The Acute Effects of Simple Sugar Ingestion on Appetite, Gut-Derived Hormone Response, and Metabolic Markers in Men
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Procedure
2.3. Sample Analysis
2.4. Statistical Analysis
3. Results
3.1. Body Mass, Urine, and Drink Analysis
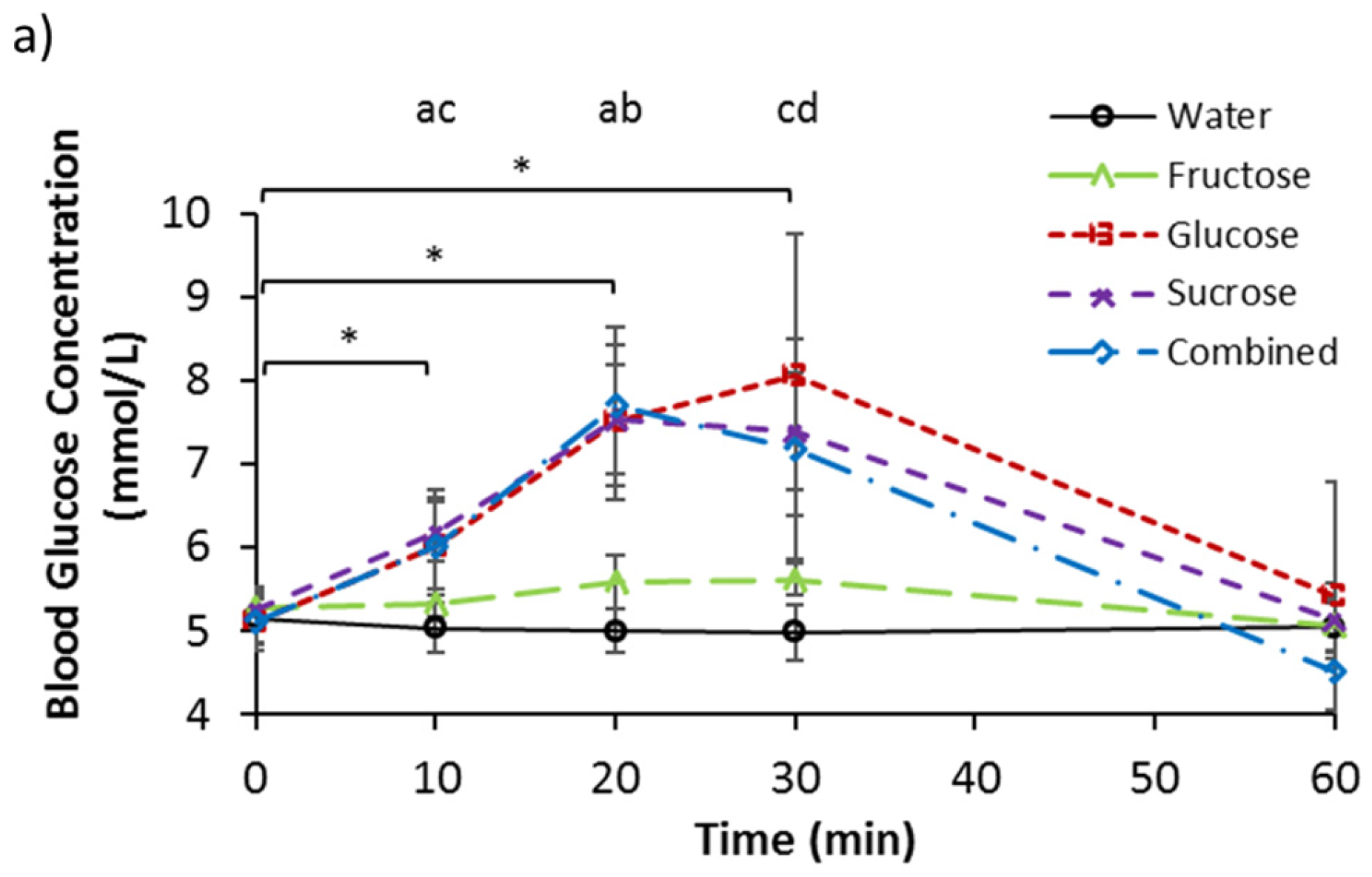
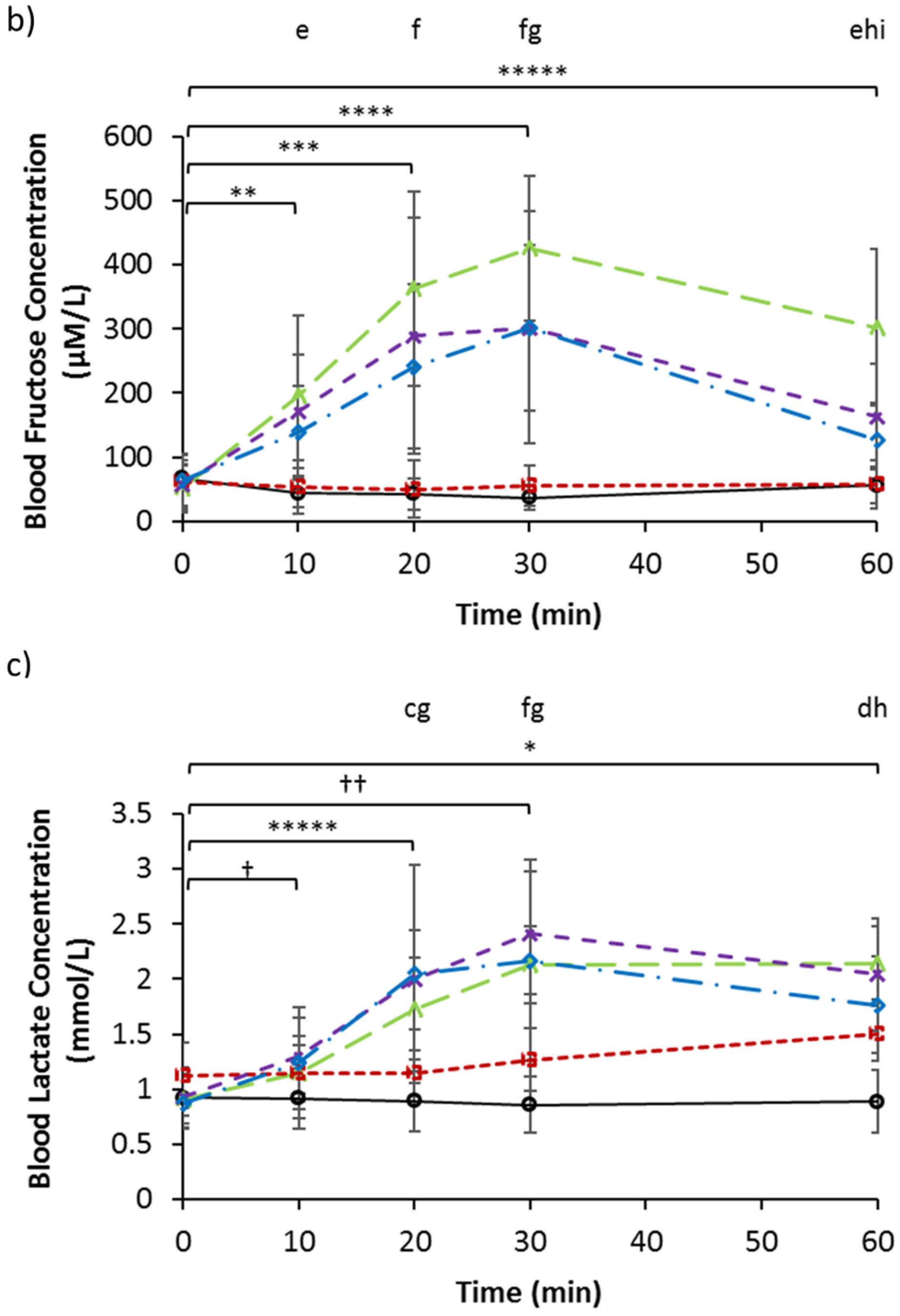
3.2. Serum Glucose, Fructose, and Lactate
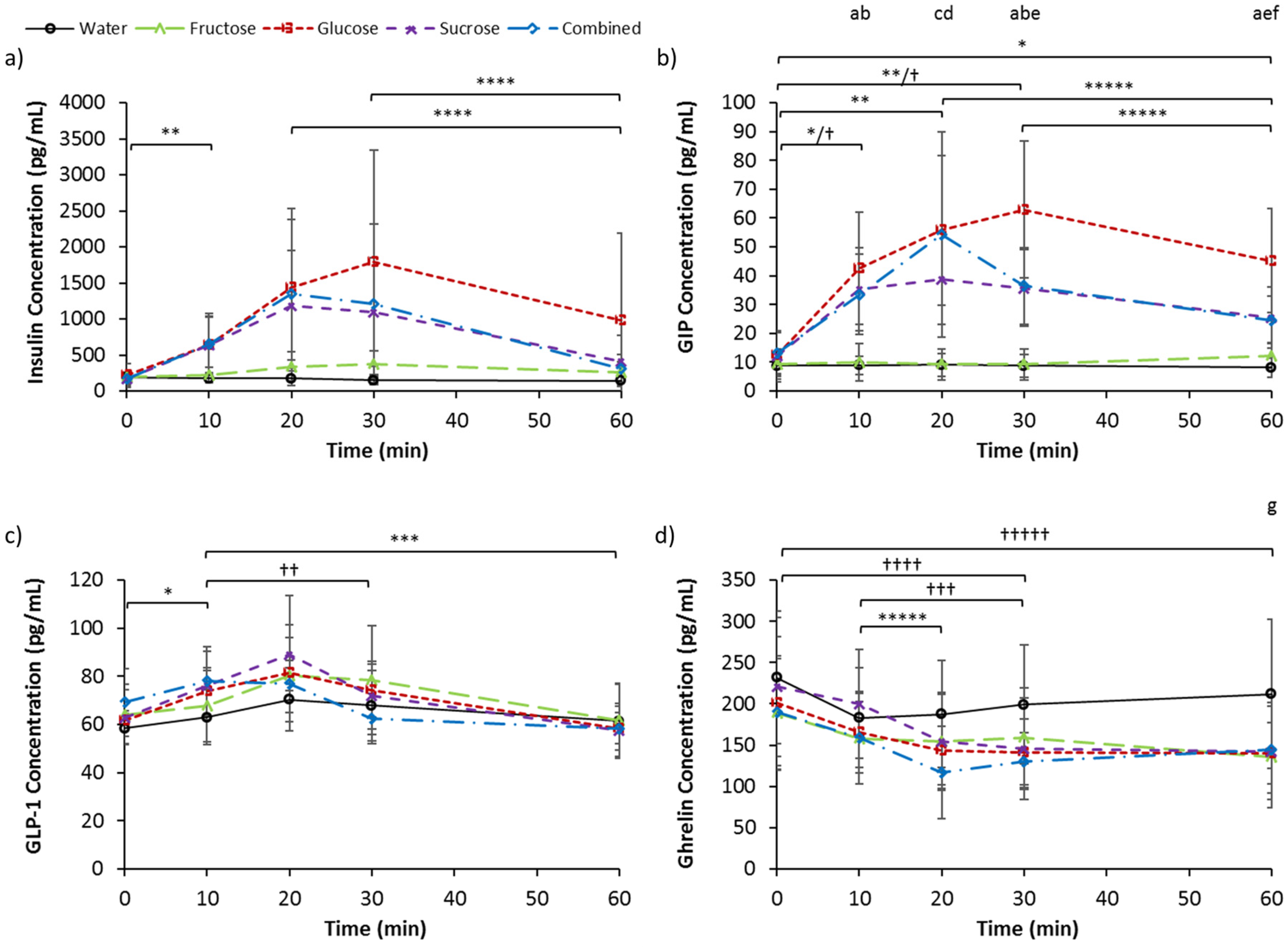
3.3. Serum Insulin, GIP, GLP-1, and Ghrelin
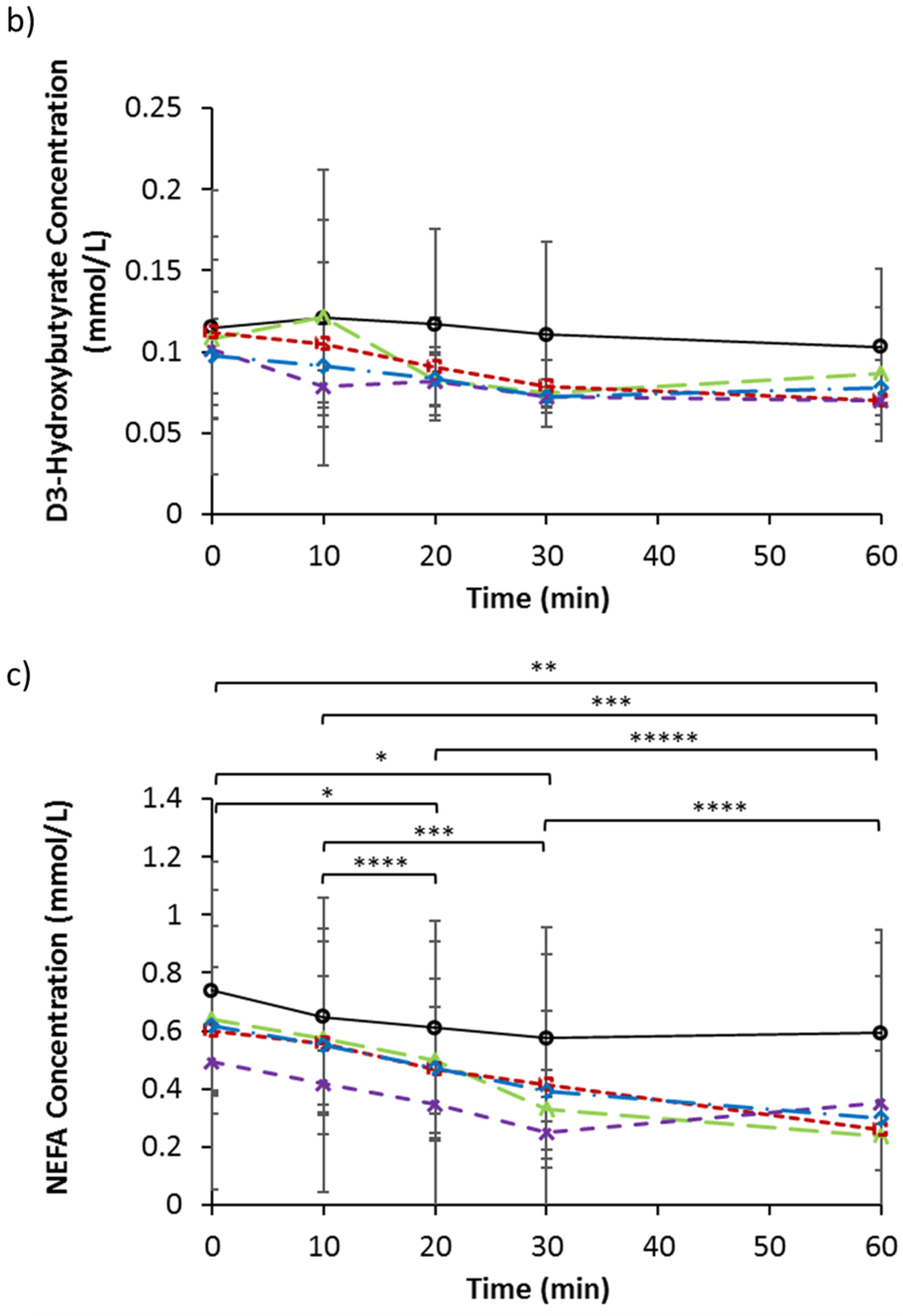
3.4. Serum Triglycerides, d-3 Hydroxybutyrate, and NEFA
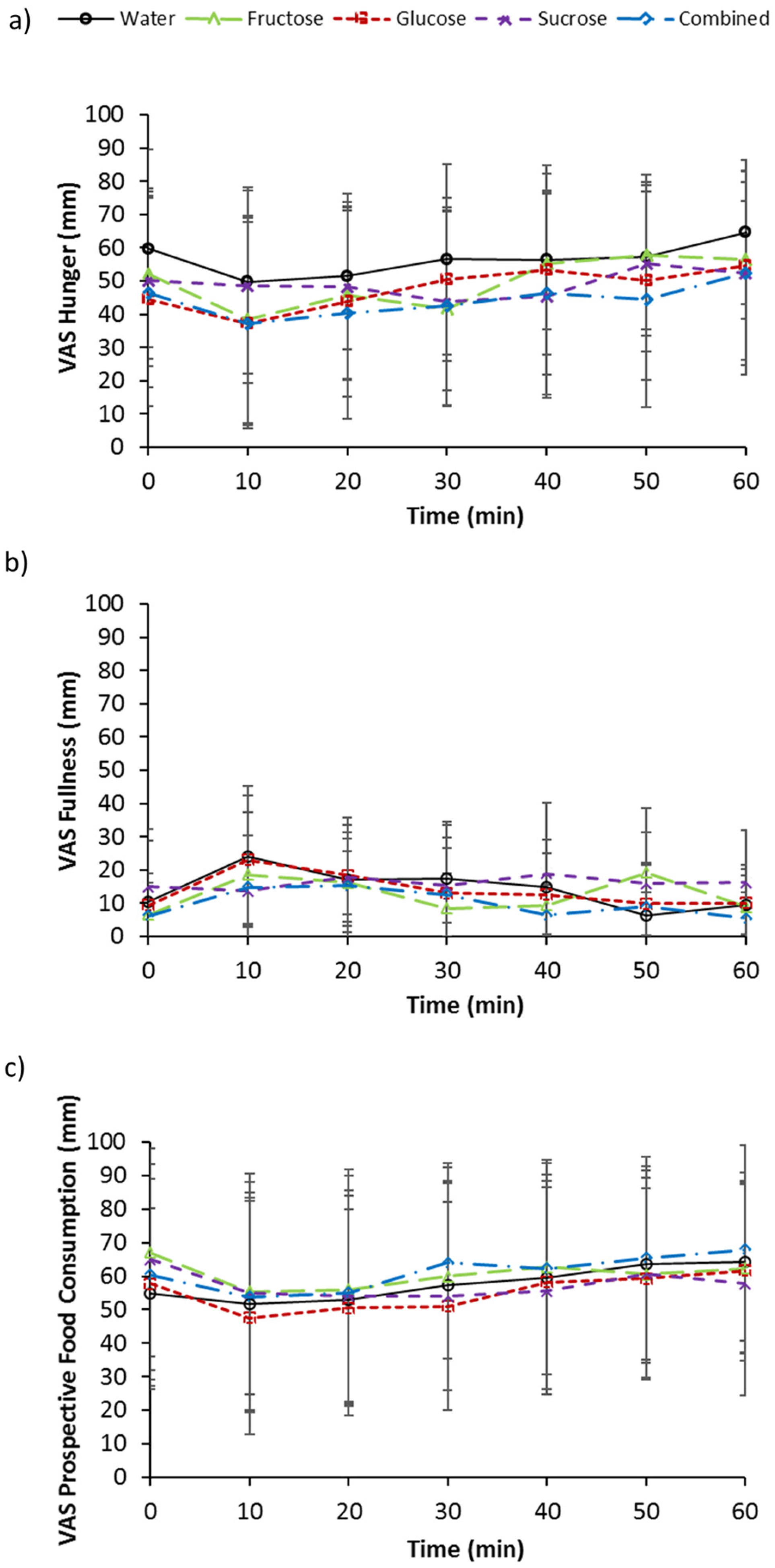
3.5. Subjective Measurements of Appetite
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Johnson, R.J.; Murray, R. Fructose, Exercise, and Health. Curr. Sports Med. Rep. 2010, 9, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, A.; Baelemans, A.; Erlanson-Albertsson, C. Effects of sucrose, glucose and fructose on peripheral and central appetite signals. Regul. Pept. 2008, 150, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.F.; Chapman, I.; Goble, E.; Wishart, J.; Wittert, G.; Morris, H.; Horowitz, M. Effects of oral fructose and glucose on plasma GLP-1 and appetite in normal subjects. Peptides 1999, 20, 545–551. [Google Scholar] [CrossRef]
