Effects of Magnesium on the Phosphate Toxicity in Chronic Kidney Disease: Time for Intervention Studies
Abstract
:1. Introduction
2. Magnesium and Vascular Calcification
2.1. Clinical Studies
2.2. Experimental Studies
3. Magnesium and Clinical Outcomes in CKD
3.1. Magnesium and Cardiovascular Outcomes in CKD
3.2. Magnesium and Phosphate Balance in the Risk of Progression of CKD
4. Low Dietary Magnesium Intake of Hemodialysis Patients
4.1. Causes of Magnesium Deficiency
4.2. Imbalance between Magnesium and Phosphate in Hemodialysis Patients
5. How Do We Increase the Magnesium Status of Dialysis Patients?
6. Conclusions
Conflicts of Interest
References
- Rosanoff, A.; Weaver, C.M.; Rude, R.K. Suboptimal magnesium status in the United States: Are the health consequences underestimated? Nutr. Rev. 2012, 70, 153–164. [Google Scholar] [CrossRef]
- Del Gobbo, L.C.; Imamura, F.; Wu, J.H.; de Oliveira Otto, M.C.; Chiuve, S.E.; Mozaffarian, D. Circulating and dietary magnesium and risk of cardiovascular disease: A systematic review and meta-analysis of prospective studies. Am. J. Clin. Nutr. 2013, 98, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Jin, F.; Hao, Y.; Li, H.; Tang, T.; Wang, H.; Yan, W.; Dai, K. Magnesium and the risk of cardiovascular events: A meta-analysis of prospective cohort studies. PLoS ONE 2013, 8, e57720. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Liang, C.; Li, M.; Montgomery, S.; Fall, K.; Aaseth, J.; Cao, Y. Dose-response relationship between dietary magnesium intake and cardiovascular mortality: A systematic review and dose-based meta-regression analysis of prospective studies. J. Trace Elem. Med. Biol. 2016, 38, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Sarrafzadegan, N.; Khosravi-Boroujeni, H.; Lotfizadeh, M.; Pourmogaddas, A.; Salehi-Abargouei, A. Magnesium status and the metabolic syndrome: A systematic review and meta-analysis. Nutrition 2016, 32, 409–417. [Google Scholar] [CrossRef] [PubMed]
- La, S.A.; Lee, J.Y.; Kim do, H.; Song, E.L.; Park, J.H.; Ju, S.Y. Magnesium Levels in Adults with Metabolic Syndrome: A Meta-Analysis. Biol. Trace Elem. Res. 2016, 170, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.Y.; Choi, W.S.; Ock, S.M.; Kim, C.M.; Kim, D.H. Dietary magnesium intake and metabolic syndrome in the adult population: Dose-response meta-analysis and meta-regression. Nutrients 2014, 6, 6005–6019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Y.; Del Gobbo, L.C.; Rosanoff, A.; Wang, J.; Zhang, W.; Song, Y. Effects of Magnesium Supplementation on Blood Pressure: A Meta-Analysis of Randomized Double-Blind Placebo-Controlled Trials. Hypertension 2016, 68, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Watutantrige, S.F.; Luchini, C.; Solmi, M.; Sartore, G.; Sergi, G.; Manzato, E.; Barbagallo, M.; Maggi, S.; Stubbs, B. Effect of magnesium supplementation on glucose metabolism in people with or at risk of diabetes: A systematic review and meta-analysis of double-blind randomized controlled trials. Eur. J. Clin. Nutr. 2016, 70, 1354–1359. [Google Scholar] [CrossRef] [PubMed]
