Orange Pomace Improves Postprandial Glycemic Responses: An Acute, Randomized, Placebo-Controlled, Double-Blind, Crossover Trial in Overweight Men
Abstract
:1. Introduction
2. Experimental Section
2.1. Subjects
2.2. Study Design
2.3. Study Beverages and Meals
2.4. Determination of Serum Glucose and Insulin
2.5. Statistical Analysis
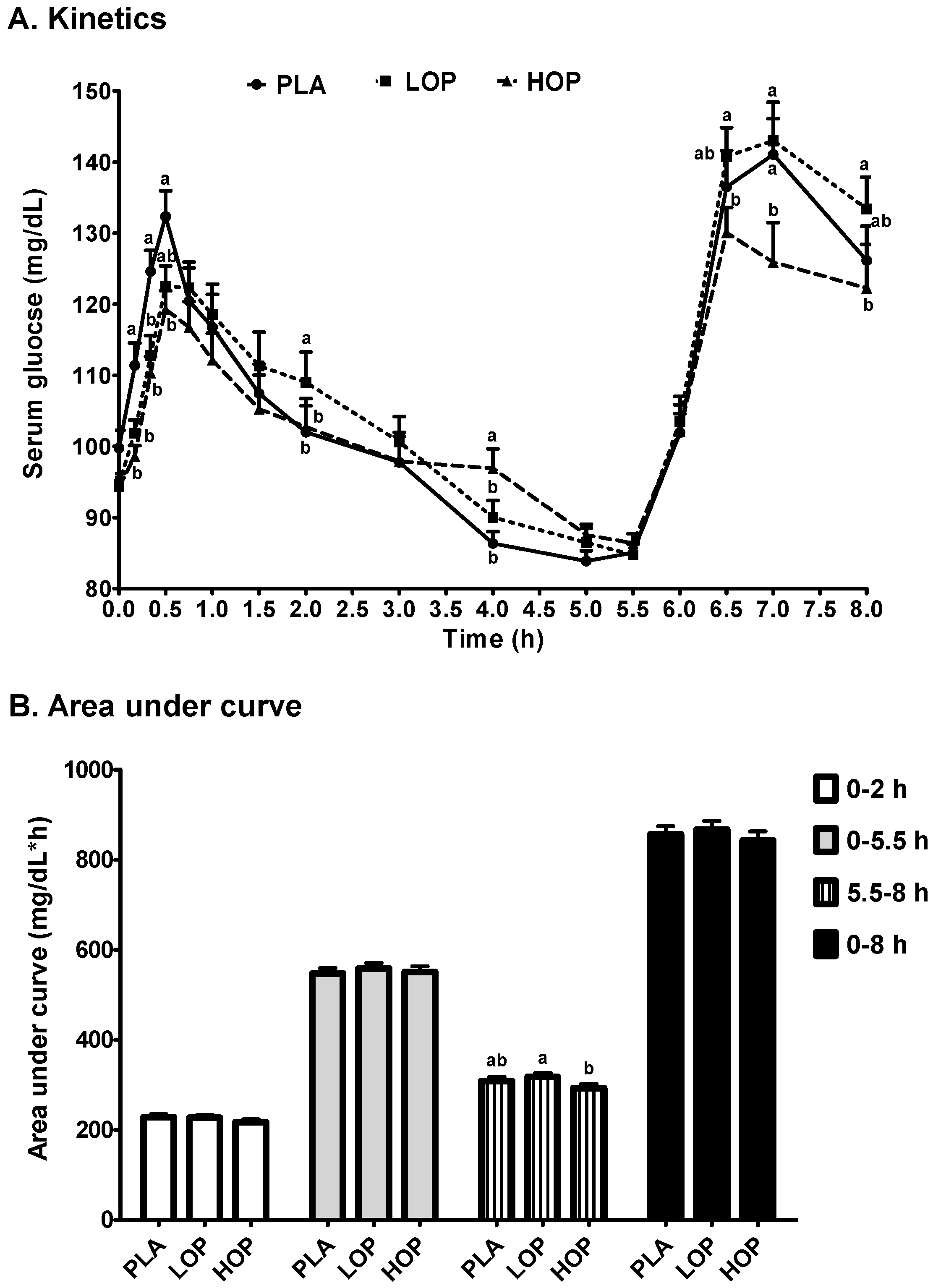
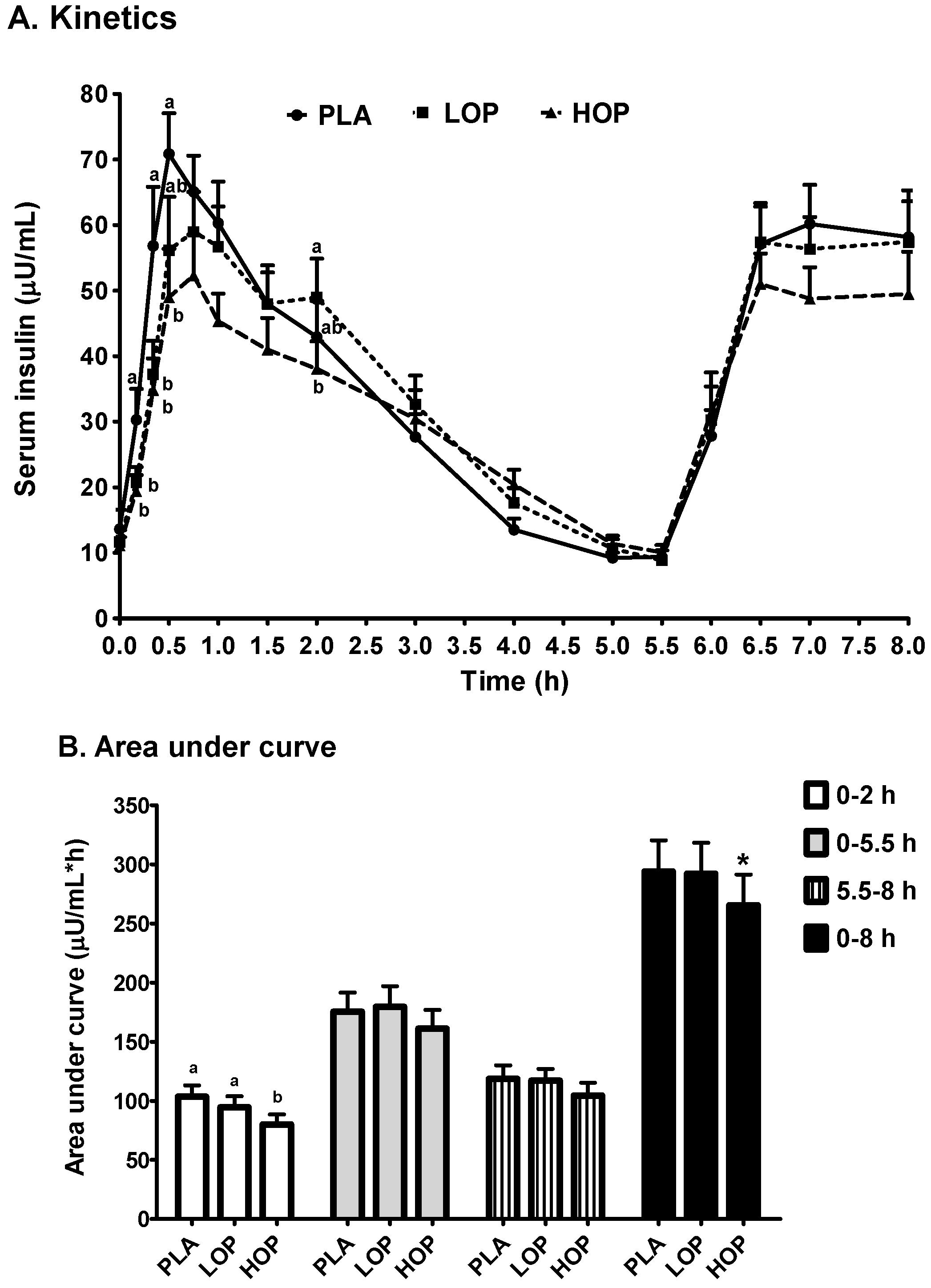
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shay, C.M.; Stamler, J.; Dyer, A.R.; Brown, I.J.; Chan, Q.; Elliott, P.; Zhao, L.; Okuda, N.; Miura, K.; Daviglus, M.L.; et al. Nutrient and food intakes of middle-aged adults at low risk of cardiovascular disease: The international study of macro-/micronutrients and blood pressure (INTERMAP). Eur. J. Nutr. 2012, 51, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Carter, P.; Gray, L.J.; Troughton, J.; Khunti, K.; Davies, M.J. Fruit and vegetable intake and incidence of type 2 diabetes mellitus: Systematic review and meta-analysis. BMJ 2010, 341, c4229. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B. Plant-based foods and prevention of cardiovascular disease: An overview. Am. J. Clin. Nutr. 2003, 78 (Suppl. S3), 544s–551s. [Google Scholar] [PubMed]
- Liu, R.H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003, 78 (Suppl. S3), 517s–520s. [Google Scholar] [PubMed]
- Brown, L.; Rosner, B.; Willett, W.W.; Sacks, F.M. Cholesterol-lowering effects of dietary fiber: A meta-analysis. Am. J. Clin. Nutr. 1999, 69, 30–42. [Google Scholar] [PubMed]
- Silva, F.M.; Kramer, C.K.; de Almeida, J.C.; Steemburgo, T.; Gross, J.L.; Azevedo, M.J. Fiber intake and glycemic control in patients with type 2 diabetes mellitus: A systematic review with meta-analysis of randomized controlled trials. Nutr. Rev. 2013, 71, 790–801. [Google Scholar] [CrossRef] [PubMed]
- Weickert, M.O.; Mohlig, M.; Koebnick, C.; Holst, J.J.; Namsolleck, P.; Ristow, M.; Osterhoff, M.; Rochlitz, H.; Rudovich, N.; Spranger, J.; et al. Impact of cereal fibre on glucose-regulating factors. Diabetologia 2005, 48, 2343–2353. [Google Scholar] [CrossRef] [PubMed]
- Whelton, S.P.; Hyre, A.D.; Pedersen, B.; Yi, Y.; Whelton, P.K.; He, J. Effect of dietary fiber intake on blood pressure: A meta-analysis of randomized, controlled clinical trials. J. Hhypertens. 2005, 23, 475–481. [Google Scholar] [CrossRef]
