015. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.W.; Zhu, Y.X.; Liu, H.Y.; Liu, J.; Zhu, X.Q.; Zhou, J.N.; Liu, R.Y. Decreased cerebral blood flow velocity in apolipoprotein E epsilon4 allele carriers with mild cognitive impairment. Eur. J. Neurol. 2007, 14, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Matteis, M.; Troisi, E.; Monaldo, B.C.; Caltagirone, C.; Silvestrini, M. Age and sex differences in cerebral hemodynamics: A transcranial Doppler study. Stroke 1998, 29, 963–967. [Google Scholar] [CrossRef] [PubMed]
- Bromberger, J.T.; Kravitz, H.M.; Chang, Y.F.; Cyranowski, J.M.; Brown, C.; Matthews, K.A. Major depression during and after the menopausal transition: Study of Women’s Health across the Nation (SWAN). Psychol. Med. 2011, 41, 1879–1888. [Google Scholar] [CrossRef] [PubMed]
- Neu, P.; Schlattmann, P.; Schilling, A.; Hartmann, A. Cerebrovascular reactivity in major depression: A pilot study. Psychosom. Med. 2004, 66, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.T.; Maki, P.M.; McDermott, M.P. Cognition and mood in perimenopause: A systematic review and meta-analysis. J. Steroid Biochem. Mol. Biol. 2014, 142, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.H.X.; Evans, H.M.; Howe, P.R.C. Poor cerebrovascular function is an early marker of cognitive decline in healthy postmenopausal women. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2016, 2, 162–168. [Google Scholar] [CrossRef]
- Xia, N.; Forstermann, U.; Li, H. Resveratrol and endothelial nitric oxide. Molecules 2014, 19, 16102–16121. [Google Scholar] [CrossRef] [PubMed]
- Kodali, M.; Parihar, V.K.; Hattiangady, B.; Mishra, V.; Shuai, B.; Shetty, A.K. Resveratrol prevents age-related memory and mood dysfunction with increased hippocampal neurogenesis and microvasculature, and reduced glial activation. Sci. Rep. 2015, 5, 8075. [Google Scholar] [CrossRef] [PubMed]
- Schmatz, R.; Mazzanti, C.M.; Spanevello, R.; Stefanello, N.; Gutierres, J.; Correa, M.; da Rosa, M.M.; Rubin, M.A.; Chitolina Schetinger, M.R.; Morsch, V.M. Resveratrol prevents memory deficits and the increase in acetylcholinesterase activity in streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 2009, 610, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Dal-Pan, A.; Pifferi, F.; Marchal, J.; Picq, J.L.; Aujard, F.; Consortium, R. Cognitive performances are selectively enhanced during chronic caloric restriction or resveratrol supplementation in a primate. PLoS ONE 2011, 6, e16581. [Google Scholar] [CrossRef] [PubMed]
- Witte, A.V.; Kerti, L.; Margulies, D.S.; Floel, A. Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. J. Neurosci. 2014, 34, 7862–7870. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Wightman, E.L.; Reay, J.L.; Lietz, G.; Okello, E.J.; Wilde, A.; Haskell, C.F. Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: A double-blind, placebo-controlled, crossover investigation. Am. J. Clin. Nutr. 2010, 91, 1590–1597. [Google Scholar] [CrossRef] [PubMed]