- Cunha, A.R.; D’El-Rei, J.; Medeiros, F.; Umbelino, B.; Oigman, W.; Touyz, R.M.; Neves, M.F. Oral magnesium supplementation improves endothelial function and attenuates subclinical atherosclerosis in thiazide-treated hypertensive women. J. Hypertens. 2017, 35, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Shechter, M.; Sharir, M.; Labrador, M.J.; Forrester, J.; Silver, B.; Bairey Merz, C.N. Oral magnesium therapy improves endothelial function in patients with coronary artery disease. Circulation 2000, 102, 2353–2358. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A. Endothelial cells and magnesium: Implications in atherosclerosis. Clin. Sci. 2012, 122, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, X.; Qi, H.; Wang, J.; Wang, M.; Zhang, Y.; Yan, H.; Zhuang, S. Correlation of serum magnesium with cardiovascular risk factors in maintenance hemodialysis patients—A cross-sectional study. Magnes. Res. 2013, 26, 100–108. [Google Scholar] [PubMed]
- Kyriazis, J.; Kalogeropoulou, K.; Bilirakis, L.; Smirnioudis, N.; Pikounis, V.; Stamatiadis, D.; Liolia, E. Dialysate magnesium level and blood pressure. Kidney Int. 2004, 66, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Pletka, P.; Bernstein, D.S.; Hampers, C.L.; Merrill, J.P.; Sherwood, L.M. Relationship between magnesium and secondary hyperparathyroidism during long-term hemodialysis. Metabolism 1974, 23, 619–630. [Google Scholar] [CrossRef]
- Quitterer, U.; Hoffmann, M.; Freichel, M.; Lohse, M.J. Paradoxical block of parathormone secretion is mediated by increased activity of Gα subunits. J. Biol. Chem. 2001, 276, 6763–6769. [Google Scholar] [CrossRef] [PubMed]
- Russo, D.; Corrao, S.; Battaglia, Y.; Andreucci, M.; Caiazza, A.; Carlomagno, A.; Lamberti, M.; Pezone, N.; Pota, A.; Russo, L.; et al. Progression of coronary artery calcification and cardiac events in patients with chronic renal disease not receiving dialysis. Kidney Int. 2011, 80, 112–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, R.; Lemos, M.M.; Manfredi, S.R.; Draibe, S.A.; Canziani, M.E. Impact of cardiovascular calcification in nondialyzed patients after 24 months of follow-up. Clin. J. Am. Soc. Nephrol. 2010, 5, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; Sang, Y.; Ballew, S.H.; Shlipak, M.; Katz, R.; Rosas, S.E.; Peralta, C.A.; Woodward, M.; Kramer, H.J.; Jacobs, D.R.; et al. Subclinical atherosclerosis measures for cardiovascular prediction in CKD. J. Am. Soc. Nephrol. 2015, 26, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, M.; Iseki, K.; Tamashiro, M.; Fujimoto, N.; Higa, N.; Touma, T.; Takishita, S. Impact of high coronary artery calcification score (CACS) on survival in patients on chronic hemodialysis. Clin. Exp. Nephrol. 2004, 8, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Block, G.A.; Raggi, P.; Bellasi, A.; Kooienga, L.; Spiegel, D.M. Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int. 2007, 71, 438–441. [Google Scholar] [CrossRef] [PubMed]
