S?Allylmercaptocysteine Attenuates Cisplatin?Induced Nephrotoxicity through Suppression of Apoptosis, Oxidative Stress, and Inflammation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture and Drug Treatment
2.3. Cell Viability Assay
2.4. DAPI Staining
2.5. Flow Cytometric Analysis
2.6. Animals and Drug Treatment
2.7. Renal Function Monitoring
2.8. Measurement of Oxidative Stress Markers and Antioxidant Enzymes Activities
2.9. Measurement of Kidney TNF-α and IL-1β Levels
2.10. Detection of Apoptosis
2.11. Histopathological Examination and Immunohistochemistry for Detection of NF-κB
2.12. Western Blot Analysis
2.13. Statistical Analysis
3. Results
3.1. SAMCAttenuates Cisplatin-Induced Cytotoxicity in HK-2 Cells
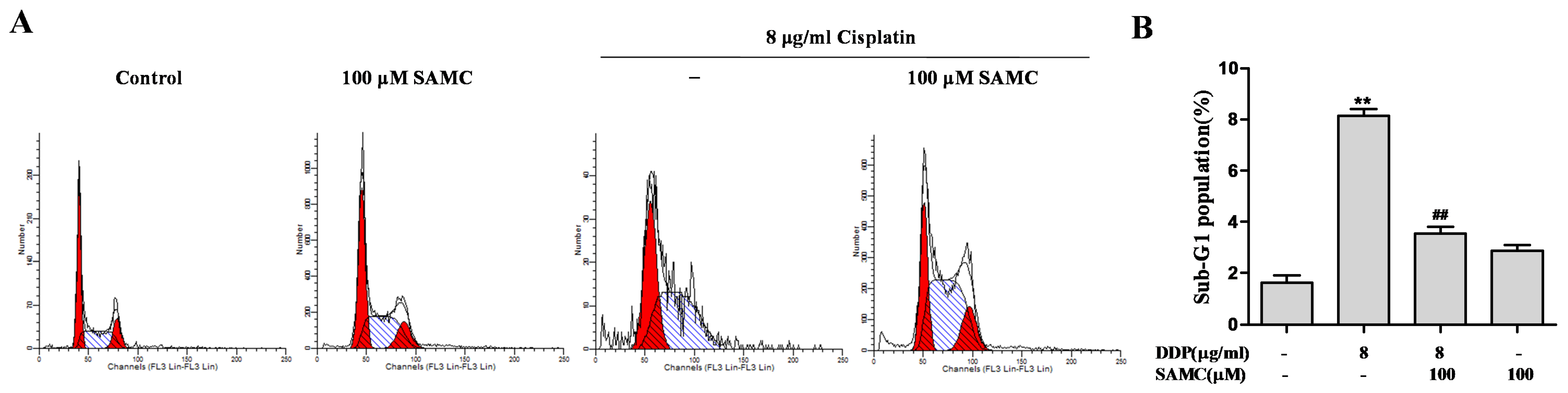
3.2. SAMCRescues HK-2 Cells from Cisplatin-Induced Apoptosis