- Post, R.E.; Mainous, A.G.; King, D.E.; Simpson, K.N. Dietary fiber for the treatment of type 2 diabetes mellitus: A meta-analysis. J. Am. Board Fam. Med. 2012, 25, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Weickert, M.O.; Pfeiffer, A.F. Metabolic effects of dietary fiber consumption and prevention of diabetes. J. Nutr. 2008, 138, 439–442. [Google Scholar] [PubMed]
- Turner, N.D.; Lupton, J.R. Dietary fiber. Adv. Nutr. 2011, 2, 151–152. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sinha, A.K.; Makkar, H.P.; de Boeck, G.; Becker, K. Dietary roles of non-starch polysaccharides in human nutrition: A review. Crit. Rev. Food Sci. Nutr. 2012, 52, 899–935. [Google Scholar] [CrossRef] [PubMed]
- Logan, K.; Wright, A.J.; Goff, H.D. Correlating the structure and in vitro digestion viscosities of different pectin fibers to in vivo human satiety. Food Funct. 2015, 6, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Palafox-Carlos, H.; Ayala-Zavala, J.F.; Gonzalez-Aguilar, G.A. The role of dietary fiber in the bioaccessibility and bioavailability of fruit and vegetable antioxidants. J. Food Sci. 2011, 76, R6–R15. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Morand, C.; Gil-Izquierdo, A.; Bouteloup-Demange, C.; Remesy, C. Bioavailability in humans of the flavanones hesperidin and narirutin after the ingestion of two doses of orange juice. Eur. J. Clin. Nutr. 2003, 57, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Priscilla, D.H.; Roy, D.; Suresh, A.; Kumar, V.; Thirumurugan, K. Naringenin inhibits α-glucosidase activity: A promising strategy for the regulation of postprandial hyperglycemia in high fat diet fed streptozotocin induced diabetic rats. Chem. Biol. Interact. 2014, 210, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, M.H.; Lamport, D.J.; Dodd, G.F.; Saunders, C.; Harkness, L.; Butler, L.T.; Spencer, J.P. Flavonoid-rich orange juice is associated with acute improvements in cognitive function in healthy middle-aged males. Eur. J. Nutr. 2016, 55, 2021–2029. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Rendeiro, C.; Kristek, A.; Sargent, L.J.; Saunders, C.; Harkness, L.; Rowland, I.; Jackson, K.G.; Spencer, J.P.; Lovegrove, J.A. Addition of Orange Pomace to Orange Juice Attenuates the Increases in Peak Glucose and Insulin Concentrations after Sequential Meal Ingestion in Men with Elevated Cardiometabolic Risk. J. Nutr. 2016, 146, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Seltzer, H.S.; Allen, E.W.; Herron, A.L., Jr.; Brennan, M.T. Insulin secretion in response to glycemic stimulus: Relation of delayed initial release to carbohydrate intolerance in mild diabetes mellitus. J. Clin. Investig. 1967, 46, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Bonora, E. Postprandial peaks as a risk factor for cardiovascular disease: Epidemiological perspectives. Int. J. Clin. Pract. Suppl. 2002, 129, 5–11. [Google Scholar]
- Ceriello, A. Postprandial hyperglycemia and diabetes complications: Is it time to treat? Diabetes 2005, 54, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hanefeld, M.; Fischer, S.; Julius, U.; Schulze, J.; Schwanebeck, U.; Schmechel, H.; Ziegelasch, H.J.; Lindner, J. Risk factors for myocardial infarction and death in newly detected NIDDM: The Diabetes Intervention Study, 11-year follow-up. Diabetologia 1996, 39, 1577–1583. [Google Scholar] [CrossRef] [PubMed]
- Cavalot, F.; Pagliarino, A.; Valle, M.; Di Martino, L.; Bonomo, K.; Massucco, P.; Anfossi, G.; Trovati, M. Postprandial blood glucose predicts cardiovascular events and all-cause mortality in type 2 diabetes in a 14-year follow-up: Lessons from the San Luigi Gonzaga Diabetes Study. Diabetes Care 2011, 34, 2237–2243. [Google Scholar] [CrossRef] [PubMed]
- Juvonen, K.R.; Salmenkallio-Marttila, M.; Lyly, M.; Liukkonen, K.H.; Lahteenmaki, L.; Laaksonen, D.E.; Uusitupa, M.I.; Herzig, K.H.; Poutanen, K.S.; Karhunen, L.J. Semisolid meal enriched in oat bran decreases plasma glucose and insulin levels, but does not change gastrointestinal peptide responses or short-term appetite in healthy subjects. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.R.; Baka, A.; Bjorck, I.; Delzenne, N.; Gao, D.; Griffiths, H.R.; Hadjilucas, E.; Juvonen, K.; Lahtinen, S.; Lansink, M.; et al. Impact of Diet Composition on Blood Glucose Regulation. Crit. Rev. Food Sci. Nutr. 2016, 56, 541–590. [Google Scholar] [CrossRef] [PubMed]