- Bowen, J.; Noakes, M.; Clifton, P.M. Appetite hormones and energy intake in obese men after consumption of fructose, glucose and whey protein beverages. Int. J. Obes. 2007, 31, 1696–1703. [Google Scholar] [CrossRef] [PubMed]
- Teff, K.L.; Grudziak, J.; Townsend, R.R.; Dunn, T.N.; Grant, R.W.; Adams, S.H.; Keim, N.L.; Cummings, B.P.; Stanhope, K.L.; Havel, P.J. Endocrine and Metabolic Effects of Consuming Fructose- and Glucose-Sweetened Beverages with Meals in Obese Men and Women: Influence of Insulin Resistance on Plasma Triglyceride Responses. J. Clin. Endocrinol. Metab. 2009, 94, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Teff, K.L.; Elliott, S.S.; Tschop, M.; Kieffer, T.J.; Rader, D.; Heiman, M.; Townsend, R.R.; Keim, N.L.; D’alessio, D.; Havel, P.J. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J. Clin. Endocrinol. Metab. 2004, 89, 2963–2972. [Google Scholar] [CrossRef] [PubMed]
- Marriott, B.P.; Cole, N.; Lee, E. National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J. Nutr. 2009, 139, S1228–S1235. [Google Scholar] [CrossRef] [PubMed]
