Dietary Fatty Acids and Changes in Blood Lipids during Adolescence: The Role of Substituting Nutrient Intakes
Abstract
:1. Introduction
2. Materials and Methods
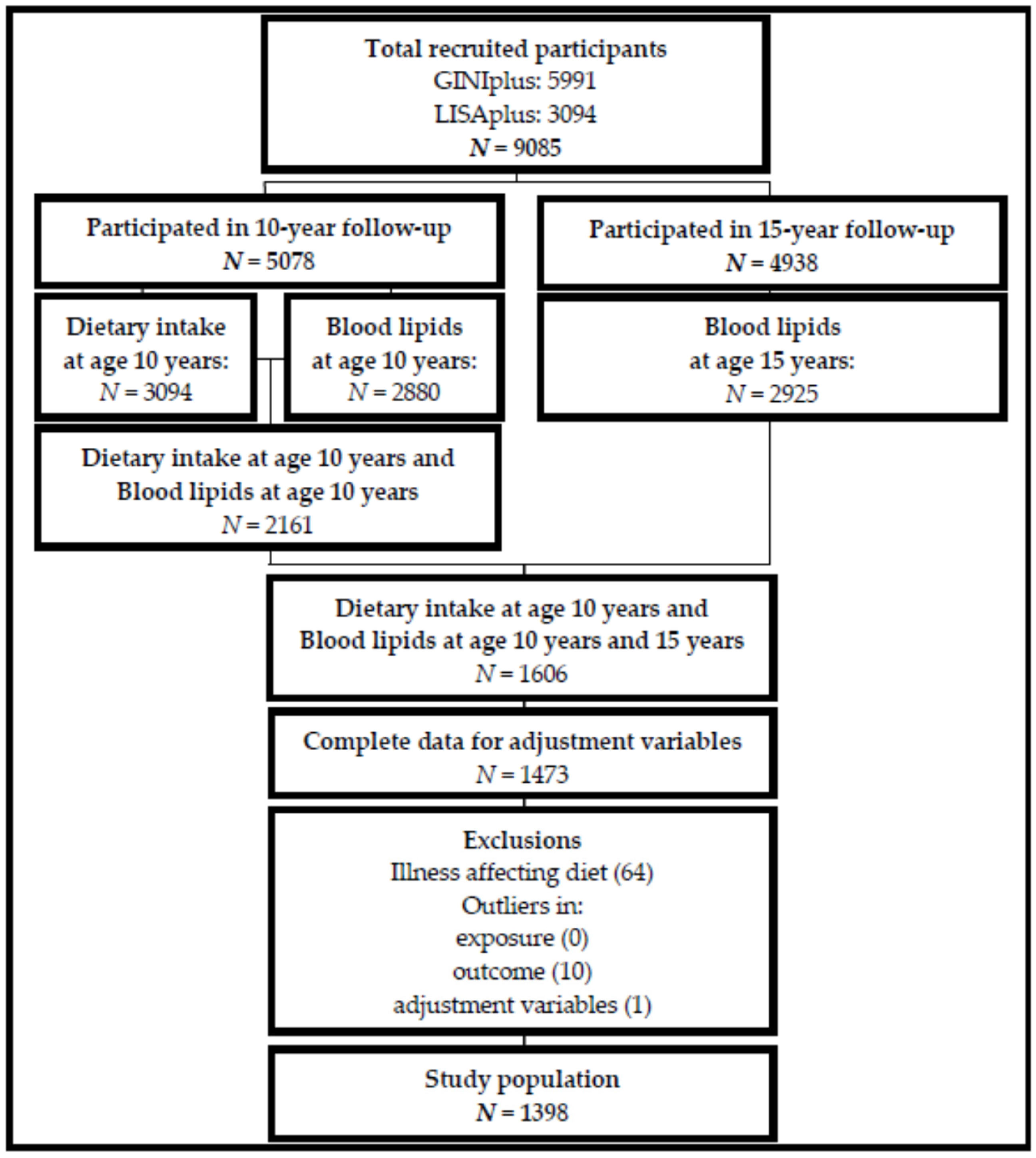
2.1. Participants
2.2. Dietary Intake
2.3. Blood Lipids
2.4. Statistical Analyses
3. Results
3.1. Study Population
3.2. Single Nutrient Models
3.3. Substitution Models
4. Discussion
4.1. Single Nutrient Model
4.2. Substitution Model
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kannel, W.B.; Dawber, T.R.; Kagan, A.; Revotskie, N.; Stokes, J., 3rd. Factors of risk in the development of coronary heart disease—Six year follow-up experience. The framingham study. Ann. Intern. Med. 1961, 55, 33–50. [Google Scholar] [CrossRef]
- Keys, A.; Anderson, J.T.; Grande, F. Serum cholesterol response to changes in the diet: Iv. Particular saturated fatty acids in the diet. Metab. Clin. Exp. 1965, 14, 776–787. [Google Scholar] [CrossRef]
- World Health Organization. Diet, Nutrition and the Prevention of Chronic Diseases; World Health Organization Technical Report Series; World Health Organization: Geneva, Switzerland, 2003; Volume 916, pp. 1–149. [Google Scholar]
- Siri-Tarino, P.W.; Sun, Q.; Hu, F.B.; Krauss, R.M. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am. J. Clin. Nutr. 2010, 91, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Mente, A.; de Koning, L.; Shannon, H.S.; Anand, S.S. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch. Intern. Med. 2009, 169, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Warnakula, S.; Kunutsor, S.; Crowe, F.; Ward, H.A.; Johnson, L.; Franco, O.H.; Butterworth, A.S.; Forouhi, N.G.; Thompson, S.G.; et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: A systematic review and meta-analysis. Ann. Intern. Med. 2014, 160, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Hodson, L.; Skeaff, C.M.; Chisholm, W.A. The effect of replacing dietary saturated fat with polyunsaturated or monounsaturated fat on plasma lipids in free-living young adults. Eur. J. Clin. Nutr. 2001, 55, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Mensink, R.P.; Zock, P.L.; Kester, A.D.; Katan, M.B. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to hdl cholesterol and on serum lipids and apolipoproteins: A meta-analysis of 60 controlled trials. Am. J. Clin. Nutr. 2003, 77, 1146–1155. [Google Scholar] [PubMed]
- Food and Agriculture Organization. Fats and Fatty Acids in human Nutrition; Report of an Expert Consultation; FAO Food and Nutrition Paper; Food and Agriculture Organization: Rome, Italy, 2010; Volume 91, pp. 1–166. [Google Scholar]
- WHO Expert Committee on Prevention in Childhood; Youth of Adult Cardiovascular Diseases World Health Organization. Prevention in Childhood and Youth of Adult Cardiovascular Diseases: Time for Action/Report of a Who Expert Committee; World Health Organization: Geneva, Switzerland, 1990. [Google Scholar]
