The Root Extract of Pueraria lobata and Its Main Compound, Puerarin, Prevent Obesity by Increasing the Energy Metabolism in Skeletal Muscle
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparations
2.2. HPLC Analysis
2.3. Animals and Experimental Design
2.4. Glucose Tolerance Test
2.5. Serum Analysis
2.6. Histological Analysis
2.7. Lipid Accumulation Analysis in Liver
2.8. C2C12 Cell Cultures and Treatment
2.9. Western Blot
2.10. Immunofluorescence Analysis
2.11. ATP Contents
2.12. Statistical Analysis
3. Results
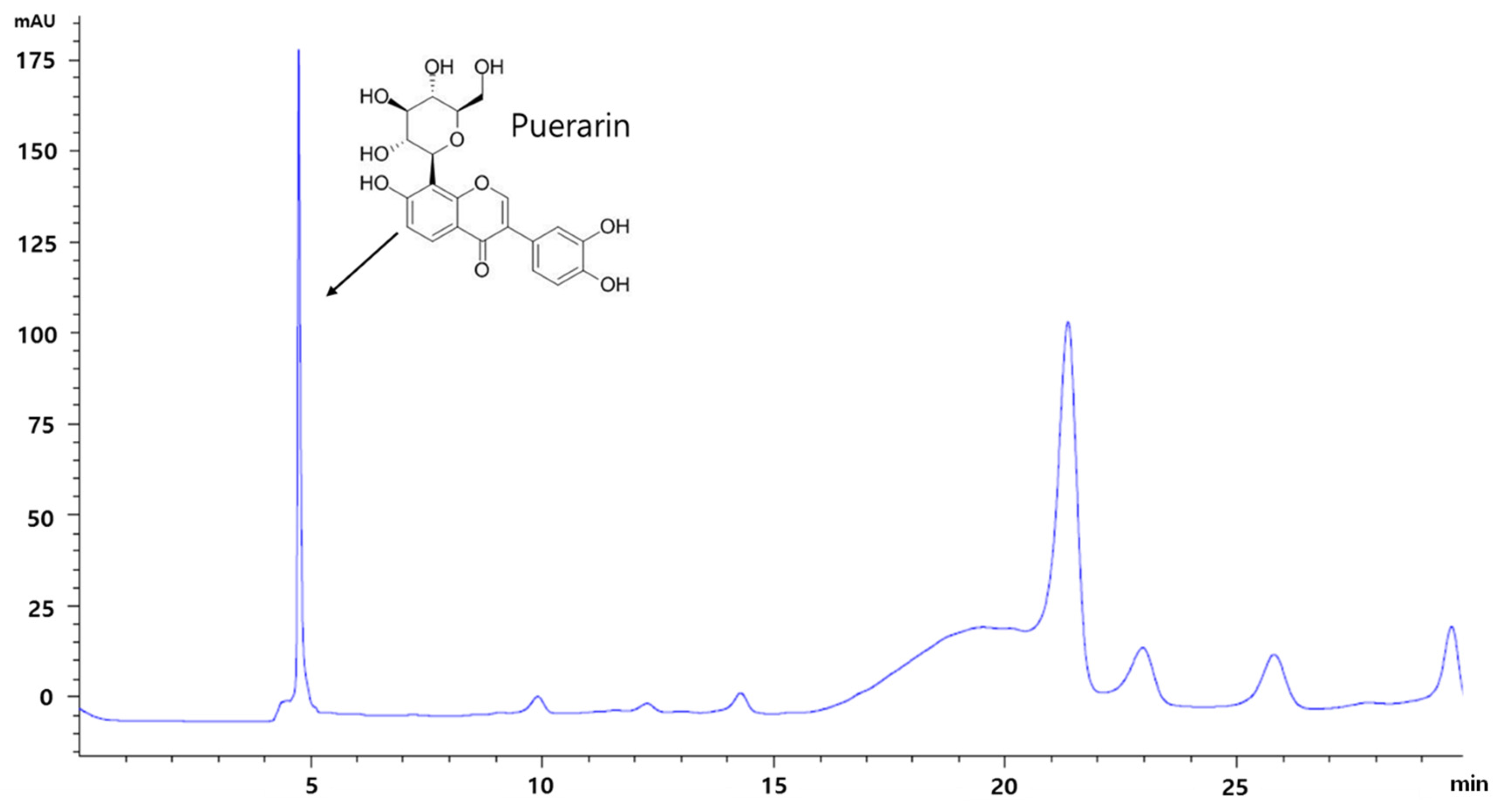
3.1. HPLC Analysis of RP Extract
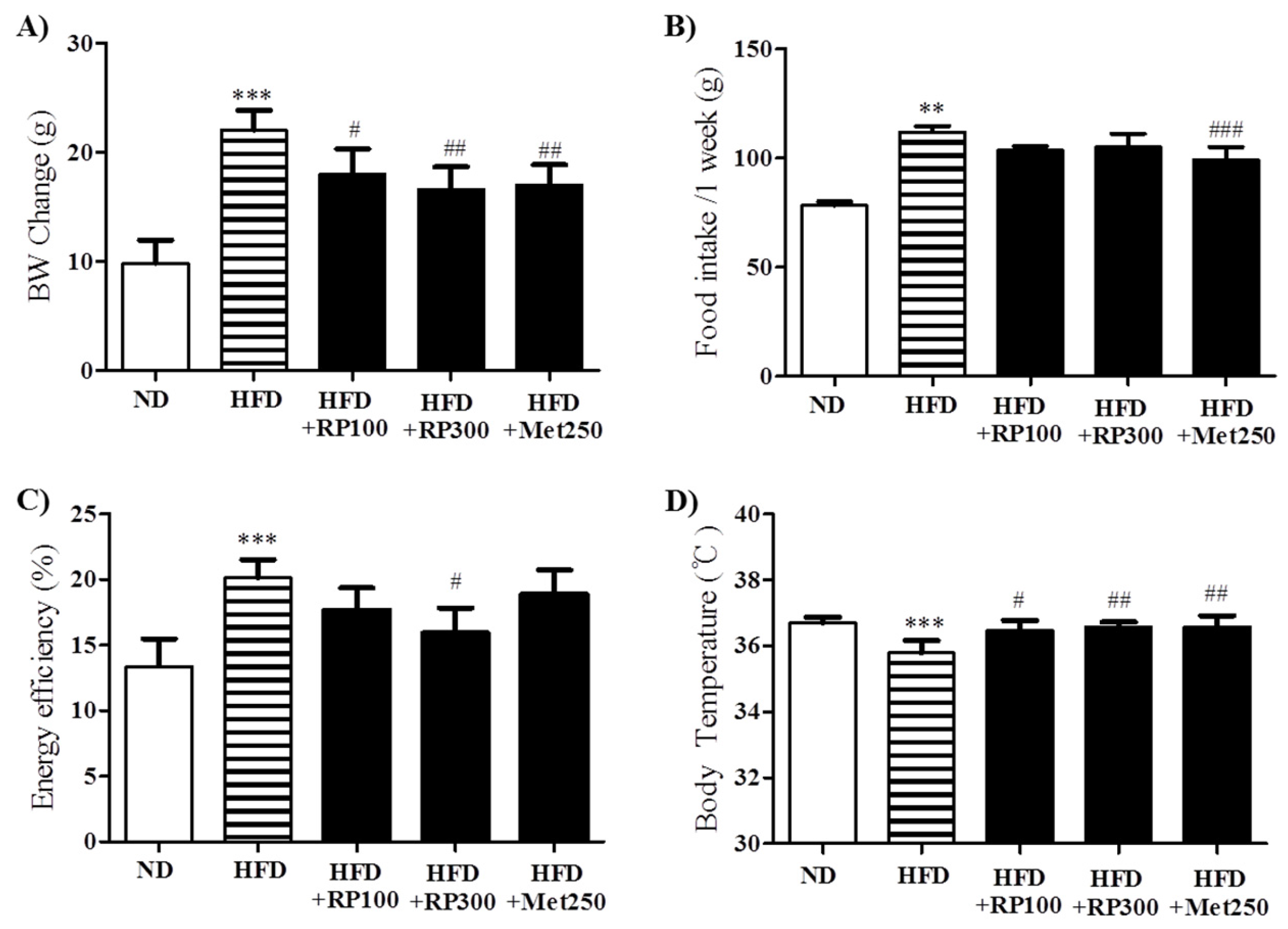