- Scientific Advisory Committee on Nutrition. Carbohydrates and Health Report. 2015. Available online: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/445503/SACN_Carbohydrates_and_Health.pdf (accessed on 1 July 2016). [Google Scholar]
- Scientific Advisory Committee on Nutrition. Dietary Reference Values for Energy. 2011. Available online: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/339317/SACN_Dietary_Reference_Values_for_Energy.pdf (accessed on 1 July 2016). [Google Scholar]
- Vos, M.B.; Lavine, J.E. Dietary fructose in nonalcoholic fatty liver disease. Hepatology 2013, 57, 2525–2531. [Google Scholar] [CrossRef] [PubMed]
- Tappy, L.; Le, K.A. Does fructose consumption contribute to non-alcoholic fatty liver disease? Clin. Res. Hepatol. Gastroenterol. 2012, 36, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Y. Review article: Fructose in non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2012, 35, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, K.L.; Bremer, A.A.; Medici, V.; Nakajima, K.; Ito, Y.; Nakano, T.; Chen, G.; Fong, T.H.; Lee, V.; Menorca, R.I.; et al. Consumption of fructose and high fructose corn syrup increase postprandial triglycerides, LDL-cholesterol, and apolipoprotein-B in young men and women. J. Clin. Endocrinol. Metab. 