1. Introduction
For several years, populations of developing countries including Mexico and Latin America have been undergoing changes in dietary habits [
1]. The nutrition transition comprising changes in food patterns and dietary intake is linked to increased energy-dense sources, such as sugars, edible oils, and processed foods, whereas consumption of complex carbohydrates, fiber, and micronutrient-rich foods including fruits and vegetables is often low. These facts, coupled with reduced physical activity, increase obesity, bone density problems and the risk of non-communicable chronic diseases among the population [
2,
3].
Micronutrients such as Ca, P, Mg, Sr, vitamin D and vitamin K play an important role in bone health [
4,
5] and are required for bone mineralization and growth. An adequate intake of calcium during childhood and adolescence is necessary to achieve the peak bone mass (PBM), which is related to the adolescent growth spurt. A low PBM is a contributing factor for determining risk of fractures in children and osteoporosis in adulthood [
6]. In North America and Europe, the main dietary sources of calcium are milk and dairy products. In contrast, in Mexico and Central America the major calcium supplies within the diet are nixtamalized corn products (foodstuffs derived from the thermo-alkaline treatment of corn grains) and vegetables as a result of their low cost compared with animal products [
7]. In Mexico, micronutrient deficiencies (vitamin A, folate, zinc, iron and calcium) are important public health problems, affecting the most vulnerable age groups including children between 5 and 11 years of age [
8].
In plant foods, calcium bioavailability decreases due to the presence of oxalic, phytic and uronic acids. These compounds bind to calcium, forming insoluble complexes which cannot be absorbed in the intestine [
9].
Opuntia ficus indica cladodes (modified flattened stems in cacti) are part of the usual diet of the Mexican population and represent a potential source of calcium in the human diet [
10]. It has been demonstrated that calcium content in cladodes increases with maturity, while the concentration of oxalates shows a cyclic tendency [
11,
12]; consequently, we hypothesized that calcium bioavailability of
O. ficus indica cladodes depends on the growth stage of the plant. This topic has not been previously investigated. Considering the above mentioned, the objective of this study was to evaluate the effect of
O. ficus indica at different maturity stages as a calcium source within the diet on mechanical, microstructural properties, bone mineral density and mineral content of the femur in growing rats.
4. Discussion
Although calcium and phosphorus are the main minerals related to bone health, minerals such as potassium and magnesium have gained importance recently since greater intakes of these micronutrients are associated with lower declines in BMD [
24,
25]. The results derived from this study agree with previous reports, which indicate that late maturity stage cladodes of
O. ficus indica have high levels of calcium, phosphorus, potassium and magnesium, [
11]. This suggests that their consumption in diet might help the maintenance of BMD. All experimental groups fed with
O. ficus indica as the only calcium source consumed less food compared with the control group. This result can be attributed to the satiating effect induced by
O. ficus indica fiber content, whose composition changes as a function of the plant age [
11,
26,
27]. In this study, the groups fed with N-200 an N-600 diet showed higher food efficiency compared with the control group. It is well documented that dietary fiber cannot be digested in the small intestine, while in the large intestine this fiber is fermented, producing a short-chain fatty acid that can be reabsorbed into the bloodstream and increase the caloric potential of experimental diets prepared with cactus
O. ficus indica [
27,
28]. Food efficiency in terms of weight gain (g)/food intake (g) ratio values found in this work are similar to those reported by Lobo et al. [
16] for experimental animals of the same age. However, once adulthood is reached, the food efficiency diminishes by nearly 50%, this fact can be ascribed to the growing reduction (weight and size), whereas the food consumption at this stage remained constant. Differences in weight and thickness of femurs in the group fed with N-600 diet in comparison to bones in the group fed with the N-60 diet can be a result of variations in mineral content in
O. ficus indica at different maturity stages (see
Table 3), specifically, Mg and K, which contribute to bone formation [
24,
25,
29]. Furthermore, identification of CaHCO
3, CaCO
3, CaMg(CO
3)
2 and MgO (bioavailable for the human body as source of Ca and Mg) in cladodes at late maturity stages [
30] support these results. In addition, high calcium oxalate content in cladodes at early maturity stages reported previously [
11] hinder calcium bioavailability [
31]. Under long-term low levels of calcium intake, the bone supplies calcium through the process of bone resorption, which explains less thickening and reduced bone mass gain to maintain homeostasis of the organism resulting in bone loss [
32].