- Juhola, J.; Magnussen, C.G.; Viikari, J.S.; Kahonen, M.; Hutri-Kahonen, N.; Jula, A.; Lehtimaki, T.; Akerblom, H.K.; Pietikainen, M.; Laitinen, T.; et al. Tracking of serum lipid levels, blood pressure, and body mass index from childhood to adulthood: The cardiovascular risk in young finns study. J. Pediatr. 2011, 159, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Nicklas, T.A.; von Duvillard, S.P.; Berenson, G.S. Tracking of serum lipids and lipoproteins from childhood to dyslipidemia in adults: The bogalusa heart study. Int. J. Sports Med. 2002, 23 (Suppl. 1), S39–S43. [Google Scholar] [CrossRef] [PubMed]
- Webber, L.S.; Srinivasan, S.R.; Wattigney, W.A.; Berenson, G.S. Tracking of serum lipids and lipoproteins from childhood to adulthood. The bogalusa heart study. Am. J. Epidemiol. 1991, 133, 884–899. [Google Scholar] [PubMed]
- Meredith, C.N.; Dwyer, J.T. Nutrition and exercise: Effects on adolescent health. Annu. Rev. Public Health 1991, 12, 309–333. [Google Scholar] [CrossRef] [PubMed]
- Rogol, A.D.; Roemmich, J.N.; Clark, P.A. Growth at puberty. J. Adolesc. Health 2002, 31, 192–200. [Google Scholar] [CrossRef]
- Praagman, J.; de Jonge, E.A.; Kiefte-de Jong, J.C.; Beulens, J.W.; Sluijs, I.; Schoufour, J.D.; Hofman, A.; van der Schouw, Y.T.; Franco, O.H. Dietary saturated fatty acids and coronary heart disease risk in a dutch middle-aged and elderly population. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2011–2018. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hruby, A.; Bernstein, A.M.; Ley, S.H.; Wang, D.D.; Chiuve, S.E.; Sampson, L.; Rexrode, K.M.; Rimm, E.B.; Willett, W.C.; et al. Saturated fats compared with unsaturated fats and sources of carbohydrates in relation to risk of coronary heart disease: A prospective cohort study. J. Am. Coll. Cardiol. 2015, 66, 1538–1548. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, M.U.; O'Reilly, E.J.; Heitmann, B.L.; Pereira, M.A.; Balter, K.; Fraser, G.E.; Goldbourt, U.; Hallmans, G.; Knekt, P.; Liu, S.; et al. Major types of dietary fat and risk of coronary heart disease: A pooled analysis of 11 cohort studies. Am. J. Clin. Nutr. 2009, 89, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.; Agostoni, C.; Elmadfa, I.; Hulshof, K.; Krause, E.; Livingstone, B.; Socha, P.; Pannemans, D.; Samartin, S. Dietary intake and nutritional status of children and adolescents in europe. Br. J. Nutr. 2004, 92 (Suppl. 2), S147–S211. [Google Scholar] [CrossRef] [PubMed]
- Luepker, R.V.; Perry, C.L.; McKinlay, S.M.; Nader, P.R.; Parcel, G.S.; Stone, E.J.; Webber, L.S.; Elder, J.P.; Feldman, H.A.; Johnson, C.C.; et al. Outcomes of a field trial to improve children’s dietary patterns and physical activity. The child and adolescent trial for cardiovascular health. Catch collaborative group. J. Am. Med. Assoc. 1996, 275, 768–776. [Google Scholar] [CrossRef]
- Cheng, H.H.; Wen, Y.Y.; Chen, C. Serum fatty acid composition in primary school children is associated with serum cholesterol levels and dietary fat intake. Eur. J. Clin. Nutr. 2003, 57, 1613–1620. [Google Scholar] [CrossRef] [PubMed]