- Shantouf, R.S.; Budoff, M.J.; Ahmadi, N.; Ghaffari, A.; Flores, F.; Gopal, A.; Noori, N.; Jing, J.; Kovesdy, C.P.; Kalantar-Zadeh, K. Total and individual coronary artery calcium scores as independent predictors of mortality in hemodialysis patients. Am. J. Nephrol. 2010, 31, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Coen, G.; Pierantozzi, A.; Spizzichino, D.; Sardella, D.; Mantella, D.; Manni, M.; Pellegrino, L.; Romagnoli, A.; Pacifici, R.; Zuccaro, P.; et al. Risk factors of one year increment of coronary calcifications and survival in hemodialysis patients. BMC Nephrol. 2010, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.T.; Grabher, J.J.; LeGeros, R.Z. Effects of magnesium on calcium phosphate formation. Magnesium 1988, 7, 123–132. [Google Scholar] [PubMed]
- Meema, H.E.; Oreopoulos, D.G.; Rapoport, A. Serum magnesium level and arterial calcification in end-stage renal disease. Kidney Int. 1987, 32, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Ishimura, E.; Okuno, S.; Kitatani, K.; Tsuchida, T.; Yamakawa, T.; Shioi, A.; Inaba, M.; Nishizawa, Y. Significant association between the presence of peripheral vascular calcification and lower serum magnesium in hemodialysis patients. Clin. Nephrol. 2007, 68, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Tzanakis, I.; Pras, A.; Kounali, D.; Mamali, V.; Kartsonakis, V.; Mayopoulou-Symvoulidou, D.; Kallivretakis, N. Mitral annular calcifications in haemodialysis patients: A possible protective role of magnesium. Nephrol. Dial. Transplant. 1997, 12, 2036–2037. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, Y.; Hamano, T.; Nakano, C.; Obi, Y.; Matsui, I.; Kusunoki, Y.; Mori, D.; Oka, T.; Hashimoto, N.; Takabatake, Y.; et al. Association between density of coronary artery calcification and serum magnesium levels among patients with chronic kidney disease. PLoS ONE 2016, 11, e0163673. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, D.M.; Farmer, B. Long-term effects of magnesium carbonate on coronary artery calcification and bone mineral density in hemodialysis patients: A pilot study. Hemodial. Int. 2009, 13, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Tzanakis, I.P.; Stamataki, E.E.; Papadaki, A.N.; Giannakis, N.; Damianakis, N.E.; Oreopoulos, D.G. Magnesium retards the progress of the arterial calcifications in hemodialysis patients: A pilot study. Int. Urol. Nephrol. 2014, 46, 2199–2205. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, O.; Syono, T.; Nakagawa, K.; Nishian, Y.; Takenaka, Y.; Takamitsu, Y. Influence of magnesium deficiency on concentration of calcium in soft tissue of uremic rats. Ren. Fail. 1996, 18, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Gorgels, T.G.; Waarsing, J.H.; de Wolf, A.; ten Brink, J.B.; Loves, W.J.; Bergen, A.A. Dietary magnesium, not calcium, prevents vascular calcification in a mouse model for pseudoxanthoma elasticum. J. Mol. Med. 2010, 88, 467–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montezano, A.C.; Zimmerman, D.; Yusuf, H.; Burger, D.; Chignalia, A.Z.; Wadhera, V.; van Leeuwen, F.N.; Touyz, R.M. Vascular smooth muscle cell differentiation to an osteogenic phenotype involves TRPM7 modulation by magnesium. Hypertension 2010, 56, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Kircelli, F.; Peter, M.E.; Sevinc Ok, E.; Celenk, F.G.; Yilmaz, M.; Steppan, S.; Asci, G.; Ok, E.; Passlick-Deetjen, J. Magnesium reduces calcification in bovine vascular smooth muscle cells in a dose-dependent manner. Nephrol. Dial. Transplant. 