- Karhunen, L.J.; Juvonen, K.R.; Flander, S.M.; Liukkonen, K.H.; Lahteenmaki, L.; Siloaho, M.; Laaksonen, D.E.; Herzig, K.H.; Uusitupa, M.I.; Poutanen, K.S. A psyllium fiber-enriched meal strongly attenuates postprandial gastrointestinal peptide release in healthy young adults. J. Nutr. 2010, 140, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.M.; Kramer, C.K.; Crispim, D.; Azevedo, M.J. A high-glycemic index, low-fiber breakfast affects the postprandial plasma glucose, insulin, and ghrelin responses of patients with type 2 diabetes in a randomized clinical trial. J. Nutr. 2015, 145, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Tappy, L.; Gugolz, E.; Wursch, P. Effects of breakfast cereals containing various amounts of β-glucan fibers on plasma glucose and insulin responses in NIDDM subjects. Diabetes Care 1996, 19, 831–834. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Stote, K.S.; Behall, K.M.; Spears, K.; Vinyard, B.; Conway, J.M. Glucose and insulin responses to whole grain breakfasts varying in soluble fiber, β-glucan: A dose response study in obese women with increased risk for insulin resistance. Eur. J. Nutr. 2009, 48, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, M.; Edelbroek, M.A.; Wishart, J.M.; Straathof, J.W. Relationship between oral glucose tolerance and gastric emptying in normal healthy subjects. Diabetologia 1993, 36, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Mourot, J.; Thouvenot, P.; Couet, C.; Antoine, J.M.; Krobicka, A.; Debry, G. Relationship between the rate of gastric emptying and glucose and insulin responses to starchy foods in young healthy adults. Am. J. Clin. Nutr. 1988, 48, 1035–1040. [Google Scholar] [PubMed]
- Schulze, C.; Bangert, A.; Kottra, G.; Geillinger, K.E.; Schwanck, B.; Vollert, H.; Blaschek, W.; Daniel, H. Inhibition of the intestinal sodium-coupled glucose transporter 1 (SGLT1) by extracts and polyphenols from apple reduces postprandial blood glucose levels in mice and humans. Mol. Nutr. Food Res. 2014, 58, 1795–1808. [Google Scholar] [CrossRef] [PubMed]
- Johnston, K.L.; Clifford, M.N.; Morgan, L.M. Possible role for apple juice phenolic compounds in the acute modification of glucose tolerance and gastrointestinal hormone secretion in humans. J. Sci. Food Agric. 2002, 82, 1800–1805. [Google Scholar] [CrossRef]
- Nauck, M.A.; Kemmeries, G.; Holst, J.J.; Meier, J.J. Rapid tachyphylaxis of the glucagon-like peptide 1-induced deceleration of gastric emptying in humans. Diabetes 2011, 60, 1561–1565. [Google Scholar] [CrossRef] [PubMed]