2011, 96, E1596–E1605. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, K.L.; Schwarz, J.M.; Keim, N.L.; Griffen, S.C.; Bremer, A.A.; Graham, J.L.; Hatcher, B.; Cox, C.L.; Dyachenko, A.; Zhang, W.; et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J. Clin. Investig. 2009, 119, 1322–1334. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, K.L.; Griffen, S.C.; Bair, B.R.; Swarbrick, M.M.; Keim, N.L.; Havel, P.J. Twenty-four-hour endocrine and metabolic profiles following consumption of high-fructose corn syrup-, sucrose-, fructose-, and glucose-sweetened beverages with meals. Am. J. Clin. Nutr. 2008, 87, 1194–1203. [Google Scholar] [PubMed]
- Ngo Sock, E.T.; Lê, K.A.; Ith, M.; Kreis, R.; Boesch, C.; Tappy, L. Effects of a short-term overfeeding with fructose or glucose in healthy young males. Br. J. Nutr. 2010, 103, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Bidwell, A.J.; Holmstrup, M.E.; Doyle, R.P.; Fairchild, T.J. Assessment of endothelial function and blood metabolite status following acute ingestion of a fructose-containing beverage. Acta Physiol. 2010, 200, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Parks, E.J.; Skokan, L.E.; Timlin, M.T.; Dingfelder, C.S. Dietary sugars stimulate fatty acid synthesis in adults. J. Nutr. 2008, 138, 1039–1046. [Google Scholar] [PubMed]
- Sun, S.Z.; Empie, M.W. Fructose metabolism in humans—What isotopic tracer studies tell us. Nutr. Metab. 2012, 9, 89. [Google Scholar] [CrossRef] [PubMed]
- Delarue, J.; Normand, S.; Pachiaudi, C.; Beylot, M.; Lamisse, F.; Riou, J.P. The contribution of naturally labeled C-13 fructose to glucose appearance in humans. Diabetologia 1993, 36, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Mueller, W.M.; Stanhope, K.L.; Gregoire, F.; Evans, J.L.; Havel, P.J. Effects of metformin and vanadium on leptin secretion from cultured rat adipocytes. Obes. Res. 2000, 8, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Truswell, A.S.; Seach, J.M.; Thorburn, A.W. Incomplete absorption of pure fructose in healthy-subjects and the facilitating effect of glucose. Am. J. Clin. Nutr. 1988, 48, 1424–1430. [Google Scholar] [PubMed]