As expected, considering greater dimensions of femurs (weight and thickness), the mechanical parameters (
Fmax,
Pmax and
E) of the femurs in groups fed with cladodes at late maturity stages (N-400 and N-600) were higher than in groups fed with cladodes at early maturity stages (N-60 and N-200). These findings might be partly ascribed to the higher levels of oxalates contained in N-60 and N-200 diets, which impair calcium absorption. Under normal conditions, the hydroxyapatite (HAP) molecules are vertically oriented to the longitudinal axis of the femur to maximize bone resistance [
33]. It is widely reported that magnesium competes with calcium for the active growth sites of HAP crystals [
34]. It has been demonstrated that when magnesium competes with calcium for the binding sites in HAP, crystal lattices are modified, which consequently influences the mechanical properties of bones [
35]. Nevertheless, even when significant differences were observed in the magnesium content; modifications in the load to failure of bones can be attributed to the calcium content and the Ca/P ratio in the femurs as it is explained below [
36].
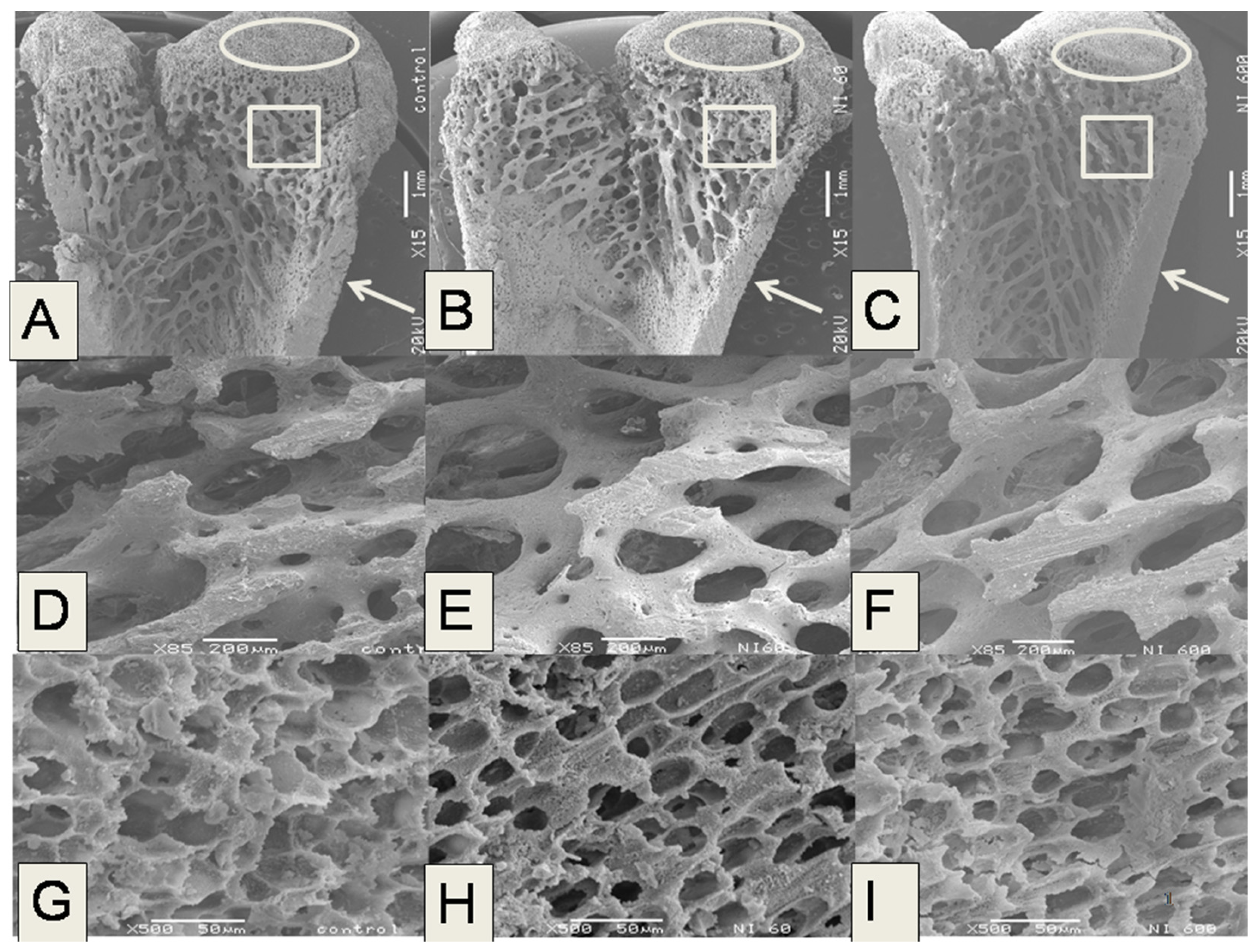
The microstructural analysis of the femurs in the experimental groups fed with N-60 and N-200 demonstrated that these bones showed lower trabecular bone areas, Ct.Wi and Tb.Th than femurs of rats fed with control, N-400 and N-600 diets. The trabecular bone located at the ends of the femur contributes to absorb and distribute the loads applied at a point along this bone [
20,
37]. Therefore, lower trabecular surface areas have been linked to an increase in fracture risk and osteoporosis. In addition, some authors have found that significant bone loss is more evident in the trabecular bone compartments, since this type of bone tissue is the metabolically most active compartment of the skeleton [
38]. In the case of the present study the most significant changes in microstructure were observed in trabecular and cortical bones.
Figure 2 shows that N-200 was the least effective sample in providing absorbable calcium, this is probably due to a combination of several factors that include: (a) a lower level of soluble dietary fiber (SDF), which is known to facilitate calcium absorption; and (b) a variation in content of bioavailable calcium salts, which are associated to SDF, as was previously reported by our research group [
10,
11]. Microstructural characteristics of the femurs were positively related to the mechanical properties. These findings are in accordance with previous reports stating that osteon morphology has an important influence on facture resistance of cortical bone [
39]. Recently, polyphenols and flavonoids were found in
O. ficus indica cladodes, the content of these phytochemicals modifies at different ages [