- Wightman, E.L.; Reay, J.L.; Haskell, C.F.; Williamson, G.; Dew, T.P.; Kennedy, D.O. Effects of resveratrol alone or in combination with piperine on cerebral blood flow parameters and cognitive performance in human subjects: A randomised, double-blind, placebo-controlled, cross-over investigation. Br. J. Nutr. 2014, 112, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.H.; Nealon, R.S.; Scholey, A.; Howe, P.R. Low dose resveratrol improves cerebrovascular function in type 2 diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Kodl, C.T.; Seaquist, E.R. Cognitive dysfunction and diabetes mellitus. Endocr. Rev. 2008, 29, 494–511. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.H.; Raederstorff, D.; Howe, P.R. Acute resveratrol consumption improves neurovascular coupling capacity in adults with type 2 diabetes mellitus. Nutrients 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G.; Bland, J.M. Treatment allocation by minimisation. BMJ 2005, 330, 843. [Google Scholar] [CrossRef] [PubMed]
- Evans, H.M.; Howe, P.R.; Wong, R.H. Clinical evaluation of effects of chronic resveratrol supplementation on cerebrovascular function, cognition, mood, physical function and general well-being in post-menopausal women-rationale and study design. Nutrients 2016, 8, 150. [Google Scholar] [CrossRef] [PubMed]
- Bravo, G.; Hebert, R. Age- and education-specific reference values for the Mini-Mental and modified Mini-Mental State Examinations derived from a non-demented elderly population. Int. J. Geriatr. Psychiatry 1997, 12, 1008–1018. [Google Scholar] [CrossRef]
- Schmidt, M. Rey Auditory and Verbal Learning Test: A Handbook; Western Psychological Services: Los Angeles, CA, USA, 1996. [Google Scholar]
- Adlam, A.L.; Patterson, K.; Bozeat, S.; Hodges, J.R. The Cambridge semantic memory test battery: Detection of semantic deficits in semantic dementia and Alzheimer’s disease. Neurocase 2010, 16, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Lovato, N.; Lack, L.; Wright, H.; Kemps, E.; Cant, M.; Humphreys, J. A preliminary assessment of the reliability and validity of a computerized working memory task. Percept. Mot. Skills 2013, 116, 368–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Cubillo, I.; Perianez, J.A.; Adrover-Roig, D.; Rodriguez-Sanchez, J.M.; Rios-Lago, M.; Tirapu, J.; Barceló, F. Construct validity of the Trail Making Test: Role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. J. Int. Neuropsychol. Soc. 2009, 15, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Nunes, T.; Almeida, L.; Rocha, J.F.; Falcao, A.; Fernandes-Lopes, C.; Loureiro, A.I.; Wright, L.; Vaz-da-Silva, M.; Soares-da-Silva, P. Pharmacokinetics of trans-resveratrol following repeated administration in healthy elderly and young subjects. J. Clin. Pharmacol. 2009, 49, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Obrist, W.D. Electroencephalographic Changes in Normal Aging and Dementia. In Brain Function in Old Age; Hoffmeister, F., Müller, C., Eds.; Springer: Berlin/Heidelberg, Germany, 1979; pp. 102–111. [Google Scholar]