2012, 27, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Salem, S.; Bruck, H.; Bahlmann, F.H.; Peter, M.; Passlick-Deetjen, J.; Kretschmer, A.; Steppan, S.; Volsek, M.; Kribben, A.; Nierhaus, M.; et al. Relationship between magnesium and clinical biomarkers on inhibition of vascular calcification. Am. J. Nephrol. 2012, 35, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Louvet, L.; Büchel, J.; Steppan, S.; Passlick-Deetjen, J.; Massy, Z.A. Magnesium prevents phosphate-induced calcification in human aortic vascular smooth muscle cells. Nephrol. Dial. Transplant. 2013, 28, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Montes de Oca, A.; Guerrero, F.; Martinez-Moreno, J.M.; Madueño, J.A.; Herencia, C.; Peralta, A.; Almaden, Y.; Lopez, I.; Aguilera-Tejero, E.; Gundlach, K.; et al. Magnesium inhibits Wnt/β-catenin activity and reverses the osteogenic transformation of vascular smooth muscle cells. PLoS ONE 2014, 9, e89525. [Google Scholar] [CrossRef] [PubMed]
- Louvet, L.; Bazin, D.; Büchel, J.; Steppan, S.; Passlick-Deetjen, J.; Massy, Z.A. Characterisation of calcium phosphate crystals on calcified human aortic vascular smooth muscle cells and potential role of magnesium. PLoS ONE 2015, 10, e0115342. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Bai, Y.; Jin, J.; Zhang, J.; Zhang, S.; Cui, L.; Zhang, H. Magnesium modulates the expression levels of calcification-associated factors to inhibit calcification in a time-dependent manner. Exp. Ther. Med. 2015, 9, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Zhang, J.; Xu, J.; Cui, L.; Zhang, H.; Zhang, S.; Feng, X. Magnesium prevents β-glycerophosphate-induced calcification in rat aortic vascular smooth muscle cells. Biomed. Rep. 2015, 3, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Louvet, L.; Metzinger, L.; Büchel, J.; Steppan, S.; Massy, Z.A. Magnesium Attenuates Phosphate-Induced Deregulation of a MicroRNA Signature and Prevents Modulation of Smad1 and Osterix during the Course of Vascular Calcification. Biomed. Res. Int. 2016, 2016, 7419524. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.R.; Hanssen, E.; McMahon, L.P.; Holt, S.G. Fetuin-A-containing calciprotein particles reduce mineral stress in the macrophage. PLoS ONE 2013, 8, e60904. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.M.; Smith, E.R.; Holt, S.G. The role of fetuin—A in mineral trafficking and deposition. Bonekey Rep. 2015, 4, 672. [Google Scholar] [CrossRef] [PubMed]
- Aghagolzadeh, P.; Bachtler, M.; Bijarnia, R.; Jackson, C.; Smith, E.R.; Odermatt, A.; Radpour, R.; Pasch, A. Calcification of vascular smooth muscle cells is induced by secondary calciprotein particles and enhanced by tumor necrosis factor-α. Atherosclerosis 2016, 251, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Pasch, A.; Farese, S.; Gräber, S.; Wald, J.; Richtering, W.; Floege, J.; Jahnen-Dechent, W. Nanoparticle-based test measures overall propensity for calcification in serum. J. Am. Soc. Nephrol. 2012, 23, 1744–1752. [Google Scholar] [CrossRef] [PubMed]