- Bel-Serrat, S.; Mouratidou, T.; Huybrechts, I.; Labayen, I.; Cuenca-Garcia, M.; Palacios, G.; Breidenassel, C.; Molnar, D.; Roccaldo, R.; Widhalm, K.; et al. Associations between macronutrient intake and serum lipid profile depend on body fat in european adolescents: The healthy lifestyle in europe by nutrition in adolescence (helena) study. Br. J. Nutr. 2014, 112, 2049–2059. [Google Scholar] [CrossRef] [PubMed]
- Niinikoski, H.; Pahkala, K.; Ala-Korpela, M.; Viikari, J.; Rönnemaa, T.; Lagström, H.; Jokinen, E.; Jula, A.; Savolainen, M.J.; Simell, O.; et al. Effect of repeated dietary counseling on serum lipoproteins from infancy to adulthood. Pediatrics 2012, 129, e704–e713. [Google Scholar] [CrossRef] [PubMed]
- Ku, C.Y.; Gower, B.A.; Nagy, T.R.; Goran, M.I. Relationships between dietary fat, body fat, and serum lipid profile in prepubertal children. Obes. Res. 1998, 6, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Nicklas, T.A.; Dwyer, J.; Feldman, H.A.; Luepker, R.V.; Kelder, S.H.; Nader, P.R. Serum cholesterol levels in children are associated with dietary fat and fatty acid intake. J. Am. Diet. Assoc. 2002, 102, 511–517. [Google Scholar] [CrossRef]
- von Berg, A.; Kramer, U.; Link, E.; Bollrath, C.; Heinrich, J.; Brockow, I.; Koletzko, S.; Grubl, A.; Filipiak-Pittroff, B.; Wichmann, H.E.; et al. Impact of early feeding on childhood eczema: Development after nutritional intervention compared with the natural course—The giniplus study up to the age of 6 years. Clin. Exp. Allergy 2010, 40, 627–636. [Google Scholar]
- Heinrich, J.; Bolte, G.; Holscher, B.; Douwes, J.; Lehmann, I.; Fahlbusch, B.; Bischof, W.; Weiss, M.; Borte, M.; Wichmann, H.E. Allergens and endotoxin on mothers’ mattresses and total immunoglobulin E in cord blood of neonates. Eur. Respir. J. 2002, 20, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Stiegler, P.; Sausenthaler, S.; Buyken, A.E.; Rzehak, P.; Czech, D.; Linseisen, J.; Kroke, A.; Gedrich, K.; Robertson, C.; Heinrich, J. A new FFQ designed to measure the intake of fatty acids and antioxidants in children. Public Health Nutr. 2010, 13, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Willett, W. Nutritional Epidemiology; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Harris, C.; Flexeder, C.; Thiering, E.; Buyken, A.; Berdel, D.; Koletzko, S.; Bauer, C.P.; Bruske, I.; Koletzko, B.; Standl, M. Changes in dietary intake during puberty and their determinants: Results from the giniplus birth cohort study. BMC Public Health 2015, 15, 841. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, B.M.; Bell, S.; Vásquez-Caicedo, A.L.; Götz, A.; Erhardt, J.; Brombach, C. Der Bundeslebensmittelschlüssel. German Nutrient Database; Federal Research Centre for Nutrition and Food (BfEL): Karlsruhe, Germany, 2005. [Google Scholar]