- Holt, S.H.; Miller, J.C.; Petocz, P. An insulin index of foods: The insulin demand generated by 1000-kJ portions of common foods. Am. J. Clin. Nutr. 1997, 66, 1264–1276. [Google Scholar] [PubMed]
- Jenkins, D.J.; Wolever, T.M.; Taylor, R.H.; Griffiths, C.; Krzeminska, K.; Lawrie, J.A.; Bennett, C.M.; Goff, D.V.; Sarson, D.L.; Bloom, S.R. Slow release dietary carbohydrate improves second meal tolerance. Am. J. Clin. Nutr. 1982, 35, 1339–1346. [Google Scholar] [PubMed]
- Brighenti, F.; Benini, L.; Del Rio, D.; Casiraghi, C.; Pellegrini, N.; Scazzina, F.; Jenkins, D.J.; Vantini, I. Colonic fermentation of indigestible carbohydrates contributes to the second-meal effect. Am. J. Clin. Nutr. 2006, 83, 817–822. [Google Scholar] [PubMed]
- Ropert, A.; Cherbut, C.; Roze, C.; Le Quellec, A.; Holst, J.J.; Fu-Cheng, X.; des Varannes, S.B.; Galmiche, J.P. Colonic fermentation and proximal gastric tone in humans. Gastroenterology 1996, 111, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Stevens, J.E.; Cukier, K.; Maddox, A.F.; Wishart, J.M.; Jones, K.L.; Clifton, P.M.; Horowitz, M.; Rayner, C.K. Effects of a protein preload on gastric emptying, glycemia, and gut hormones after a carbohydrate meal in diet-controlled type 2 diabetes. Diabetes Care 2009, 32, 1600–1602. [Google Scholar] [CrossRef] [PubMed]
- Augustin, L.S.; Kendall, C.W.; Jenkins, D.J.; Willett, W.C.; Astrup, A.; Barclay, A.W.; Bjorck, I.; Brand-Miller, J.C.; Brighenti, F.; Buyken, A.E.; et al. Glycemic index, glycemic load and glycemic response: An International Scientific Consensus Summit from the International Carbohydrate Quality Consortium (ICQC). Nutr. Metab. Cardiovasc. Dis. 2015, 25, 795–815. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, A.C.; Ostman, E.M.; Granfeldt, Y.; Bjorck, I.M. Effect of cereal test breakfasts differing in glycemic index and content of indigestible carbohydrates on daylong glucose tolerance in healthy subjects. Am. J. Clin. Nutr. 2008, 87, 645–654. [Google Scholar] [PubMed]
- Holst, J.J. The physiology of glucagon-like peptide 1. Physiol. Rev. 2007, 87, 1409–1439. [Google Scholar] [CrossRef] [PubMed]


| Fat (g) | Protein (g) | Carbohydrates (g) | Energy (kcal) | |
|---|---|---|---|---|
| Breakfast | ||||
| Croissant | 47 | 14 | 64 | 740 |
| Butter | 4 | 0 | 0 | 37 |
| Total | 51 | 14 | 64 | 777 |
| Lunch | ||||
| 2 Slices white bread | 2 | 8.5 | 50 | 237 |