- Keyzer, C.A.; de Borst, M.H.; van den Berg, E.; Jahnen-Dechent, W.; Arampatzis, S.; Farese, S.; Bergmann, I.P.; Floege, J.; Navis, G.; Bakker, S.J.; et al. Calcification Propensity and Survival among Renal Transplant Recipients. J. Am. Soc. Nephrol. 2016, 27, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, Y.; Fujii, N.; Shoji, T.; Hayashi, T.; Rakugi, H.; Isaka, Y. Hypomagnesemia is a significant predictor of cardiovascular and non-cardiovascular mortality in patients undergoing hemodialysis. Kidney Int. 2014, 85, 174–181. [Google Scholar] [CrossRef] [PubMed]
- João Matias, P.; Azevedo, A.; Laranjinha, I.; Navarro, D.; Mendes, M.; Ferreira, C.; Amaral, T.; Jorge, C.; Aires, I.; Gil, C.; et al. Lower serum magnesium is associated with cardiovascular risk factors and mortality in haemodialysis patients. Blood Purif. 2014, 38, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Fein, P.; Weiss, S.; Ramos, F.; Singh, P.; Chattopadhyay, J.; Avram, M.M. Serum magnesium concentration is a significant predictor of mortality in peritoneal dialysis patients. Adv. Perit. Dial. 2014, 30, 90–93. [Google Scholar] [PubMed]
- Li, L.; Streja, E.; Rhee, C.M.; Mehrotra, R.; Soohoo, M.; Brunelli, S.M.; Kovesdy, C.P.; Kalantar-Zadeh, K. Hypomagnesemia and Mortality in Incident Hemodialysis Patients. Am. J. Kidney Dis. 2015, 66, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Lacson, E., Jr.; Wang, W.; Ma, L.; Passlick-Deetjen, J. Serum Magnesium and Mortality in Hemodialysis Patients in the United States: A Cohort Study. Am. J. Kidney Dis. 2015, 66, 1056–1066. [Google Scholar] [CrossRef] [PubMed]
- De Roij van Zuijdewijn, C.L.; Grooteman, M.P.; Bots, M.L.; Blankestijn, P.J.; Steppan, S.; Büchel, J.; Groenwold, R.H.; Brandenburg, V.; van den Dorpel, M.A.; Ter Wee, P.M.; et al. Serum magnesium and sudden death in European hemodialysis patients. PLoS ONE 2015, 10, e0143104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurita, N.; Akizawa, T.; Fukagawa, M.; Onishi, Y.; Kurokawa, K.; Fukuhara, S. Contribution of dysregulated serum magnesium to mortality in hemodialysis patients with secondary hyperparathyroidism: A 3-year cohort study. Clin. Kidney J. 2015, 8, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Luo, Q.; Dai, Z.; Zhu, B.; Fei, J.; Xue, C.; Wu, D. Hypomagnesemia Is Associated with Increased Mortality among Peritoneal Dialysis Patients. PLoS ONE 2016, 11, e0152488. [Google Scholar] [CrossRef]
- Yang, X.; Soohoo, M.; Streja, E.; Rivara, M.B.; Obi, Y.; Adams, S.V.; Kalantar-Zadeh, K.; Mehrotra, R. Serum Magnesium Levels and Hospitalization and Mortality in Incident Peritoneal Dialysis Patients: A Cohort Study. Am. J. Kidney Dis. 2016, 68, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Ago, R.; Shindo, T.; Banshodani, M.; Shintaku, S.; Moriishi, M.; Masaki, T.; Kawanishi, H. Hypomagnesemia as a predictor of mortality in hemodialysis patients and the role of proton pump inhibitors: A cross-sectional, 1-year, retrospective cohort study. Hemodial. Int. 2016, 20, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Kanbay, M.; Yilmaz, M.I.; Apetrii, M.; Saglam, M.; Yaman, H.; Unal, H.U.; Gok, M.; Caglar, K.; Oguz, Y.; Yenicesu, M.; et al. Relationship between serum magnesium levels and cardiovascular events in chronic kidney disease patients. Am. J. Nephrol. 