- Ruitenberg, A.; den Heijer, T.; Bakker, S.L.; van Swieten, J.C.; Koudstaal, P.J.; Hofman, A.; Breteler, M.M. Cerebral hypoperfusion and clinical onset of dementia: The Rotterdam Study. Ann. Neurol. 2005, 57, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.T.; Jung, K.H.; Lee, Y.S. Decreased vasomotor reactivity in Alzheimer’s disease. J. Clin. Neurol. 2007, 3, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Stefani, A.; Sancesario, G.; Pierantozzi, M.; Leone, G.; Galati, S.; Hainsworth, A.H.; Diomedi, M. CSF biomarkers, impairment of cerebral hemodynamics and degree of cognitive decline in Alzheimer’s and mixed dementia. J. Neurol. Sci. 2009, 283, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Sackeim, H.A.; Prohovnik, I.; Moeller, J.R.; Mayeux, R.; Stern, Y.; Devanand, D.P. Regional cerebral blood flow in mood disorders. II. Comparison of major depression and Alzheimer’s disease. J. Nucl. Med. 1993, 34, 1090–1101. [Google Scholar] [PubMed]
- Reinhard, M.; Schwarzer, G.; Briel, M.; Altamura, C.; Palazzo, P.; King, A.; Bornstein, N.M.; Petersen, N.; Motschall, E.; Hetzel, A.; et al. Cerebrovascular reactivity predicts stroke in high-grade carotid artery disease. Neurology 2014, 83, 1424–1431. [Google Scholar] [CrossRef] [PubMed]
- Cantin, S.; Villien, M.; Moreaud, O.; Tropres, I.; Keignart, S.; Chipon, E.; Le Bas, J.F.; Warnking, J.; Krainik, A. Impaired cerebral vasoreactivity to CO2 in Alzheimer’s disease using BOLD fMRI. Neuroimage 2011, 58, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xu, J.; Rottinghaus, G.E.; Simonyi, A.; Lubahn, D.; Sun, G.Y.; Sun, A.Y. Resveratrol protects against global cerebral ischemic injury in gerbils. Brain Res. 2002, 958, 439–447. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, S.; Simonyi, A.; Rottinghaus, G.; Sun, G.Y.; Sun, A.Y. Resveratrol protects against neurotoxicity induced by kainic acid. Neurochem. Res. 2004, 29, 2105–2112. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-Y.; Tan, M.-S.; Yu, J.-T.; Tan, L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med. 2015, 3, 136. [Google Scholar] [PubMed]
- Xia, S.; Zhang, X.; Zheng, S.; Khanabdali, R.; Kalionis, B.; Wu, J.; Wan, W.; Tai, X. An Update on inflamm-aging: Mechanisms, prevention, and treatment. J. Immunol. Res. 2016, 2016, 8426874. [Google Scholar] [CrossRef] [PubMed]
- Woolley, C.S.; McEwen, B.S. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J. Neurosci. 1992, 12, 2549–2554. [Google Scholar] [PubMed]
- Gazzaley, A.H.; Weiland, N.G.; McEwen, B.S.; Morrison, J.H. Differential regulation of NMDAR1 mRNA and protein by estradiol in the rat hippocampus. J. Neurosci. 1996, 16, 6830–6838. [Google Scholar] [PubMed]
| Participants’ Characteristics | Placebo (n = 41) | Resveratrol (n = 38) |
|---|---|---|
| Age (years) | 61.5 ± 1.2 | 61.5 ± 1.1 |
| Years since cessation of menses | 10.9 ± 1.1 | 11.8 ± 1.5 |
| Years of formal education | 15.4 ± 0.6 | 15.5 ± 0.7 |
| 3MS score (%) | 97.8 ± 0.4 | 98.0 ± 0.3 |
| BMI (Body Mass Index) (kg/m2) | 26.6 ± 0.8 | 26.8 ± 0.8 |
| Waist circumference (cm) | 87.0 ± 1.4 | 87.5 ± 1.7 |
| Systolic blood pressure (mmHg) | 125.9 ± 2.1 | 124.7 ± 2.2 |
| Diastolic blood pressure (mmHg) | 69.5 ± 1.4 | 72.4 ± 1.3 |
| Basal mean blood flow velocity (cm/s) | 50.3 ± 2.1 | 48.4 ± 1.8 |