3.2. RP Extract Reduced Body Weight Gain
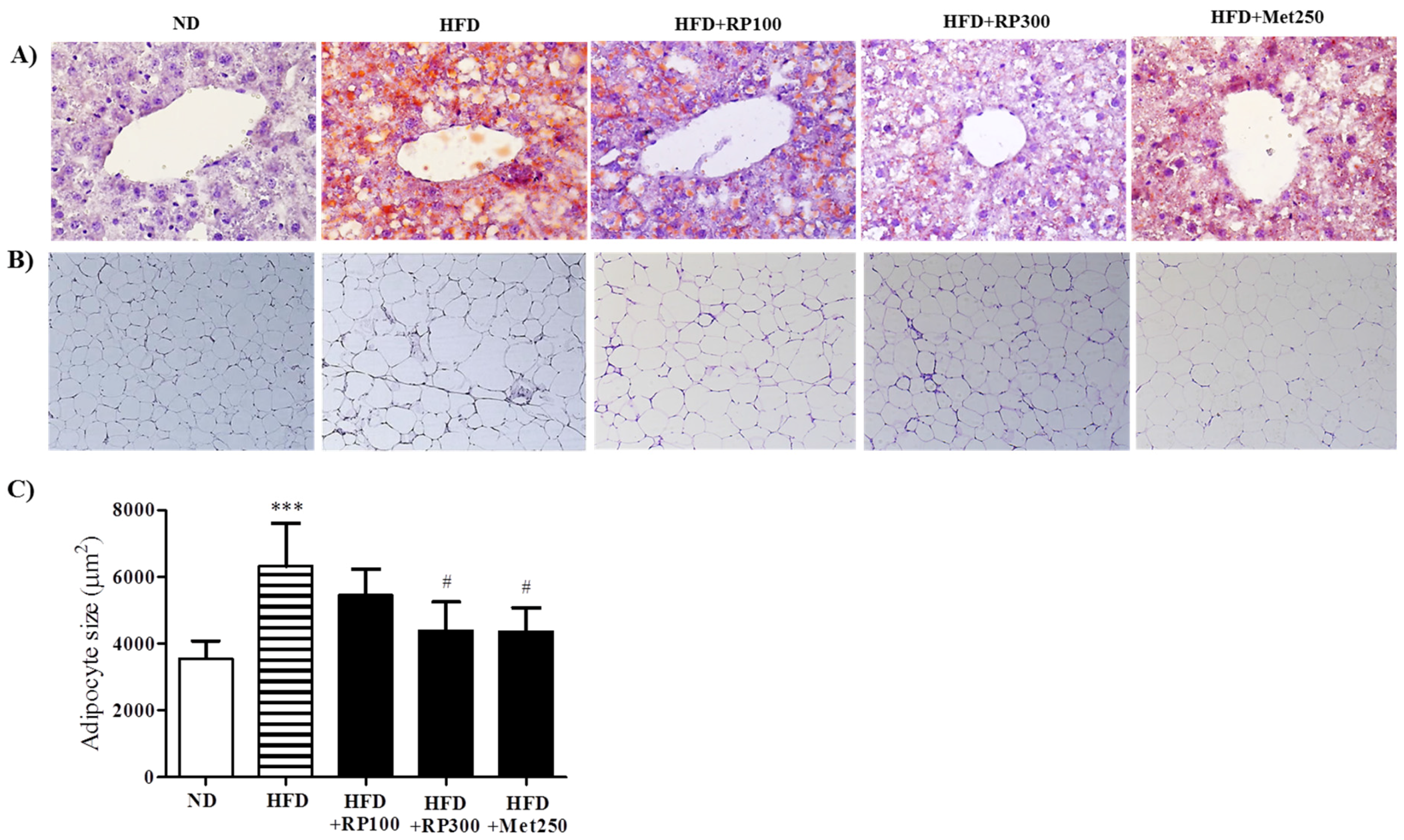
3.3. RP Extract Reduced Obesity-Induced Lipid Accumulation
3.4. RP Extract Improved Obesity-Induced Glucose Tolerance
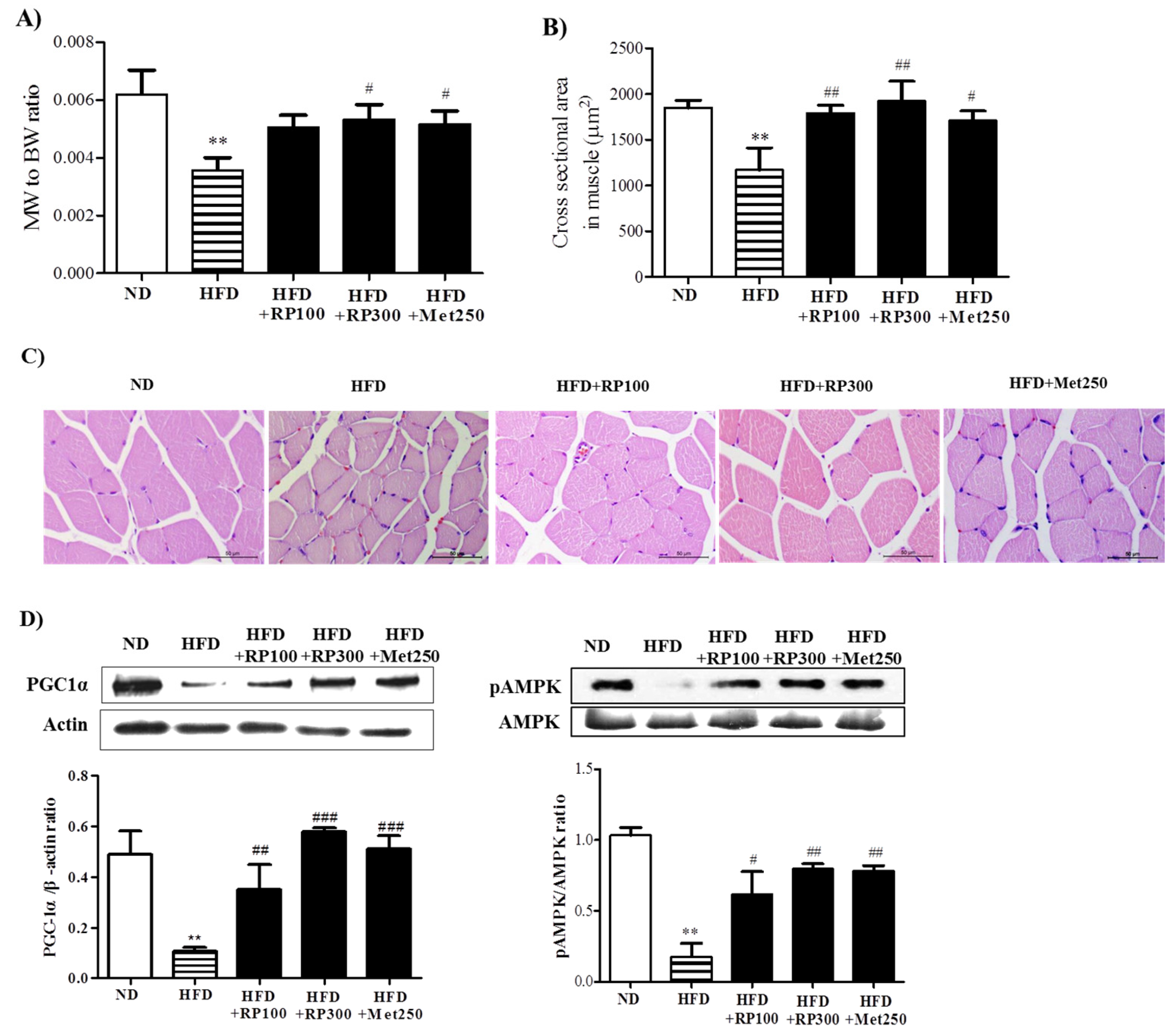
3.5. RP Extract Protected against Obesity-Induced Skeletal Muscle Atrophy and Improved Energy Metabolism