- Reed, D.; McGee, D.; Yano, K.; Hankin, J. Diet, blood pressure, and multicollinearity. Hypertension 1985, 7, 405–410. [Google Scholar] [PubMed]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Wolfram, G.; Bechthold, A.; Boeing, H.; Ellinger, S.; Hauner, H.; Kroke, A.; Leschik-Bonnet, E.; Linseisen, J.; Lorkowski, S.; Schulze, M.; et al. Evidence-based guideline of the german nutrition society: Fat intake and prevention of selected nutrition-related diseases. Ann. Nutr. Metab. 2015, 67, 141–204. [Google Scholar] [CrossRef] [PubMed]
- Guillaume, M.; Lapidus, L.; Lambert, A. Differences in associations of familial and nutritional factors with serum lipids between boys and girls: The luxembourg child study. Am. J. Clin. Nutr. 2000, 72, 384–388. [Google Scholar] [PubMed]
- Samuelson, G.; Bratteby, L.E.; Mohsen, R.; Vessby, B. Dietary fat intake in healthy adolescents: Inverse relationships between the estimated intake of saturated fatty acids and serum cholesterol. Br. J. Nutr. 2001, 85, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Altwaijri, Y.A.; Day, R.S.; Harrist, R.B.; Dwyer, J.T.; Ausman, L.M.; Labarthe, D.R. Sexual maturation affects diet–blood total cholesterol association in children: Project heartbeat! Am. J. Prev. Med. 2009, 37, S65–S70. [Google Scholar] [CrossRef] [PubMed]
- Siri-Tarino, P.W.; Chiu, S.; Bergeron, N.; Krauss, R.M. Saturated fats versus polyunsaturated fats versus carbohydrates for cardiovascular disease prevention and treatment. Ann. Rev. Nutr. 2015, 35, 517–543. [Google Scholar] [CrossRef] [PubMed]
- Parks, E.J.; Hellerstein, M.K. Carbohydrate-induced hypertriacylglycerolemia: Historical perspective and review of biological mechanisms. Am. J. Clin. Nutr. 2000, 71, 412–433. [Google Scholar] [PubMed]
- Siri-Tarino, P.W.; Sun, Q.; Hu, F.B.; Krauss, R.M. Saturated fat, carbohydrate, and cardiovascular disease. Am. J. Clin. Nutr. 2010, 91, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Panel on Dietetic Products, Nutrition and Allergies. Scientific opinion on dietary reference values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2010, 8. [Google Scholar] [CrossRef] [Green Version]
- Mensink, R.P.; Katan, M.B. Effect of dietary fatty acids on serum lipids and lipoproteins. A meta-analysis of 27 trials. Arterioscler. Thromb. 1992, 12, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Katan, M. Randomized clinical trials on the effects of dietary fat and carbohydrate on plasma lipoproteins and cardiovascular disease. Am. J. Med. 2002, 113 (Suppl. 9B), 13S–24S. [Google Scholar] [CrossRef]
- Krauss, R.M.; Blanche, P.J.; Rawlings, R.S.; Fernstrom, H.S.; Williams, P.T. Separate effects of reduced carbohydrate intake and weight loss on atherogenic dyslipidemia. Am. J. Clin. Nutr. 2006, 83, 1025–1031. [Google Scholar] [PubMed]
- Te Morenga, L.A.; Howatson, A.J.; Jones, R.M.; Mann, J. Dietary sugars and cardiometabolic risk: Systematic review and meta-analyses of randomized controlled trials of the effects on blood pressure and lipids. Am. J. Clin. Nutr. 2014, 100, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Amiel, S.A.; Sherwin, R.S.; Simonson, D.C.; Lauritano, A.A.; Tamborlane, W.V. Impaired insulin action in puberty. N. Engl. J. Med. 1986, 315, 215–219. [Google Scholar] [CrossRef] [PubMed]