| Pulsatility index | 0.8 ± 0.1 | 0.8 ± 0.1 |
| Week 0 (V1) | Week 14 (V2) | Δ (V2 − V1) | Placebo vs. Resveratrol | |||||
|---|---|---|---|---|---|---|---|---|
| Memory Domain Component Tasks | Placebo (n = 41) | Resveratrol (n = 38) | Placebo (n = 41) | Resveratrol (n = 38) | Placebo (n = 41) | Resveratrol (n = 38) | p-Value (Unadjusted) | p-Value (CES-D Covariate) |
| Verbal Memory | 50.7 ± 1.2 | 51.3 ± 1.1 | 53.2 ± 1.0 | 56.5 ± 1.1 | 2.5 ± 0.8 | 5.2 ± 0.9 | 0.024 * | 0.021 * |
| RAVLT immediate | 52.0 ± 0.8 | 52.3 ± 0.9 | 53.9 ± 0.7 | 55.5 ± 0.8 | 1.8 ± 0.6 | 3.2 ± 0.7 | 0.136 | 0.135 |
| Learning | 66.3 ± 1.7 | 71.0 ± 2.0 | 73.6 ± 1.8 | 78.0 ± 1.9 | 7.3 ± 1.0 | 7.0 ± 1.2 | 0.837 | 0.830 |
| Proactive memory | 46.1 ± 1.2 | 43.1 ± 1.3 | 43.8 ± 1.2 | 44.5 ± 1.5 | −2.3 ± 1.6 | 1.4 ± 1.4 | 0.160 | 0.155 |
| Retroactive interference | 43.8 ± 1.1 | 42.7 ± 1.1 | 44.2 ± 1.2 | 44.0 ± 1.0 | 0.4 ± 1.3 | 1.3 ± 1.4 | 0.630 | 0.637 |
| RAVLT delayed | 49.3 ± 1.6 | 50.3 ± 1.6 | 52.5 ± 1.4 | 57.5 ± 1.7 | 3.2 ± 1.2 | 7.2 ± 1.4 | 0.035 * | 0.029 * |
| Delayed recall | 42.3 ± 1.3 | 41.4 ± 1.5 | 43.0 ± 1.2 | 45.4 ± 1.2 | 0.7 ± 1.1 | 3.9 ± 1.8 | 0.116 | 0.110 |
| Delayed recognition | 56.3 ± 2.3 | 59.1 ± 2.3 | 62.1 ± 2.0 | 69.6 ± 2.6 | 5.8 ± 2.0 | 10.5 ± 2.0 | 0.097 | 0.073 |
| Semantic Memory | 75.6 ± 0.7 | 75.6 ± 0.8 | 75.9 ± 0.7 | 76.9 ± 0.8 | 0.3 ± 0.3 | 1.3 ± 0.4 | 0.032 * | 0.150 |
| Category fluency | 48.7 ± 1.3 | 48.3 ± 2.0 | 48.0 ± 1.5 | 49.2 ± 1.8 | −0.7 ± 0.7 | 0.9 ± 1.0 | 0.239 | 0.293 |
| Letter fluency | 45.2 ± 2.3 | 46.4 ± 1.8 | 45.1 ± 2.0 | 48.0 ± 2.0 | −0.1 ± 1.0 | 1.6 ± 1.4 | 0.320 | 0.348 |
| Naming | 97.0 ± 0.3 | 95.9 ± 0.5 | 97.6 ± 0.3 | 97.1 ± 0.4 | 0.6 ± 0.3 | 1.2 ± 0.4 | 0.292 | 0.290 |
| Category comprehension | 99.0 ± 0.2 | 99.0 ± 0.2 | 99.4 ± 0.2 | 99.5 ± 0.1 | 0.4 ± 0.2 | 0.6 ± 0.2 | 0.475 | 0.587 |
| Camel and cactus | 88.0 ± 0.8 | 87.6 ± 1.1 | 89.1 ± 0.7 | 89.9 ± 0.7 | 1.8 ± 0.6 | 2.3 ± 1.0 | 0.353 | 0.410 |
| Visuospatial Working Memory | ||||||||
| Double Span | 85.9 ± 0.8 | 86.0 ± 1.3 | 87.4 ± 1.0 | 87.5 ± 1.2 | 1.5 ± 0.7 | 1.6 ± 1.1 | 0.965 | 0.987 |
| Executive Function | ||||||||
| Trail Making Task 1 | ||||||||
| Time taken A (s) | 34.9 ± 1.2 | 36.0 ± 0.9 | 33.1 ± 1.2 | 33.1 ± 1.1 | −1.9 ± 1.1 | −2.9 ± 1.0 | 0.512 | 0.498 |
| Number of Errors A | 0.2 ± 0.1 | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.1 ± 0.1 | −0.1 ± 0.1 | −0.1 ± 0.1 | 0.973 | 0.959 |
| Time taken B (s) | 72.7 ± 2.9 | 75.4 ± 3.8 | 70.5 ± 2.8 | 68.6 ± 3.1 | −2.2 ± 2.9 | −6.9 ± 2.6 | 0.236 | 0.215 |
| Number of Errors B | 0.5 ± 0.1 | 0.7 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.1 | 0.1 ± 0.1 | −0.4 ± 0.2 | 0.043 * | 0.084 |
| Interference (ratio) | 2.1 ± 0.1 | 2.1 ± 0.1 | 2.2 ± 0.1 | 2.0 ± 0.1 | 0.1 ± 0.1 | −0.1 ± 0.1 | 0.578 | 0.602 |
| Overall Cognitive Performance | 70.6 ± 0.6 | 70.8 ± 0.7 | 71.6 ± 0.6 | 73.1 ± 0.7 | 1.0 ± 0.3 | 2.3 ± 0.3 | 0.004 * | 0.018 * |
| Week 0 (V1) | Week 14 (V2) | Δ (V2 − V1) | Placebo vs. Resveratrol | |||||
|---|---|---|---|---|---|---|---|---|
| Outcome Measure | Placebo (n = 33) | Resveratrol (n = 28) | Placebo (n = 33) | Resveratrol (n = 28) | Placebo (n = 33) | Resveratrol (n = 28) | p-Value | Adjusted p-Value (Age) |