- Kuhre, R.E.; Gribble, F.M.; Hartmann, B.; Reimann, F.; Windeløv, J.A.; Rehfeld, J.F.; Holst, J.J. Fructose stimulates GLP-1 but not GIP secretion in mice, rats, and humans. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 306, G622–G630. [Google Scholar] [CrossRef] [PubMed]
- Dushay, J.R.; Toschi, E.; Mitten, E.K.; Fisher, F.M.; Herman, M.A.; Maratos-Flier, E. Fructose ingestion acutely stimulates circulating FGF21 levels in humans. Mol. Metab. 2014, 4, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wu, J.; Zhu, J.; Kuei, C.; Yu, J.; Shelton, J.; Sutton, S.W.; Li, X.; Yun, S.J.; Mirzadegan, T.; et al. Lactate inhibits lipolysis in fat cells through activation of an orphan G-protein-coupled receptor, GPR81. J. Biol. Chem. 2009, 284, 2811–2828. [Google Scholar] [CrossRef] [PubMed]

| Water | Glucose | Fructose | Sucrose | Combined | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Body mass (kg) | 81.52 | 12.03 | 81.84 | 11.77 | 81.80 | 12.31 | 81.93 | 12.06 | 81.54 | 12.42 |
| Pre urine volume (mL) | 174 | 114 | 199 | 178 | 143 | 136 | 164 | 120 | 179 | 197 |
| Post urine volume (mL) | 613 | 268 | 639 | 226 | 411 | 254 | 577 | 400 | 596 | 331 |