| Variables | Total (N = 1398) | Females (N = 681) | Males (N = 717) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 10 Years | 15 Years | p-Value a | 10 Years | 15 Years | p-Value a | 10 Years | 15 Years | p-Value a | |
| Blood lipids | |||||||||
| LDL (mmol/L) | 2.1 (1.7; 2.5) | 2.3 (1.9; 2.7) | <0.01 | 2.1 (1.8; 2.5) † | 2.4 (2.0; 2.9) † | <0.01 | 2.0 (1.7; 2.5) | 2.2 (1.8; 2.6) | <0.01 |
| HDL (mmol/L) | 1.2 (1.1; 1.4) | 1.5 (1.2; 1.7) | <0.01 | 1.2 (1.1; 1.4) | 1.6 (1.4; 1.8) † | <0.01 | 1.3 (1.1; 1.5) § | 1.4 (1.2; 1.6) | <0.01 |
| TAG (mmol/L) | 1.2 (0.9; 1.6) | 1.0 (0.8; 1.4) | <0.01 | 1.2 (0.9; 1.6) | 1.0 (0.8; 1.3) | <0.01 | 1.1 (0.8; 1.6) | 1.0 (0.7; 1.4) | <0.01 |
| TOTAL:HDL | 3.8 (3.2; 4.5) | 2.9 (2.5; 3.4) | <0.01 | 3.9 (3.4; 4.6) † | 2.9 (2.5; 3.4) | <0.01 | 3.6 (3.2; 4.4) | 3.0 (2.5; 3.5) § | <0.01 |
| Fatty acids | |||||||||
| SFA (%EI) | 12.6 (10.9; 14.7) | 12.7 (10.8; 14.7) | 0.621 | 12.5 (10.7; 14.7) | 12.6 (10.6; 14.6) | 0.190 | 12.8 (11.1; 14.8) | 12.9 (10.9; 14.9) | 0.512 |
| MUFA (%EI) | 10.7 (9.3; 12.3) | 10.8 (9.4; 12.3) | 0.133 | 10.7 (9.2; 12.1) | 10.5 (9.1; 12.2) | 0.608 | 10.7 (9.5; 12.4) | 11.2 (9.6; 12.6) § | <0.01 |
| n-3 PUFA (%EI) | 0.54 (0.49; 0.62) | 0.56 (0.49; 0.65) | <0.01 | 0.55 (0.49; 0.63) | 0.57 (0.49; 0.64) | 0.009 | 0.54 (0.48; 0.62) | 0.56 (0.48; 0.65) | <0.01 |
| n-6 PUFA (%EI) | 3.7 (3.2; 4.3) | 3.9 (3.3; 4.6) | <0.01 | 3.7 (3.2; 4.3) | 3.9 (3.3; 4.7) | 0.002 | 3.7 (3.2; 4.3) | 3.9 (3.3; 4.6) | <0.01 |
| Covariates | |||||||||
| Age (years) | 10.2 (10.1; 10.3) | 15.1 (15.0; 15.3) | <0.01 | 10.2 (10.1; 10.3) | 15.1 (15; 15.3) | <0.01 | 10.2 (10.1; 10.3) | 15.1 (15; 15.3) | <0.01 |
| BMI (kg/m2) | 16.7 (15.6; 18.4) | 20.2 (18.7; 22.2) | <0.01 | 16.8 (15.6; 18.5) | 20.4 (18.9; 22.3) | <0.01 | 16.7 (15.7; 18.4) | 20 (18.6; 22.1) | <0.01 |
| Fasting (yes) | 237 (17.0) | 649 (46.4) | <0.01 b | 121 (17.8) | 309 (45.2) | <0.01 b | 116 (16.2) | 341 (47.6) | <0.01 b |
| Screen-time (high) | 134 (9.6) | 763 (55.3) | <0.01 b | 48 (7.0) | 322 (47.8) | <0.01 b | 86 (12.0) § | 442 (62.5) § | <0.01 b |
| Energy intake (kcal) | 1933 (1591; 2292) | 2011 (1584; 2532) | <0.01 | 1798 (1486; 2124) | 1734 (1360; 2115) | 0.016 | 2061 (1705; 2447) § | 2361 (1884; 2866) § | <0.01 |
| Fat (%EI) | 30.1 (26.7; 34.2) | 30.5 (27.1; 34.8) | 0.128 | 29.9 (26.1; 33.9) | 30.0 (26.5; 34.2) | 0.982 | 30.2 (27.3; 34.4) | 30.9 (27.6; 35.3) § | 0.029 |
| Carbohydrate (%EI) | 54.1 (49.6; 58.0) | 53.2 (48.6; 57.7) | 0.004 | 54.3 (49.7; 58.4) | 54.1 (49.1; 58.4) † | 0.696 | 53.7 (49.6; 57.5) | 52.4 (47.7; 56.7) | <0.01 |
| Protein (%EI) | 14.5 (12.9; 16.0) | 14.8 (13.1; 16.6) | <0.01 | 14.4 (12.8; 16.1) | 14.5 (12.7; 16.3) | 0.452 | 14.5 (13.1; 16.0) | 15.1 (13.4; 16.8) § | <0.01 |
| Study | |||||||||
| GINI observation | 452 (32.3) | 221 (32.5) | 231 (32.2) | ||||||
| GINI intervention | 437 (31.3) | 224 (32.9) | 213 (29.7) | ||||||
| LISA | 509 (36.4) | 236 (34.7) | 273 (38.1) | ||||||
| Region | |||||||||
| Munich | 807 (57.7) | 389 (57.1) | 418 (58.3) | ||||||