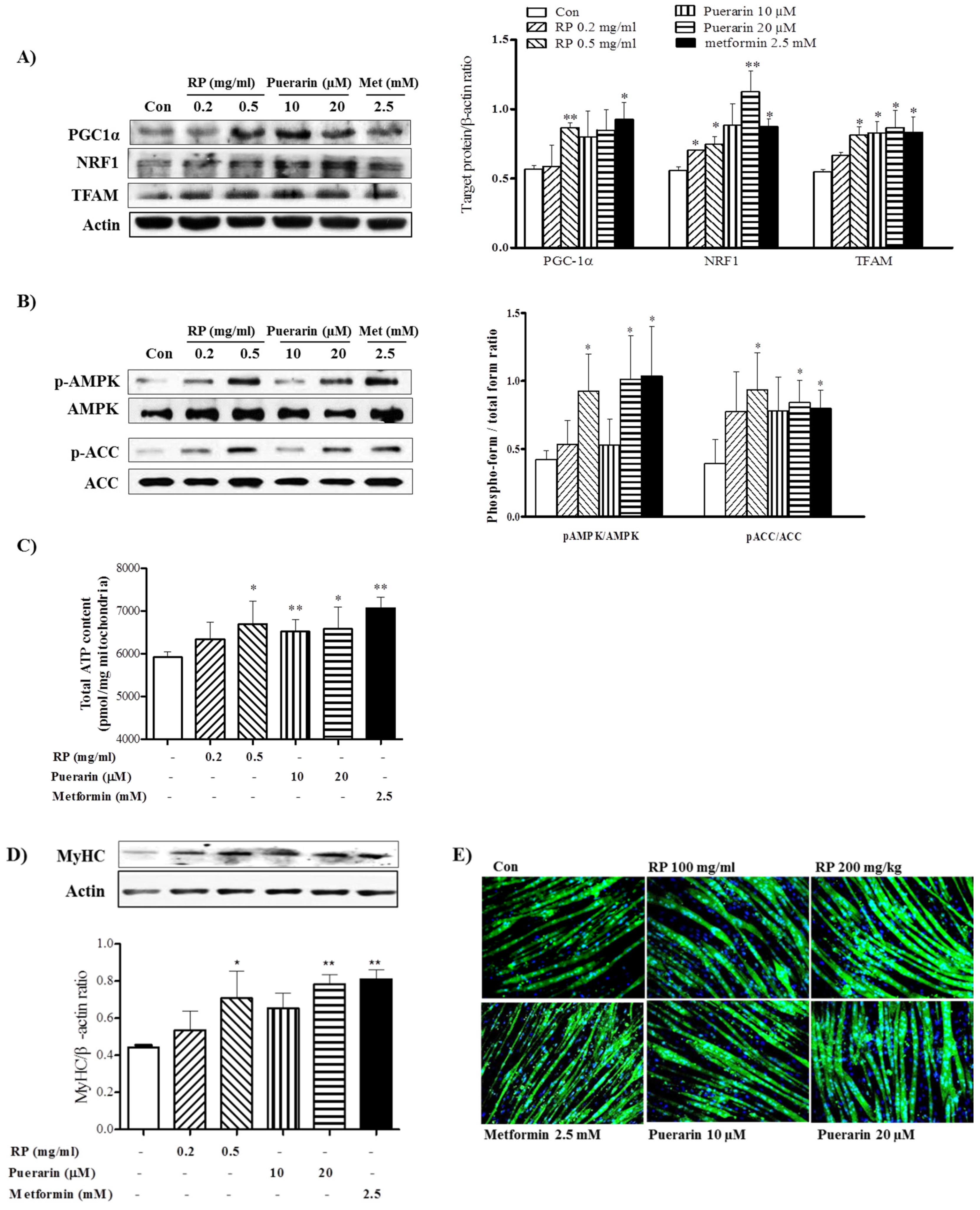
3.6. RP Extract and Puerarin Improved Mitochondrial Biogenesis and Myotube Hypertrophy in C2C12 Skeletal Muscle Cells
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Swinburn, B.A.; Sacks, G.; Hall, K.D.; McPherson, K.; Finegood, D.T.; Moodie, M.L.; Gortmaker, S.L. The global obesity pandemic: Shaped by global drivers and local environments. Lancet 2011, 378, 804–814. [Google Scholar] [CrossRef]
- Obesity and Overweight, Fact Sheet. Available online: http://www.who.int/mediacentre /factsheets/fs311/en (accessed on 3 October 2016).