| Pre urine osmolality (mOsmol/kg) | 461 | 232 | 375 | 224 | 431 | 174 | 593 | 309 | 465 | 260 |
| Post urine osmolality (mOsmol/kg) | 161 a | 101 | 137 a | 59 | 233 a | 148 | 185 a | 157 | 269 | 299 |
| Water | Glucose | Fructose | Sucrose | Combined | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Glucose (mmol/L) | 5.15 | 0.39 | 5.11 | 0.28 | 5.27 | 0.21 | 5.25 | 0.18 | 5.12 | 0.26 |
| Fructose (µM/L) | 67.34 | 19.59 | 60.70 | 41.81 | 51.75 | 37.31 | 57.95 | 36.73 | 64.62 | 40.65 |
| Lactate (mmol/L) | 0.93 | 0.27 | 1.13 | 0.30 | 0.91 | 0.23 | 0.94 | 0.18 | 0.88 | 0.24 |
| Insulin (pg/mL) | 191.4 | 88.5 | 216.9 | 163.1 | 192.1 | 102.3 | 172.4 | 103.4 | 177.7 | 89.4 |
| GIP (pg/mL) | 8.81 | 3.33 | 12.67 | 7.71 | 9.31 | 5.26 | 12.12 | 8.82 | 13.15 | 7.20 |
| GLP-1 (pg/mL) | 58.4 | 6.3 | 61.7 | 4.2 | 64.1 | 12.5 | 62.9 | 11.4 | 69.2 | 14.2 |
| Ghrelin (pg/mL) | 232.1 | 79.6 | 200.9 | 80.2 | 189.0 | 68.8 | 220.7 | 84.2 | 189.9 | 65.1 |
| Triglycerides (mmol/L) | 1.20 | 0.47 | 1.19 | 0.59 | 1.29 | 0.62 | 1.13 | 0.56 | 1.13 | 0.52 |
| d-3 Hydroxybutyrate (mmol/L) | 0.12 | 0.06 | 0.11 | 0.09 | 0.11 | 0.05 | 0.10 | 0.03 | 0.10 | 0.02 |