| Soft cheese | 13 | 3.6 | 0 | 131 |
| Crisps | 9 | 1.5 | 13 | 133 |
| Shortbread biscuits | 6 | 1.4 | 16 | 127 |
| Total | 30 | 15 | 80 | 628 |
| Attributes | PLA ^ | LOP | HOP |
|---|---|---|---|
| Serum glucose (mg/dL) | 99.8 ± 2.5 | 94.7 ± 1.4 | 94.3 ± 1.3 |
| Serum insulin (µU/mL) | 13.6 ± 3.0 | 11.7 ± 1.8 | 11.1 ± 1.2 |
| Systolic blood pressure (mmHg) | 124.3 ± 2.2 | 125.3 ± 1.9 | 123.9 ± 2.3 |
| Diastolic blood pressure (mmHg) | 76.0 ± 1.3 | 76.4 ± 1.1 | 76.3 ± 1.3 |
| Heart rate (beat/min) | 71.1 ± 2.3 | 69.5 ± 2.1 | 70.4 ± 2.4 |
| Body weight (kg) | 85.1 ± 1.3 | 85.0 ± 1.3 | 84.9 ± 1.4 |
| Body mass index (kg/m2) | 27.7 ± 0.3 | 27.6 ± 0.3 | 27.6 ± 0.3 |
| Water consumed (mL/8 h) | 404 ± 24.5 | 401 ± 20.9 | 364 ± 22.8 |
| PLA ^ | LOP | HOP | |
|---|---|---|---|
| Glucose | |||
| Cmax1 (mg/dL) | 136.6 ± 4.0 | 132.1 ± 3.3 | 127.3 ± 3.0 |
| Tmax1 (min) | 33.3 ± 2.9 a | 47.2 ± 4.3 b | 45.0 ± 3.8 ab |
| Cmax2 (mg/dL) | 148.5 ± 4.8 ab | 153.6 ± 4.8 a | 139.4 ± 5.2 b |
| Tmax2 (h) | 7.0 ± 0.1 | 6.9 ± 0.1 | 6.9 ± 0.1 |
| Slope (mg/dL/h, (Tmax1 to 5.5 h)) | −7.85 ± 0.9 a | −7.72 ± 0.7 a | −5.73 ± 0.7 b |
| Insulin | |||
| Cmax1 (µU/mL) | 83.5 ± 9.3 a | 75.0 ± 8.4 a | 62.9 ± 7.4 b |
| Tmax1 (min) | 46.0 ± 3.7 a | 59.7 ± 4.7 b | 57.4 ± 5.4 ab |
| Cmax2 (µU/mL) | 70.9 ± 6.5 | 71.7 ± 6.8 | 64.4 ± 6.9 |
| Tmax2 (h) | 7.1 ± 0.1 | 7.0 ± 0.1 | 7.1 ± 0.1 |
| Slope (µU/mL/h, (Tmax1 to 5.5 h)) | −12.95 ± 1.3 a | −12.79 ± 1.7 a | −9.70 ± 1.3 b |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-Y.O.; Rasmussen, H.; Kamil, A.; Du, P.; Blumberg, J.B. Orange Pomace Improves Postprandial Glycemic Responses: An Acute, Randomized, Placebo-Controlled, Double-Blind, Crossover Trial in Overweight Men. Nutrients 2017, 9, 130. https://doi.org/10.3390/nu9020130
Chen C-YO, Rasmussen H, Kamil A, Du P, Blumberg JB. Orange Pomace Improves Postprandial Glycemic Responses: An Acute, Randomized, Placebo-Controlled, Double-Blind, Crossover Trial in Overweight Men. Nutrients. 2017; 9(2):130. https://doi.org/10.3390/nu9020130
Chicago/Turabian StyleChen, C.-Y. Oliver, Helen Rasmussen, Alison Kamil, Peng Du, and Jeffrey B. Blumberg. 2017. "Orange Pomace Improves Postprandial Glycemic Responses: An Acute, Randomized, Placebo-Controlled, Double-Blind, Crossover Trial in Overweight Men" Nutrients 9, no. 2: 130. https://doi.org/10.3390/nu9020130