- Muoio, D.M.; Newgard, C.B. Obesity-Related derangements in metabolic regulation. Annu. Rev. Biochem 2006, 75, 367–401. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.-H.; Cypess, A.M.; Kahn, C.R. Cellular bioenergetics as a target for obesity therapy. Nat. Rev. Drug Discov. 2010, 9, 465–482. [Google Scholar] [CrossRef] [PubMed]
- Krssak, M.; Petersen, K.F.; Dresner, A.; DiPietro, L.; Vogel, S.; Rothman, D.; Shulman, G.; Roden, M. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: A 1 h nmr spectroscopy study. Diabetologia 1999, 42, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Greco, A.V.; Mingrone, G.; Giancaterini, A.; Manco, M.; Morroni, M.; Cinti, S.; Granzotto, M.; Vettor, R.; Camastra, S.; Ferrannini, E. Insulin resistance in morbid obesity reversal with intramyocellular fat depletion. Diabetes 2002, 51, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.; Wells, J.; Smith, S.; Stephan, B.; Siervo, M. Sarcopenic obesity: A critical appraisal of the current evidence. Clin. Nutr. 2012, 31, 583–601. [Google Scholar] [CrossRef] [PubMed]
- Srikanthan, P.; Hevener, A.L.; Karlamangla, A.S. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: Findings from the National Health and Nutrition Examination Survey III. PLoS ONE 2010, 5, e10805. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Ward, W.F. Pgc-1α: A key regulator of energy metabolism. Adv. Physiol. Eudc. 2006, 30, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M.; Lin, J.; Handschin, C.; Yang, W.; Arany, Z.P.; Lecker, S.H.; Goldberg, A.L.; Spiegelman, B.M. PGC-1α protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc. Natl. Acad. Sci. USA 2006, 103, 16260–16265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, H.-J.; Brandauer, J.; Goodyear, L.J. LKB1 and AMPK and the regulation of skeletal muscle metabolism. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 227. [Google Scholar] [CrossRef] [PubMed]
- Jäger, S.; Handschin, C.; St.-Pierre, J.; Spiegelman, B.M. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. Proc. Natl. Acad. Sci. USA 2007, 104, 12017–12022. [Google Scholar]
- Zhang, Z.; Lam, T.N.; Zuo, Z. Radix puerariae: An overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J. Clin. Pharmacol. 2013, 53, 787–811. [Google Scholar] [CrossRef] [PubMed]
- Keung, W.M.; Vallee, B.L. Kudzu root: An ancient chinese source of modern antidipsotropic agents. Phytochemistry 1998, 47, 499–506. [Google Scholar] [CrossRef]
- Prasain, J.K.; Peng, N.; Rajbhandari, R.; Wyss, J.M. The Chinese Pueraria root extract (Pueraria lobata) ameliorates impaired glucose and lipid metabolism in obese mice. Phytomed. Int. J. Phytother. Phytopharmacol. 2012, 20, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Lin, L.; Zhong, S.; Zhang, Q.; Li, D. Effects of puerarin on lipid accumulation and metabolism in high-fat diet-fed mice. PLoS ONE 2015, 10, e0122925. [Google Scholar] [CrossRef] [PubMed]
- Song, M.Y. The effects of cinnamomum cassia blume, aconitum carmichaeli debx, and pueraria lobata benth on glucose and energy metabolism in C2C12 myotubes. J. Korean Med. Obes. Res. 2015, 15, 131–136. [Google Scholar] [CrossRef]
- Parlee, S.D.; Lentz, S.I.; Mori, H.; MacDougald, O.A. Quantifying size and number of adipocytes in adipose tissue. Methods Enzymol. 2014, 537, 93. [Google Scholar] [PubMed]
- Cantó, C.; Auwerx, J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 2009, 20, 98. [Google Scholar] [CrossRef] [PubMed]