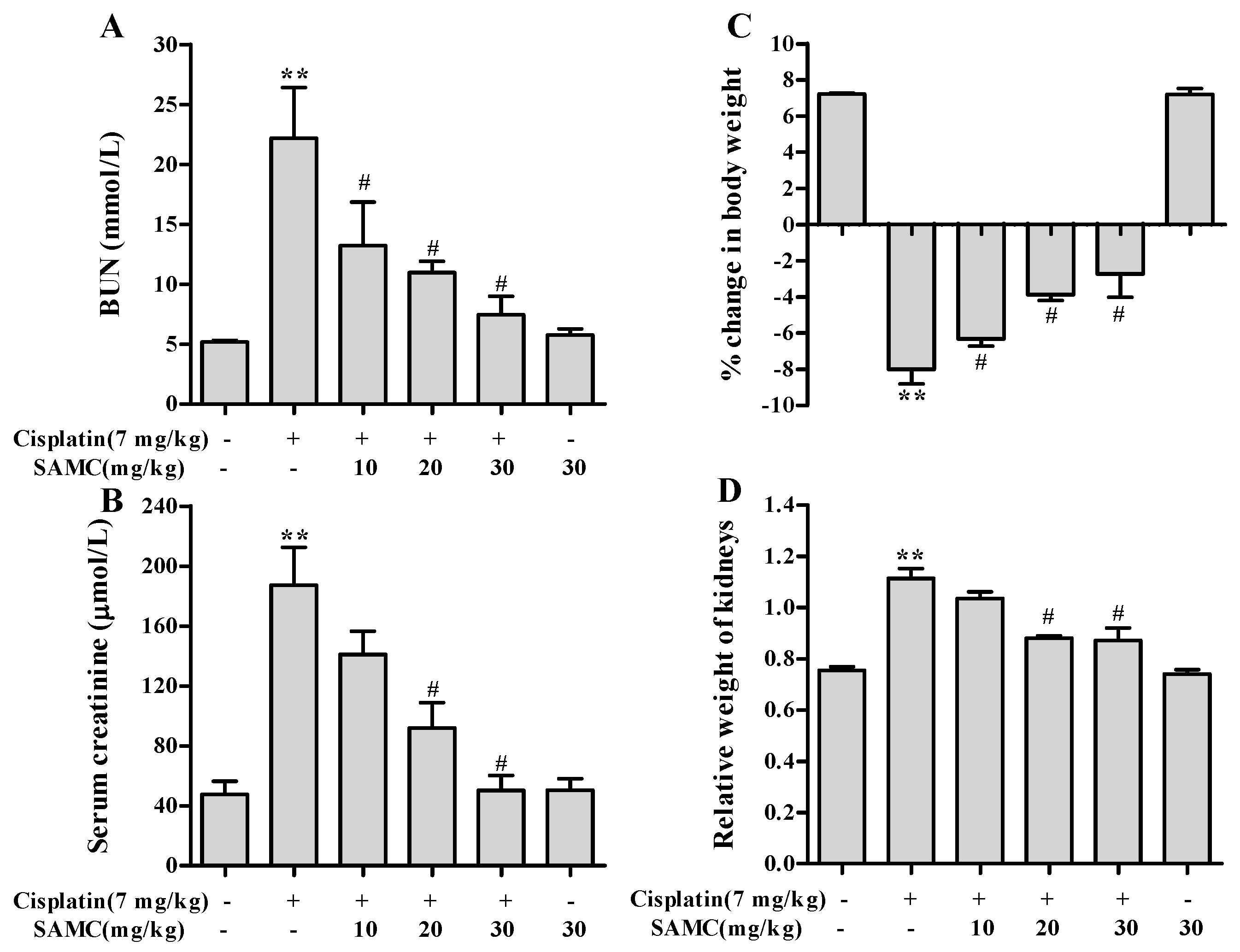
3.3. Effect of SAMC on Cisplatin-Induced Renal Injury Parameters In Vivo
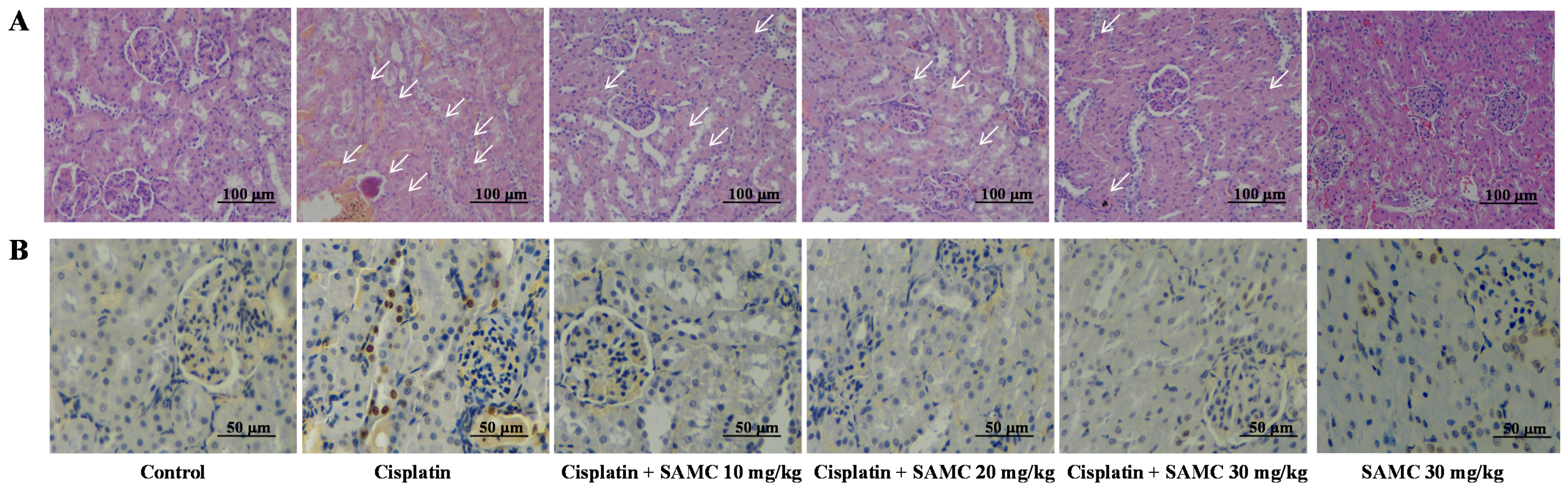
3.4. SAMC Ameliorates Tubular Necrosis and Apoptosisin Cisplatin-Induced Renal Injury
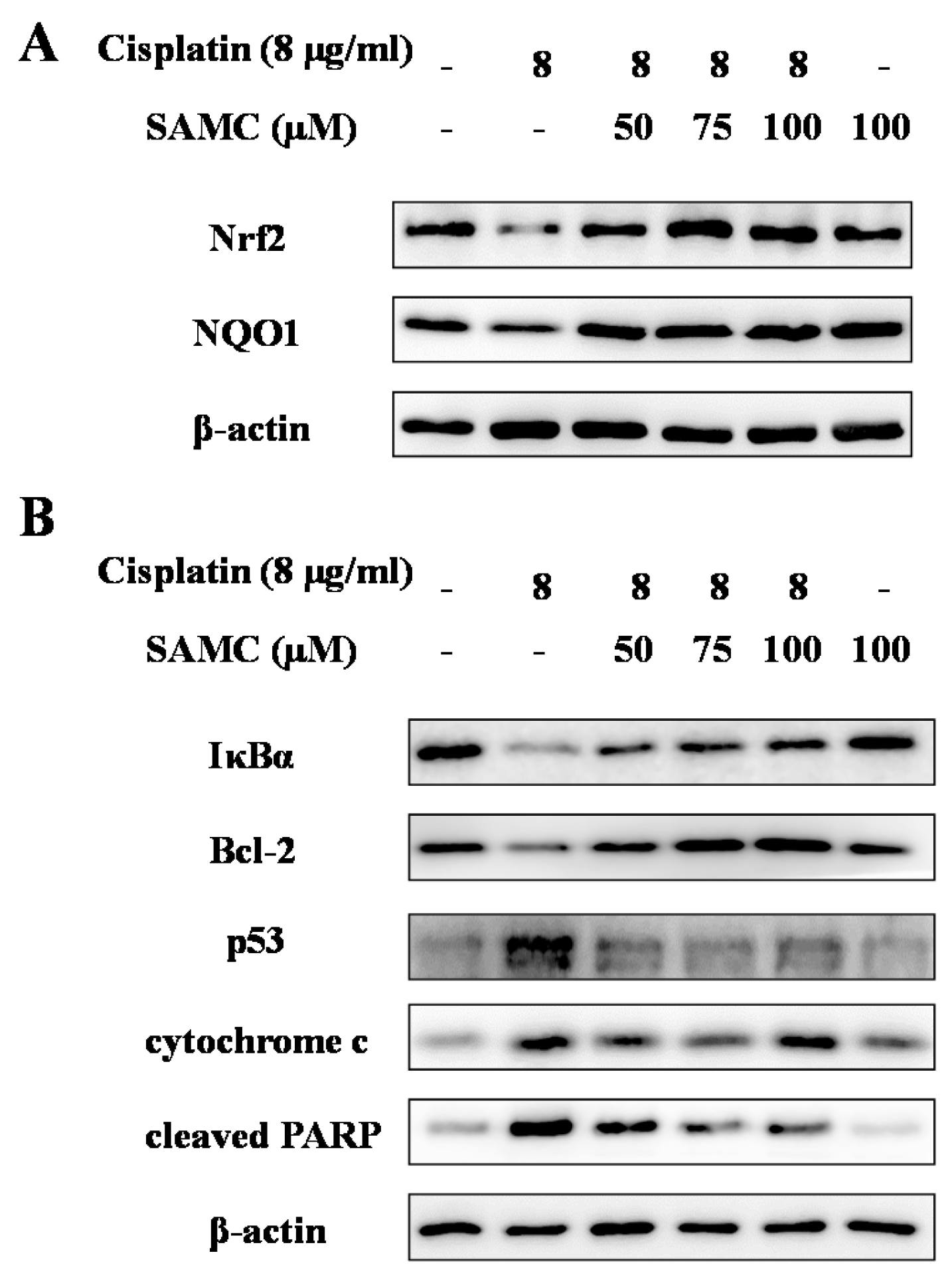