| Leipzig | 123 (8.8) | 60 (8.8) | 63 (8.8) | ||||||
| Bad Honnef | 65 (4.6) | 29 (4.3) | 36 (5.0) | ||||||
| Wesel | 403 (28.8) | 203 (29.8) | 200 (27.9) | ||||||
| Parental education (High) | 997 (71.3) | 497 (73.0) | 500 (69.7) | ||||||
| Pubertal onset (Yes) | 680 (48.6) | 507 (74.4) † | 173 (24.1) | ||||||
| Fatty Acids | ΔLDL | ΔHDL | ΔTAG | ΔTOTAL:HDL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | 95% CI | p-Value | β | 95% CI | p-Value | β | 95% CI | p-Value | β | 95% CI | p-Value | ||
| TOTAL | |||||||||||||
| SFA | MBASIC | −0.038 | −0.077; 0.001 | 0.057 | 0.005 | −0.017; 0.027 | 0.653 | −0.038 | −0.075; −0.001 | 0.042 | −0.030 | −0.075; 0.015 | 0.196 |
| MADJ | −0.036 | −0.075; 0.003 | 0.068 | 0.005 | −0.017; 0.027 | 0.638 | −0.038 | −0.075; −0.001 | 0.042 | −0.029 | −0.074; 0.016 | 0.209 | |
| MUFA | MBASIC | −0.012 | −0.048; 0.024 | 0.519 | 0.011 | −0.010; 0.031 | 0.306 | −0.012 | −0.046; 0.023 | 0.507 | −0.017 | −0.059; 0.025 | 0.428 |
| MADJ | −0.011 | −0.048; 0.025 | 0.534 | 0.011 | −0.009; 0.031 | 0.297 | −0.012 | −0.046; 0.023 | 0.503 | −0.017 | −0.059; 0.025 | 0.430 | |
| n-3 PUFA | MBASIC | −0.027 | −0.058; 0.003 | 0.075 | 0.005 | −0.012; 0.022 | 0.590 | 0.001 | −0.028; 0.030 | 0.953 | −0.017 | −0.052; 0.018 | 0.342 |
| MADJ | −0.027 | −0.057; 0.003 | 0.082 | 0.005 | −0.012; 0.022 | 0.540 | 0.000 | −0.028; 0.029 | 0.981 | −0.017 | −0.053; 0.018 | 0.332 | |
| n-6 PUFA | MBASIC | −0.003 | −0.034; 0.028 | 0.866 | 0.015 | −0.002; 0.032 | 0.089 | 0.009 | −0.020; 0.039 | 0.525 | −0.012 | −0.048; 0.024 | 0.504 |
| MADJ | −0.003 | −0.034; 0.028 | 0.849 | 0.016 | −0.002; 0.033 | 0.074 | 0.009 | −0.021; 0.038 | 0.559 | −0.013 | −0.049; 0.023 | 0.465 | |
| FEMALES | |||||||||||||
| SFA | MBASIC | −0.048 | −0.110; 0.014 | 0.131 | 0.012 | −0.022; 0.045 | 0.491 | −0.053 | −0.100; −0.006 | 0.026 | −0.057 | −0.116; 0.002 | 0.060 |
| MADJ | −0.047 | −0.110; 0.015 | 0.139 | 0.012 | −0.022; 0.045 | 0.484 | −0.053 | −0.100; −0.007 | 0.025 | −0.057 | −0.116; 0.002 | 0.060 | |
| MUFA | MBASIC | −0.004 | −0.061; 0.053 | 0.888 | 0.020 | −0.011; 0.050 | 0.211 | −0.017 | −0.060; 0.026 | 0.430 | −0.026 | −0.081; 0.028 | 0.342 |
| MADJ | −0.005 | −0.062; 0.053 | 0.876 | 0.020 | −0.011; 0.050 | 0.209 | −0.018 | −0.061; 0.025 | 0.420 | −0.027 | −0.081; 0.028 | 0.336 | |
| n-3 PUFA | MBASIC | −0.030 | −0.078; 0.018 | 0.219 | 0.011 | −0.015; 0.037 | 0.406 | 0.000 | −0.036; 0.036 | 0.990 | −0.032 | −0.077; 0.014 | 0.173 |
| MADJ | −0.030 | −0.079; 0.018 | 0.214 | 0.011 | −0.015; 0.037 | 0.403 | 0.000 | −0.036; 0.036 | 0.998 | −0.032 | −0.078; 0.014 | 0.169 | |
| n-6 PUFA | MBASIC | −0.020 | −0.068; 0.028 | 0.409 | 0.019 | −0.007; 0.044 | 0.148 | 0.016 | −0.020; 0.052 | 0.390 | −0.026 | −0.071; 0.020 | 0.269 |
| MADJ | −0.021 | −0.069; 0.027 | 0.397 | 0.020 | −0.006; 0.046 | 0.124 | 0.014 | −0.022; 0.050 | 0.446 | −0.028 | −0.074; 0.018 | 0.231 | |
| MALES | |||||||||||||
| SFA | MBASIC | −0.029 | −0.078; 0.019 | 0.237 | −0.002 | −0.031; 0.026 | 0.868 | −0.016 | −0.072; 0.04 | 0.577 | 0.002 | −0.066; 0.070 | 0.962 |