2012, 36, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, Y.; Fujii, N.; Shoji, T.; Hayashi, T.; Rakugi, H.; Iseki, K.; Tsubakihara, Y.; Isaka, Y.; Committee of Renal Data Registry of the Japanese Society for Dialysis Therapy. Magnesium modifies the cardiovascular mortality risk associated with hyperphosphatemia in patients undergoing hemodialysis: A cohort study. PLoS ONE 2014, 9, e116273. [Google Scholar] [CrossRef] [PubMed]
- Ibels, L.S.; Alfrey, A.C.; Haut, L.; Huffer, W.E. Preservation of function in experimental renal disease by dietary restriction of phosphate. N. Engl. J. Med. 1978, 298, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, M.; Dusso, A.S.; Liapis, H.; Finch, J.; Lu, Y.; Burke, S.K.; Slatopolsky, E. The effects of sevelamer hydrochloride and calcium carbonate on kidney calcification in uremic rats. J. Am. Soc. Nephrol. 2002, 13, 2299–2308. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, T.; Murakami, K.; Nakayama, H.; Kuwahara, T.; Ohnishi, Y. Role of dietary phosphorus in the progression of renal failure. Biochem. Biophys. Res. Commun. 2002, 295, 917–921. [Google Scholar] [CrossRef]
- Nagano, N.; Miyata, S.; Obana, S.; Kobayashi, N.; Fukushima, N.; Burke, S.K.; Wada, M. Sevelamer hydrochloride, a phosphate binder, protects against deterioration of renal function in rats with progressive chronic renal insufficiency. Nephrol. Dial. Transplant. 2003, 18, 2014–2023. [Google Scholar] [CrossRef] [PubMed]
- Neves, K.R.; Graciolli, F.G.; dos Reis, L.M.; Pasqualucci, C.A.; Moysés, R.M.; Jorgetti, V. Adverse effects of hyperphosphatemia on myocardial hypertrophy, renal function, and bone in rats with renal failure. Kidney Int. 2004, 66, 2237–2244. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, R.; Peralta, C.A.; Chen, S.C.; Sachs, M.; Shah, A.; Norris, K.; Saab, G.; Whaley-Connell, A.; Kestenbaum, B.; McCullough, P.A. No independent association of serum phosphorus with risk for death or progression to end-stage renal disease in a large screen for chronic kidney disease. Kidney Int. 2013, 84, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Trivedi, B.K.; Kalantar-Zadeh, K.; Kovesdy, C.P. Association of disorders in mineral metabolism with progression of chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2006, 1, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Norris, K.C.; Greene, T.; Kopple, J.; Lea, J.; Lewis, J.; Lipkowitz, M.; Miller, P.; Richardson, A.; Rostand, S.; Wang, X.; et al. Baseline predictors of renal disease progression in the African American Study of Hypertension and Kidney Disease. J. Am. Soc. Nephrol. 2006, 17, 2928–2936. [Google Scholar] [CrossRef] [PubMed]