- Zurlo, F.; Larson, K.; Bogardus, C.; Ravussin, E. Skeletal muscle metabolism is a major determinant of resting energy expenditure. J. Clin. Investig. 1990, 86, 1423. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, E.; Simonson, D.C.; Katz, L.D.; Reichard, G.; Bevilacqua, S.; Barrett, E.J.; Olsson, M.; DeFronzo, R.A. The disposal of an oral glucose load in patients with non-insulin-dependent diabetes. Metabolism 1988, 37, 79–85. [Google Scholar] [CrossRef]
- Atlantis, E.; Martin, S.A.; Haren, M.T.; Taylor, A.W.; Wittert, G.A. Inverse associations between muscle mass, strength, and the metabolic syndrome. Metabolism 2009, 58, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.M.; Campbell, F.M.; Drew, J.E.; Koch, C.; Hoggard, N.; Rees, W.D.; Kamolrat, T.; Ngo, H.T.; Steffensen, I.-L.; Gray, S.R. The development of diet-induced obesity and glucose intolerance in C57Bl/6 mice on a high-fat diet consists of distinct phases. PLoS ONE 2014, 9, e106159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Yoo, J.E.; Jung, E.H.; Yoo, D.Y. Effects of Pueraria lobata on body weight and gene expression in obese rats muscle with estrogen difficiency. J. Korean Obstet. Gynecol. 2012, 25, 71–84. [Google Scholar]
- Attele, A.S.; Zhou, Y.-P.; Xie, J.-T.; Wu, J.A.; Zhang, L.; Dey, L.; Pugh, W.; Rue, P.A.; Polonsky, K.S.; Yuan, C.-S. Antidiabetic effects of panax ginseng berry extract and the identification of an effective component. Diabetes 2002, 51, 1851–1858. [Google Scholar] [CrossRef] [PubMed]
- Landsberg, L.; Young, J.B.; Leonard, W.R.; Linsenmeier, R.A.; Turek, F.W. Is obesity associated with lower body temperatures? Core temperature: A forgotten variable in energy balance. Metabolism 2009, 58, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Glueck, C.J.; Fontaine, R.N.; Wang, P.; Subbiah, M.T.R.; Weber, K.; Lllig, E.; Streicher, P.; Sieve-Smith, L.; Tracy, T.M.; Lang, J.E.; et al. Metformin Reduces weight, centripetal obesity, insulin, leptin, and low-density lipoprotein cholesterol in nondiabetic, morbidly obese subjects with body mass index greater than 30. Metabolism 2001, 50, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Rouru, J.; Huupponen, R.; Pesonen, U.; Koulu, M. Subchronic treatment with metformin produces anorectic effect and reduces hyperinsulinemia in genetically obese Zucker rats. Life Sci. 1992, 50, 1813–1820. [Google Scholar] [CrossRef]
- Rouru, J.; Pesonen, U.; Koulu, M.; Huupponen, R.; Santti, E.; Virtanen, K.; Rouvari, T.; Jhanwar-Uniyal, M. Anorectic effect of metformin in obese Zucker rats: Lack of evidence for the involvement of neuropeptide y. Eur. J. Pharmacol. 1995, 273, 99–106. [Google Scholar] [CrossRef]
- Lv, W.-S.; Wen, J.-P.; Li, L.; Sun, R.-X.; Wang, J.; Xian, Y.-X.; Cao, C.-X.; Wang, Y.-L.; Gao, Y.-Y. The effect of metformin on food intake and its potential role in hypothalamic regulation in obese diabetic rats. Brain Res. 2012, 1444, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Le, N.H.; Kim, C.-S.; Park, T.; Park, J.H.Y.; Sung, M.-K.; Lee, D.G.; Hong, S.-M.; Choe, S.-Y.; Goto, T.; Kawada, T. Quercetin protects against obesity-induced skeletal muscle inflammation and atrophy. Mediators Inflamm. 2014. [Google Scholar] [CrossRef] [PubMed]
- Dedieu, S.; Mazeres, G.; Cottin, P.; Brustis, J.-J. Involvement of myogenic regulator factors during fusion in the cell line C2C12. Int. J. Dev. Biol. 2002, 46, 235–241. [Google Scholar] [PubMed]
- Suwa, M.; Egashira, T.; Nakano, H.; Sasaki, H.; Kumagai, S. Metformin increases the PGC-1α protein and oxidative enzyme activities possibly via ampk phosphorylation in skeletal muscle in vivo. J. Appl. Physiol. 2006, 101, 1685–1692. [Google Scholar] [CrossRef] [PubMed]