| MADJ | −0.026 | −0.075; 0.022 | 0.282 | −0.003 | −0.031; 0.026 | 0.863 | −0.015 | −0.072; 0.041 | 0.591 | 0.003 | −0.065; 0.071 | 0.929 | |
| MUFA | MBASIC | −0.021 | −0.066; 0.025 | 0.372 | 0.000 | −0.027; 0.027 | 0.975 | −0.002 | −0.055; 0.051 | 0.952 | −0.001 | −0.065; 0.063 | 0.972 |
| MADJ | −0.019 | −0.065; 0.026 | 0.400 | 0.001 | −0.026; 0.027 | 0.964 | −0.002 | −0.056; 0.051 | 0.927 | −0.001 | −0.065; 0.063 | 0.968 | |
| n-3 PUFA | MBASIC | −0.026 | −0.064; 0.012 | 0.181 | −0.003 | −0.025; 0.02 | 0.818 | 0.005 | −0.040; 0.049 | 0.826 | 0.001 | −0.053; 0.055 | 0.971 |
| MADJ | −0.025 | −0.063; 0.013 | 0.199 | −0.001 | −0.023; 0.022 | 0.952 | 0.003 | −0.042; 0.048 | 0.891 | 0.000 | −0.054; 0.054 | 0.992 | |
| n-6 PUFA | MBASIC | 0.011 | −0.029; 0.051 | 0.583 | 0.010 | −0.013; 0.034 | 0.392 | 0.001 | −0.045; 0.048 | 0.953 | 0.000 | −0.056; 0.056 | 0.991 |
| MADJ | 0.013 | −0.027; 0.052 | 0.534 | 0.012 | −0.011; 0.036 | 0.299 | −0.001 | −0.047; 0.046 | 0.975 | −0.001 | −0.057; 0.055 | 0.971 | |
| Substituting Nutrient | ΔLDL | ΔHDL | ΔTAG | ΔTOTAL:HDL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | 95% CI | p-Value | β | 95% CI | p-Value | β | 95% CI | p-Value | β | 95% CI | p-Value | ||
| TOTAL | |||||||||||||
| MUFA | MBASIC | −0.037 | −0.085; 0.011 | 0.134 | 0.002 | −0.025; 0.029 | 0.897 | −0.041 | −0.087; 0.005 | 0.077 | −0.027 | −0.083; 0.029 | 0.346 |
| MADJ | −0.034 | −0.082; 0.014 | 0.163 | 0.002 | −0.025; 0.029 | 0.894 | −0.041 | −0.086; 0.005 | 0.081 | −0.025 | −0.082; 0.031 | 0.378 | |
| n-3 PUFA | MBASIC | −0.027 | −0.064; 0.011 | 0.164 | −0.003 | −0.024; 0.018 | 0.752 | 0.002 | −0.033; 0.037 | 0.913 | −0.008 | −0.052; 0.036 | 0.715 |
| MADJ | −0.026 | −0.063; 0.012 | 0.179 | −0.003 | −0.024; 0.018 | 0.775 | 0.002 | −0.034; 0.037 | 0.916 | −0.008 | −0.052; 0.036 | 0.723 | |
| n-6 PUFA | MBASIC | 0.018 | −0.019; 0.056 | 0.341 | 0.016 | −0.005; 0.037 | 0.143 | 0.019 | −0.017; 0.055 | 0.303 | −0.001 | −0.045; 0.043 | 0.978 |
| MADJ | 0.017 | −0.021; 0.054 | 0.384 | 0.016 | −0.005; 0.038 | 0.129 | 0.018 | −0.018; 0.054 | 0.324 | −0.002 | −0.047; 0.042 | 0.916 | |
| CHO | MBASIC | 0.063 | 0.000; 0.127 | 0.050 | −0.001 | −0.036; 0.035 | 0.970 | 0.057 | −0.003; 0.117 | 0.061 | 0.043 | −0.031; 0.117 | 0.257 |
| MADJ | 0.060 | −0.004; 0.123 | 0.064 | −0.001 | −0.037; 0.034 | 0.947 | 0.057 | −0.003; 0.117 | 0.063 | 0.041 | −0.033; 0.115 | 0.278 | |
| FEMALES | |||||||||||||
| MUFA | MBASIC | −0.053 | −0.130; 0.024 | 0.175 | 0.005 | −0.037; 0.046 | 0.825 | −0.065 | −0.122; −0.007 | 0.029 | −0.054 | −0.127; 0.020 | 0.153 |
| MADJ | −0.052 | −0.129; 0.025 | 0.188 | 0.005 | −0.037; 0.046 | 0.824 | −0.064 | −0.122; −0.007 | 0.029 | −0.053 | −0.127; 0.020 | 0.154 | |
| n-3 PUFA | MBASIC | −0.020 | −0.080; 0.039 | 0.502 | −0.004 | −0.036; 0.028 | 0.822 | 0.002 | −0.043; 0.047 | 0.928 | −0.003 | −0.060; 0.053 | 0.907 |
| MADJ | −0.020 | −0.079; 0.039 | 0.510 | −0.004 | −0.036; 0.028 | 0.788 | 0.003 | −0.042; 0.048 | 0.895 | −0.002 | −0.059; 0.055 | 0.942 | |