- Voormolen, N.; Noordzij, M.; Grootendorst, D.C.; Beetz, I.; Sijpkens, Y.W.; van Manen, J.G.; Boeschoten, E.W.; Huisman, R.M.; Krediet, R.T.; Dekker, F.W.; et al. High plasma phosphate as a risk factor for decline in renal function and mortality in pre-dialysis patients. Nephrol. Dial. Transplant. 2007, 22, 2909–2916. [Google Scholar] [CrossRef] [PubMed]
- Chue, C.D.; Edwards, N.C.; Davis, L.J.; Steeds, R.P.; Townend, J.N.; Ferro, C.J. Serum phosphate but not pulsewave velocity predicts decline in renal function in patients with earlychronic kidney disease. Nephrol. Dial. Transplant. 2011, 26, 2576–2582. [Google Scholar] [CrossRef] [PubMed]
- O’Seaghdha, C.M.; Hwang, S.J.; Muntner, P.; Melamed, M.L.; Fox, C.S. Serum phosphorus predicts incident chronic kidney disease and end-stage renal disease. Nephrol. Dial. Transplant. 2011, 26, 2885–2890. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C.; Ruggenenti, P.; Perna, A.; Leonardis, D.; Tripepi, R.; Tripepi, G.; Mallamaci, F.; Remuzzi, G.; REIN Study Group. Phosphate may promote CKD progression and attenuate renoprotective effect of ACE inhibition. J. Am. Soc. Nephrol. 2011, 22, 1923–1930. [Google Scholar] [CrossRef] [PubMed]
- Bellasi, A.; Mandreoli, M.; Baldrati, L.; Corradini, M.; Di Nicolò, P.; Malmusi, G.; Santoro, A. Chronic kidney disease progression and outcome according to serum phosphorus in mild-to-moderate kidney dysfunction. Clin. J. Am. Soc. Nephrol. 2011, 6, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Sim, J.J.; Bhandari, S.K.; Smith, N.; Chung, J.; Liu, I.L.; Jacobsen, S.J.; Kalantar-Zadeh, K. Phosphorus and risk of renal failure in subjects with normal renal function. Am. J. Med. 2013, 126, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Chartsrisak, K.; Vipattawat, K.; Assanatham, M.; Nongnuch, A.; Ingsathit, A.; Domrongkitchaiporn, S.; Sumethkul, V.; Distha-Banchong, S. Mineral metabolism and outcomes in chronic kidney disease stage 2–4 patients. BMC Nephrol. 2013, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Da, J.; Xie, X.; Wolf, M.; Disthabanchong, S.; Wang, J.; Zha, Y.; Lv, J.; Zhang, L.; Wang, H. Serum Phosphorus and Progression of CKD and Mortality: A Meta-analysis of Cohort Studies. Am. J. Kidney Dis. 2015, 66, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, Y.; Shoji, T.; Hayashi, T.; Suzuki, A.; Shimizu, M.; Mitsumoto, K.; Kawabata, H.; Niihata, K.; Okada, N.; Isaka, Y.; et al. Hypomagnesemia in type 2 diabetic nephropathy: A novel predictor of end-stage renal disease. Diabetes Care 2012, 35, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Van Laecke, S.; Nagler, E.V.; Verbeke, F.; Van Biesen, W.; Vanholder, R. Hypomagnesemia and the risk of death and GFR decline in chronic kidney disease. Am. J. Med. 2013, 126, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Tin, A.; Grams, M.E.; Maruthur, N.M.; Astor, B.C.; Couper, D.; Mosley, T.H.; Selvin, E.; Coresh, J.; Kao, W.H. Results from the Atherosclerosis Risk in Communities study suggest that low serum magnesium is associated with incident kidney disease. Kidney Int. 2015, 87, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Rebholz, C.M.; Tin, A.; Liu, Y.; Kuczmarski, M.F.; Evans, M.K.; Zonderman, A.B.; Crews, D.C. Dietary Magnesium and Kidney Function Decline: The Healthy Aging in Neighborhoods of Diversity across the Life Span Study. Am. J. Nephrol. 2016, 44, 381–387. [Google Scholar] [CrossRef] [PubMed]