- Jornayvaz, F.R.; Shulman, G.I. Regulation of mitochondrial biogenesis. Essays Biochem. 2010, 47, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wu, H.; Tarr, P.T.; Zhang, C.-Y.; Wu, Z.; Boss, O.; Michael, L.F.; Puigserver, P.; Isotani, E.; Olson, E.N. Transcriptional co-activator Pgc-1α drives the formation of slow-twitch muscle fibres. Pflugers Arch. 2002, 418, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Sacheck, J.M.; Hyatt, J.-P.K.; Raffaello, A.; Jagoe, R.T.; Roy, R.R.; Edgerton, V.R.; Lecker, S.H.; Goldberg, A.L. Rapid disuse and denervation atrophy involve transcriptional changes similar to those of muscle wasting during systemic diseases. FASEB J. 2007, 21, 140–155. [Google Scholar] [CrossRef] [PubMed]
- Sáinz, N.; Rodríguez, A.; Catalán, V.; Becerril, S.; Ramírez, B.; Gómez-Ambrosi, J.; Frühbeck, G. Leptin administration favors muscle mass accretion by decreasing FoxO3a and increasing PGC-1α in ob/ob mice. PLoS ONE 2009, 4, e6808. [Google Scholar] [CrossRef] [PubMed]
- Arany, Z. Pgc-1 coactivators and skeletal muscle adaptations in health and disease. Curr. Opin. Genet. Dev. 2008, 18, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhao, Y.; Li, R.; Gong, J.; Zheng, Y.; Wang, Y. PGC-1α is associated with C2C12 myoblast differentiation. Cent. Eur. J. Biol. 2014, 9, 1030–1036. [Google Scholar] [CrossRef]

| ND | HFD | HFD + RP 100 | HFD + RP 300 | HFD + Met 250 | |
|---|---|---|---|---|---|
| Tissue weight (g) | |||||
| Liver | 1.50 ± 0.06 | 2.24 ± 0.35 *** | 2.14 ± 0.17 | 1.57 ± 0.22 ## | 1.46 ± 0.17 ### |
| Pancreas | 0.33 ± 0.05 | 0.51 ± 0.03 *** | 0.34 ± 0.02 ### | 0.34 ± 0.03 ### | 0.35 ± 0.05 ### |
| Epididymal fat | 0.59 ± 0.05 | 1.40 ± 0.15 *** | 1.12 ± 0.15 # | 1.00 ± 0.15 ## | 0.92 ± 0.09 ### |
| Serum | |||||
| ALT (IU/L) | 39.1 ± 5.24 | 81.0 ± 9.40 *** | 54.6 ± 10.43 ### | 32.80 ± 5.48 ### | 49.9 ± 3.50 ### |
| AST (IU/L) | 50.7 ± 2.87 | 81.8 ± 5.94 *** | 66.90 ± 5.76 ## | 46.10 ± 7.72 ### | 59.3 ± 2.11 ### |
| TG (mg/dL) | 250.10 ± 3.11 | 248.66 ± 3.08 | 245.89 ± 6.93 | 240.83 ± 2.92 | 243.19 ± 1.82 |
| TC (mg/dL) | 205.27 ± 14.58 | 242.67 ± 12.94 ** | 229.33 ± 10.97 | 196.33 ± 22.03 ## | 218.33 ± 13.79 |
| HDL-C (mg/dL) | 72.81 ± 8.72 | 43.90 ± 12.87 ** | 65.54 ± 10.98 # | 106.51 ± 10.62 ### | 67.77 ± 4.20 # |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, H.W.; Kang, A.N.; Kang, S.Y.; Park, Y.-K.; Song, M.Y. The Root Extract of Pueraria lobata and Its Main Compound, Puerarin, Prevent Obesity by Increasing the Energy Metabolism in Skeletal Muscle. Nutrients 2017, 9, 33. https://doi.org/10.3390/nu9010033
Jung HW, Kang AN, Kang SY, Park Y-K, Song MY. The Root Extract of Pueraria lobata and Its Main Compound, Puerarin, Prevent Obesity by Increasing the Energy Metabolism in Skeletal Muscle. Nutrients. 2017; 9(1):33. https://doi.org/10.3390/nu9010033
Chicago/Turabian StyleJung, Hyo Won, An Na Kang, Seok Yong Kang, Yong-Ki Park, and Mi Young Song. 2017. "The Root Extract of Pueraria lobata and Its Main Compound, Puerarin, Prevent Obesity by Increasing the Energy Metabolism in Skeletal Muscle" Nutrients 9, no. 1: 33. https://doi.org/10.3390/nu9010033
APA StyleJung, H. W., Kang, A. N., Kang, S. Y., Park, Y.-K., & Song, M. Y. (2017). The Root Extract of Pueraria lobata and Its Main Compound, Puerarin, Prevent Obesity by Increasing the Energy Metabolism in Skeletal Muscle. Nutrients, 9(1), 33. https://doi.org/10.3390/nu9010033