| n-6 PUFA | MBASIC | −0.004 | −0.062; 0.054 | 0.896 | 0.015 | −0.016; 0.046 | 0.341 | 0.034 | −0.009; 0.078 | 0.125 | −0.005 | −0.060; 0.050 | 0.856 |
| MADJ | −0.005 | −0.063; 0.053 | 0.868 | 0.017 | −0.014; 0.048 | 0.292 | 0.032 | −0.012; 0.075 | 0.155 | −0.008 | −0.064; 0.047 | 0.774 | |
| CHO | MBASIC | 0.127 | 0.023; 0.231 | 0.017 | −0.003 | −0.059; 0.053 | 0.916 | 0.097 | 0.019; 0.175 | 0.015 | 0.114 | 0.014; 0.213 | 0.025 |
| MADJ | 0.125 | 0.021; 0.229 | 0.019 | −0.004 | −0.060; 0.052 | 0.891 | 0.098 | 0.020; 0.176 | 0.014 | 0.115 | 0.015; 0.215 | 0.024 | |
| MALES | |||||||||||||
| MUFA | MBASIC | −0.027 | −0.088; 0.033 | 0.373 | −0.001 | −0.036; 0.035 | 0.976 | −0.015 | −0.085; 0.055 | 0.668 | −0.006 | −0.091; 0.079 | 0.898 |
| MADJ | −0.024 | −0.084; 0.036 | 0.435 | −0.001 | −0.037; 0.035 | 0.948 | −0.015 | −0.085; 0.056 | 0.683 | −0.004 | −0.089; 0.082 | 0.934 | |
| n-3 PUFA | MBASIC | −0.043 | −0.091; 0.005 | 0.079 | −0.008 | −0.037; 0.020 | 0.570 | 0.006 | −0.050; 0.062 | 0.833 | −0.009 | −0.076; 0.059 | 0.796 |
| MADJ | −0.043 | −0.090; 0.005 | 0.081 | −0.007 | −0.035; 0.021 | 0.630 | 0.005 | −0.050; 0.061 | 0.851 | −0.009 | −0.077; 0.058 | 0.788 | |
| n-6 PUFA | MBASIC | 0.041 | −0.009; 0.090 | 0.106 | 0.017 | −0.012; 0.046 | 0.256 | −0.004 | −0.061; 0.054 | 0.898 | 0.001 | −0.069; 0.071 | 0.985 |
| MADJ | 0.041 | −0.008; 0.090 | 0.103 | 0.018 | −0.011; 0.048 | 0.224 | −0.004 | −0.062; 0.053 | 0.880 | 0.000 | −0.070; 0.070 | 0.995 | |
| CHO | MBASIC | 0.017 | −0.059; 0.093 | 0.658 | −0.001 | −0.046; 0.044 | 0.961 | 0.021 | −0.068; 0.109 | 0.647 | −0.008 | −0.115; 0.099 | 0.884 |
| MADJ | 0.012 | −0.064; 0.088 | 0.755 | 0.000 | −0.045; 0.045 | 0.991 | 0.020 | −0.069; 0.108 | 0.663 | −0.011 | −0.118; 0.096 | 0.842 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harris, C.; Buyken, A.; Koletzko, S.; Von Berg, A.; Berdel, D.; Schikowski, T.; Koletzko, B.; Heinrich, J.; Standl, M. Dietary Fatty Acids and Changes in Blood Lipids during Adolescence: The Role of Substituting Nutrient Intakes. Nutrients 2017, 9, 127. https://doi.org/10.3390/nu9020127
Harris C, Buyken A, Koletzko S, Von Berg A, Berdel D, Schikowski T, Koletzko B, Heinrich J, Standl M. Dietary Fatty Acids and Changes in Blood Lipids during Adolescence: The Role of Substituting Nutrient Intakes. Nutrients. 2017; 9(2):127. https://doi.org/10.3390/nu9020127
Chicago/Turabian StyleHarris, Carla, Anette Buyken, Sibylle Koletzko, Andrea Von Berg, Dietrich Berdel, Tamara Schikowski, Berthold Koletzko, Joachim Heinrich, and Marie Standl. 2017. "Dietary Fatty Acids and Changes in Blood Lipids during Adolescence: The Role of Substituting Nutrient Intakes" Nutrients 9, no. 2: 127. https://doi.org/10.3390/nu9020127
APA StyleHarris, C., Buyken, A., Koletzko, S., Von Berg, A., Berdel, D., Schikowski, T., Koletzko, B., Heinrich, J., & Standl, M. (2017). Dietary Fatty Acids and Changes in Blood Lipids during Adolescence: The Role of Substituting Nutrient Intakes. Nutrients, 9(2), 127. https://doi.org/10.3390/nu9020127