40]. Regarding this, polyphenols protect bone health through modulation of osteoblastogenesis, osteoclastogenesis and osteoimmunological action [
41]. Undoubtedly, variations of chemical components (oxalates, polyphenols and flavonoids) in
O. ficus indica at different maturity stages are responsible for the differences in calcium bioavailability and consequently, within the bone’s properties.
The mineral content in the bone, specifically Ca and P, constitute a widely accepted criterion for assessing bone health [
22,
36]. According to
Table 6, the bones of rats fed with
O. ficus indica at late development stage (N-400 and N-600) had higher calcium content than the bones of rats fed with
O. ficus indica at early development stage. Taking into account that the calcium content in all diets was adjusted to the same concentration (5 g /kg diet), the differences found in the calcium content in the femurs of experimental groups fed with cladodes at different development stages can be explained by chemical composition of
O. ficus indica used in the preparation of diets, specifically reduced anti-nutritional compounds content (oxalates) [
12,
31], as well as the formation of crystalline compounds (calcium carbonate, calcium-magnesium bicarbonate and magnesium oxide, etc.) in the cladodes at late development stages [
10,
30]. It is important to note that these crystalline compounds are associated with beneficial effects on bone health [
24,
42]. Moreover, it has been reported that dietary fiber composition influences short-chain fatty acid (SCFA) formation through fiber fermentation caused by colonic bacteria in the large intestine. The SCFAs lower the intestinal pH, which in turn dissolves insoluble mineral salts, particularly calcium, magnesium and iron, increasing their absorption [
16,
43,
44,
45].
P and Ca are the most studied minerals to assess the bone health [
46]. Likewise, monitoring the relationship between calcium and phosphorus (Ca/P ratio) in the diet helps to detect changes that occur in normal and diseased bone due to increased calcium intake, as well as high dietary calcium/phosphorus ratios which have favorable effects on bone mass [
47]. Low values of Ca/P ratio within the bones of rats fed with N-60 diet, as well as high values of this ratio in the bones of rats fed with control and N-600 diets demonstrate that the Ca/P ratio in the diet is strongly associated with Ca/P ratio in bone. These results confirm that the bone Ca/P ratio is a good index of bone quality, as has been reported previously [
48]. Regarding this, other researchers have suggested that a very low dietary Ca/P ratio (0.25) triggers high concentrations of serum parathyroid hormone (PHT) persistently, which increases the bone’s resorption and hence turnover; this physiological adaptation probably holds at any life stage [
46,
49].