| RAVLT immediate | 16.8 ± 1.6 | 17.4 ± 2.3 | 16.4 ± 1.5 | 21.9 ± 2.2 | −0.4 ± 1.6 | 4.5 ± 1.3 | 0.020 * | 0.033 |
| RAVLT delayed | 12.9 ± 1.5 | 15.2 ± 1.4 | 13.5 ± 1.3 | 16.5 ± 1.7 | 0.6 ± 1.0 | 1.3 ± 1.2 | 0.686 | 0.695 |
| Category fluency | 16.1 ± 1.6 | 6.9 ± 1.9 | 14.6 ± 0.9 | 10.1 ± 1.7 | −1.4 ± 0.9 | 3.2 ± 1.7 | 0.016 * | 0.013 * |
| Letter fluency | 9.4 ± 1.9 | 9.6 ± 1.8 | 7.4 ± 1.4 | 19.3 ± 1.7 | −1.9 ± 1.9 | −0.3 ± 1.7 | 0.449 | 0.415 |
| Naming | 7.4 ± 1.8 | 9.7 ± 1.7 | 8.9 ± 1.4 | 17.3 ± 1.5 | −1.5 ± 1.6 | −2.4 ± 1.6 | 0.097 | 0.088 |
| Category comprehension | 11.4 ± 1.6 | 9.1 ± 1.5 | 12.2 ± 1.7 | 9.3 ± 1.4 | 0.8 ± 1.6 | 0.2 ± 1.3 | 0.761 | 0.676 |
| Camel and cactus | 7.7 ± 1.5 | 5.1 ± 1.2 | 4.8 ± 1.0 | 8.0 ± 1.5 | −2.9 ± 1.5 | 2.8 ± 1.2 | 0.006 * | 0.007 * |
| Double Span | 5.3 ± 1.1 | 9.5 ± 1.5 | 6.3 ± 1.3 | 9.6 ± 1.4 | 1.0 ± 1.1 | 0.1 ± 1.4 | 0.606 | 0.542 |
| TMT | 14.0 ± 2.0 | 15.8 ± 1.8 | 11.8 ± 1.9 | 17.9 ± 2.1 | −2.2 ± 1.3 | 2.1 ± 1.7 | 0.047 | 0.046 |
| Overall cognitive CVR | 9.6 ± 0.9 | 10.8 ± 0.8 | 9.2 ± 0.8 | 12.6 ± 1.0 | −0.4 ± 0.5 | 1.7 ± 0.5 | 0.008 * | 0.002 * |
| Week 0 (V1) | Week 14 (V2) | Δ (V2 − V1) | Placebo vs. Resveratrol | ||||
|---|---|---|---|---|---|---|---|
| Mood Questionnaire Component | Placebo (n = 41) | Resveratrol (n = 38) | Placebo (n = 41) | Resveratrol (n = 38) | Placebo (n = 41) | Resveratrol (n = 38) | p-Value |
| CES-D † | 8.2 ± 1.2 | 8.5 ± 1.2 | 9.7 ± 1.4 | 7.74 ± 1.17 | 1.5 ± 1.4 | −0.8 ± 0.9 | 0.104 |
| POMS | 10.7 ± 3.9 | 11.5 ± 4.4 | 8.6 ± 4.8 | 3.0 ± 4.0 | −2.0 ± 2.6 | −8.5 ± 2.5 | 0.085 |
| Anxiety | 6.4 ± 0.8 | 7.4 ± 1.0 | 6.2 ± 0.8 | 5.2 ± 0.8 | −0.3 ± 0.6 | −2.2 ± 0.6 | 0.025 * |
| Depression | 4.2 ± 0.9 | 5.6 ± 1.1 | 4.1 ± 1.2 | 4.3 ± 0.9 | −0.2 ± 0.9 | −1.4 ± 0.7 | 0.290 |
| Anger | 5.0 ± 1.0 | 5.5 ± 1.0 | 4.2 ± 1.0 | 4.0 ± 0.9 | −0.8 ± 0.7 | −1.5 ± 0.7 | 0.458 |
| Fatigue | 6.6 ± 1.0 | 5.9 ± 0.8 | 6.7 ± 1.1 | 4.6 ± 0.8 | 0.1 ± 0.7 | −1.3 ± 9.7 | 0.159 |
| Confusion | 5.4 ± 0.6 | 5.3 ± 0.6 | 5.2 ± 0.6 | 4.5 ± 0.5 | −0.2 ± 0.4 | −0.7 ± 0.4 | 0.404 |
| Vigour ‡ | −17.0 ± 1.0 | −18.0 ± 1.0 | −17.8 ± 1.1 | −19.5 ± 1.0 | −0.8 ± 0.7 | −1.5 ± 0.9 | 0.486 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evans, H.M.; Howe, P.R.C.; Wong, R.H.X. Effects of Resveratrol on Cognitive Performance, Mood and Cerebrovascular Function in Post-Menopausal Women; A 14-Week Randomised Placebo-Controlled Intervention Trial. Nutrients 2017, 9, 27. https://doi.org/10.3390/nu9010027
Evans HM, Howe PRC, Wong RHX. Effects of Resveratrol on Cognitive Performance, Mood and Cerebrovascular Function in Post-Menopausal Women; A 14-Week Randomised Placebo-Controlled Intervention Trial. Nutrients. 2017; 9(1):27. https://doi.org/10.3390/nu9010027
Chicago/Turabian StyleEvans, Hamish M., Peter R. C. Howe, and Rachel H. X. Wong. 2017. "Effects of Resveratrol on Cognitive Performance, Mood and Cerebrovascular Function in Post-Menopausal Women; A 14-Week Randomised Placebo-Controlled Intervention Trial" Nutrients 9, no. 1: 27. https://doi.org/10.3390/nu9010027