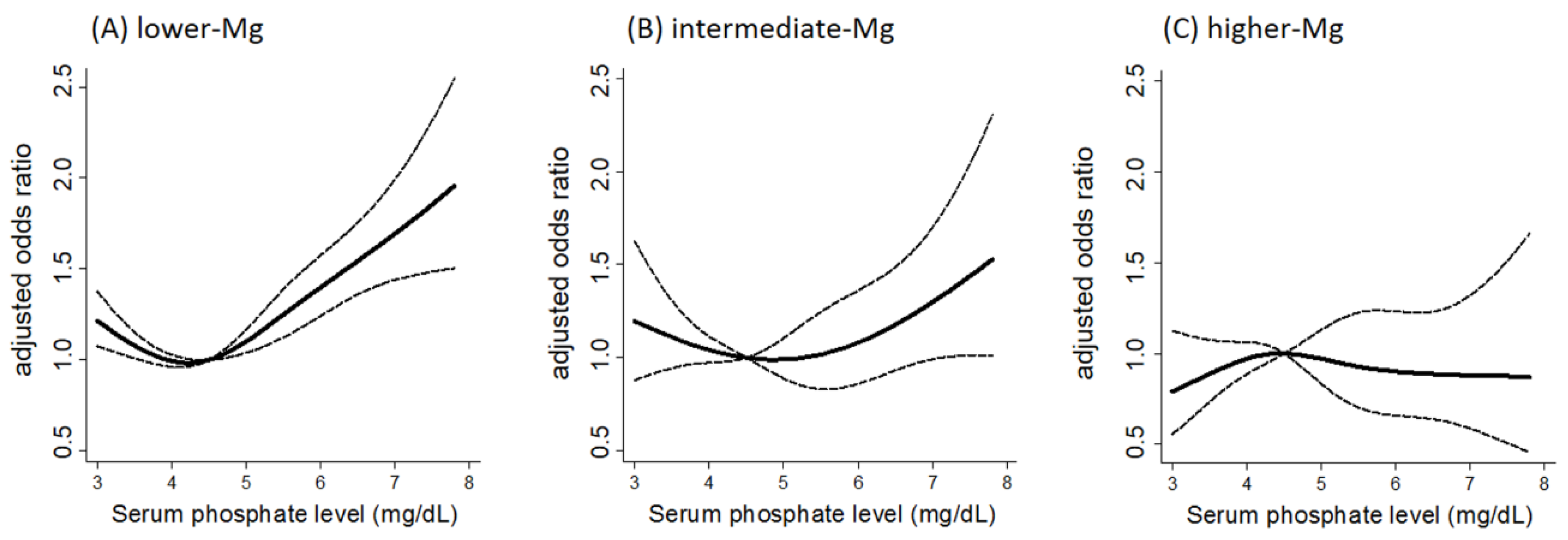
- Sakaguchi, Y.; Iwatani, H.; Hamano, T.; Tomida, K.; Kawabata, H.; Kusunoki, Y.; Shimomura, A.; Matsui, I.; Hayashi, T.; Tsubakihara, Y.; et al. Magnesium modifies the association between serum phosphate and the risk of progression to end-stage kidney disease in patients with non-diabetic chronic kidney disease. Kidney Int. 2015, 88, 833–842. [Google Scholar] [CrossRef] [PubMed]
- De Baaij, J.H.; Hoenderop, J.G.; Bindels, R.J. Magnesium in man: Implications for health and disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, A.; Ohkido, I.; Yokoyama, K.; Mafune, A.; Urashima, M.; Yokoo, T. Proton Pump Inhibitor Use and Magnesium Concentrations in Hemodialysis Patients: A Cross-Sectional Study. PLoS ONE 2015, 10, e0143656. [Google Scholar] [CrossRef] [PubMed]
- Gommers, L.M.; Hoenderop, J.G.; Bindels, R.J.; de Baaij, J.H. Hypomagnesemia in Type 2 Diabetes: A Vicious Circle? Diabetes 2016, 65, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Wyskida, K.; Witkowicz, J.; Chudek, J.; Więcek, A. Daily magnesium intake and hypermagnesemia in hemodialysis patients with chronic kidney disease. J. Ren. Nutr. 2012, 22, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Luis, D.; Zlatkis, K.; Comenge, B.; García, Z.; Navarro, J.F.; Lorenzo, V.; Carrero, J.J. Dietary Quality and Adherence to Dietary Recommendations in Patients Undergoing Hemodialysis. J. Ren. Nutr. 2016, 26, 190–195. [Google Scholar] [CrossRef] [PubMed]
- De Schutter, T.M.; Behets, G.J.; Geryl, H.; Peter, M.E.; Steppan, S.; Gundlach, K.; Passlick-Deetjen, J.; D’Haese, P.C.; Neven, E. Effect of a magnesium-based phosphate binder on medial calcification in a rat model of uremia. Kidney Int. 2013, 83, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- De Francisco, A.L.; Leidig, M.; Covic, A.C.; Ketteler, M.; Benedyk-Lorens, E.; Mircescu, G.M.; Scholz, C.; Ponce, P.; Passlick-Deetjen, J. Evaluation of calcium acetate/magnesium carbonate as a phosphate binder compared with sevelamer hydrochloride in haemodialysis patients: A controlled randomized study (CALMAG study) assessing efficacy and tolerability. Nephrol. Dial. Transplant. 2010, 25, 3707–3717. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakaguchi, Y.; Hamano, T.; Isaka, Y. Effects of Magnesium on the Phosphate Toxicity in Chronic Kidney Disease: Time for Intervention Studies. Nutrients 2017, 9, 112. https://doi.org/10.3390/nu9020112
Sakaguchi Y, Hamano T, Isaka Y. Effects of Magnesium on the Phosphate Toxicity in Chronic Kidney Disease: Time for Intervention Studies. Nutrients. 2017; 9(2):112. https://doi.org/10.3390/nu9020112
Chicago/Turabian StyleSakaguchi, Yusuke, Takayuki Hamano, and Yoshitaka Isaka. 2017. "Effects of Magnesium on the Phosphate Toxicity in Chronic Kidney Disease: Time for Intervention Studies" Nutrients 9, no. 2: 112. https://doi.org/10.3390/nu9020112