The minerals found in trace amounts in the skeleton, such as magnesium and potassium, also play an important role in the bone health [
24,
25]. Regarding the magnesium content in the bones it is interesting to note that even when the Mg content in diets prepared with
O. ficus indica increased from 2.3 up to 3.5 times compared to the control diet, the magnesium content in bones of experimental animals was (
p ≤ 0.05) lower than or equal to the control group. These results are in accordance with previous reports related to the magnesium consumption, where a high Mg intake (2–5 times Mg requirements) in growing rats had no effect on Mg content in tibia in short term studies (4 weeks) [
50]. Modifications in macro-structural parameters of bones in growing rats (mid-lateral diameters and the ratios dry weight/length of humeri) have been observed in extreme conditions, such as long term studies (7 months) and excessive Mg supplementation (4-fold Mg requirements) [
51]. At this point, it is important to denote that the effect of Mg in bone health has not been fully clarified, since some researchers have reported that magnesium supplementation (3 times Mg requirements) reduces calcium retention in growing male rats as a result of Mg inhibitory effect on calcium absorption in intestine, and promotes the secretion of endogenous calcium into intestine [
52]; while other studies have revealed that Mg supplementation promotes bone formation, prevents bone resorption and increases the dynamic strength of the bones in ovariectomized (OVX) rats [
53].
On the other hand, concerning potassium, this study showed that different K content in diets containing
O. ficus indica (from 2.3 to 4.9 times compared to the control diet) affected content of this mineral within the bones of experimental groups; although the K consumption in diets was not correlated with the K content in femurs. In this regard, it has been demonstrated in long-term studies that high dietary potassium intake shows positive association with bone density in elderly women, suggesting that increasing the consumption of food rich in potassium may play a role in osteoporosis prevention [
54]. It has been shown that supplementation with alkaline potassium salts such as potassium bicarbonate (KHCO
3), potassium citrate (C
6H
5K
3O
7) and potassium chloride (KCl) leads to significant reduction in renal calcium excretion since potassium promotes an alkaline environment and reduces bone resorption in humans [
25,
55,
56,
57]. Regarding this, it is worth mentioning that KCl has been previously identified in
O. ficus indica cladodes [
30].
The evaluation of BMD is widely accepted to diagnose bone health and to assess the effectiveness of treatments for skeletal diseases [
58,
59]. The groups fed with the control and N-600 diets showed the highest values of BMD and in both groups the gain of BMD was fastest in the pubertal stage. This effect may be related, as already mentioned above, with a lower concentration of oxalates [
12], and the presence of phytochemicals i.e., isoflavones and phytosterols in cladodes, which influence the bone mass gain trough the regulation of the bone cells (osteoblasts and osteoclasts) responsible of bone formation and resorption, respectively [
60,
61].
On the other hand, the groups fed with N-60, N-200 and N-400 diets showed an abrupt increase in BMD at the adolescence stage. This increase can be explained by adaptation process of experimental animals to dietary fiber consumption, as well as to the fiber composition, which, in the case of O. ficus indica, is modified depending on the maturity stage, as previously mentioned.
Finally, the results derived from this research revealed a strong correlation between the mechanical properties and BMD. These results are in accordance with previous findings stating that prior knowledge of cancellous bone density is a reasonable prediction of mechanical stiffness and strength of bone [
62]. The results from this study have also demonstrated that along with mechanical properties, the microstructural parameters (Ct.Wi, Tb.Wi and Tb.Sp) are correlated with BMD, as previously reported [
17,
39]. A significant finding in this research is that Ca and K content in bone are correlated with BMD. In the case of calcium, this is due to calcium intake through diet or supplements increasing bone density and retarding the loss of bone stock [
63]. With respect to potassium some authors suggest that potassium intake has a beneficial effect on bone mass in adult men, elderly men and women, adult men and peri- and early post-menopausal women [
61]. Probably, this is due to an anion-independent effect of potassium on calcium excretion and bone metabolism [
64].