| NEFA (mmol/L) | 0.74 | 0.35 | 0.60 | 0.22 | 0.64 | 0.32 | 0.50 | 0.12 | 0.62 | 0.56 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yau, A.M.W.; McLaughlin, J.; Gilmore, W.; Maughan, R.J.; Evans, G.H. The Acute Effects of Simple Sugar Ingestion on Appetite, Gut-Derived Hormone Response, and Metabolic Markers in Men. Nutrients 2017, 9, 135. https://doi.org/10.3390/nu9020135
Yau AMW, McLaughlin J, Gilmore W, Maughan RJ, Evans GH. The Acute Effects of Simple Sugar Ingestion on Appetite, Gut-Derived Hormone Response, and Metabolic Markers in Men. Nutrients. 2017; 9(2):135. https://doi.org/10.3390/nu9020135
Chicago/Turabian StyleYau, Adora M. W., John McLaughlin, William Gilmore, Ronald J. Maughan, and Gethin H. Evans. 2017. "The Acute Effects of Simple Sugar Ingestion on Appetite, Gut-Derived Hormone Response, and Metabolic Markers in Men" Nutrients 9, no. 2: 135. https://doi.org/10.3390/nu9020135
APA StyleYau, A. M. W., McLaughlin, J., Gilmore, W., Maughan, R. J., & Evans, G. H. (2017). The Acute Effects of Simple Sugar Ingestion on Appetite, Gut-Derived Hormone Response, and Metabolic Markers in Men. Nutrients, 9(2), 135. https://doi.org/10.3390/nu9020135