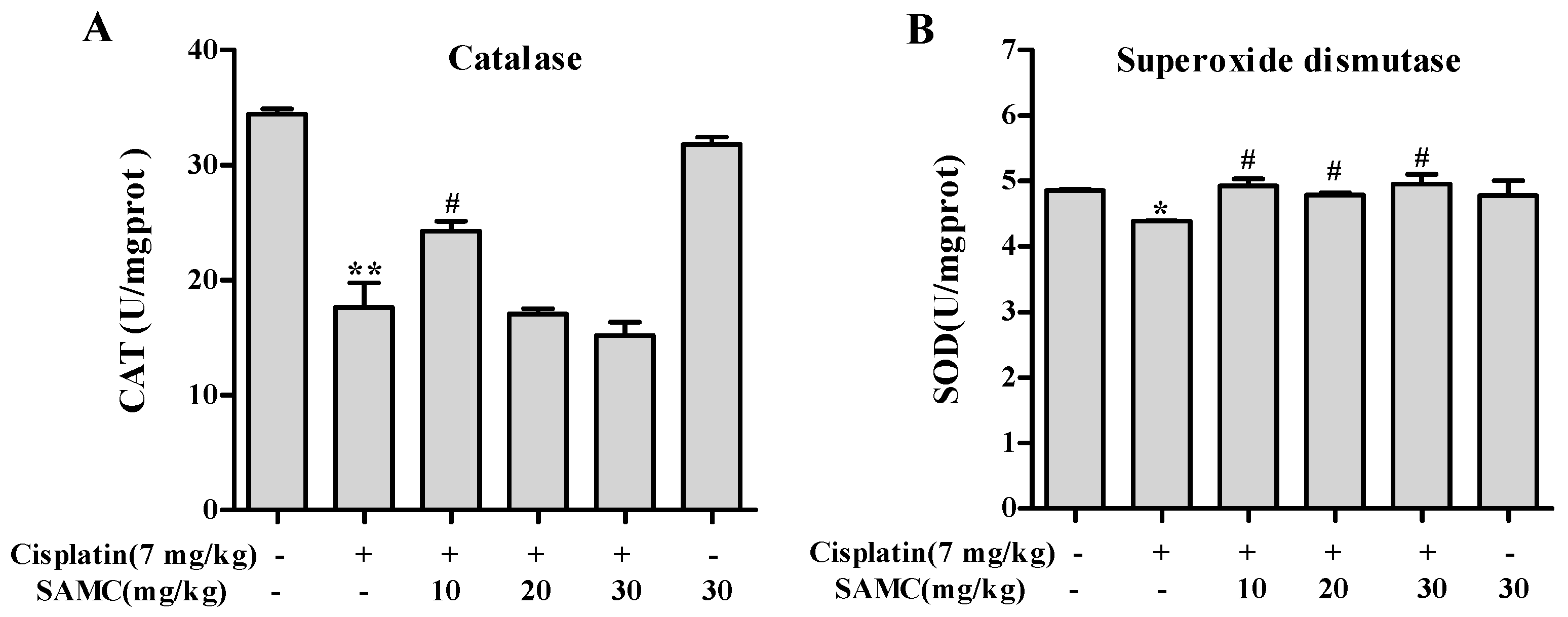
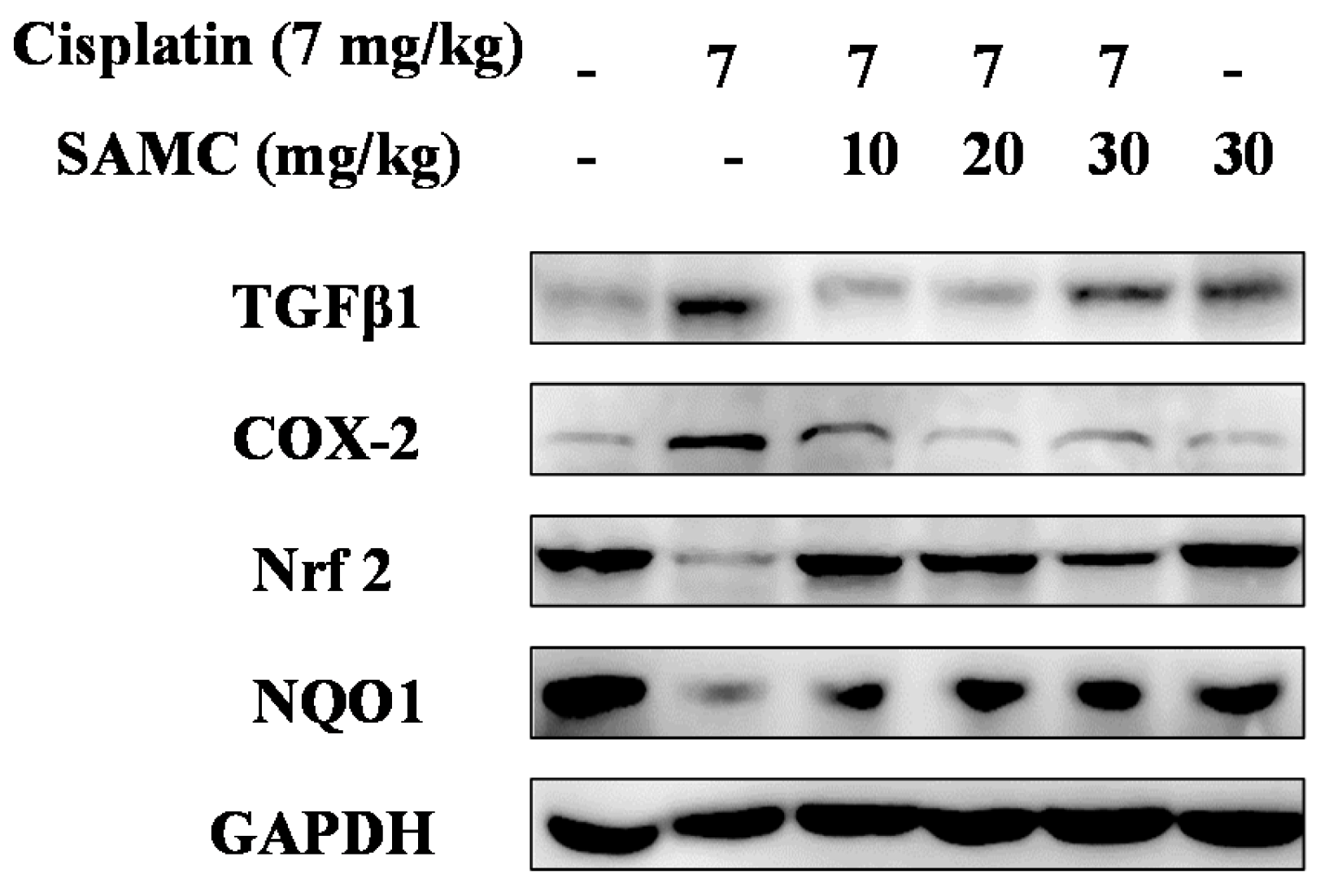
3.5. SAMC Attenuates Cisplatin-Induced Oxidative Stress and BoostsRenal Antioxidant Defenses
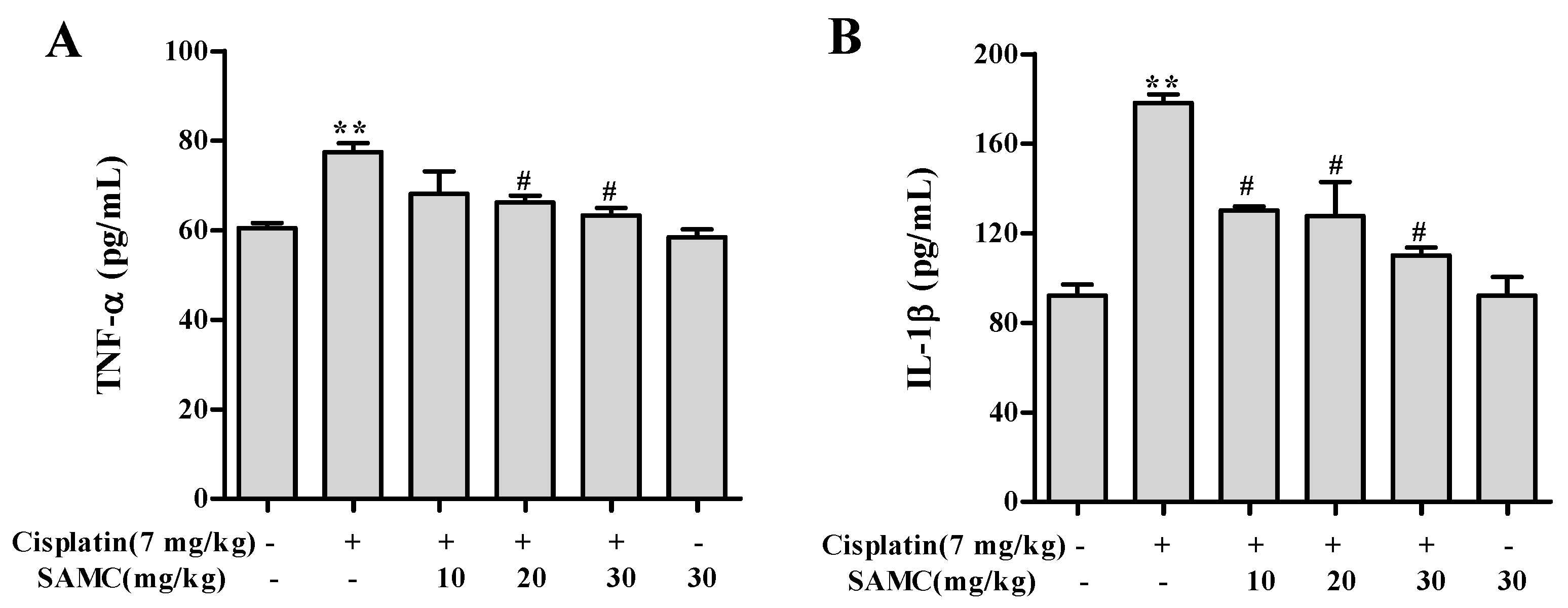
3.6. SAMCSuppresses the Inflammatory Events in Cisplatin-Induced Nephrotoxicity
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Osanto, S.; Bukman, A.; Van Hoek, F.; Sterk, P.J.; De Laat, J.A.; Hermans, J. Long-term effects of chemotherapy in patients with testicular cancer. J. Clin. Oncol. 1992, 10, 574–579. [Google Scholar] [PubMed]
- Hartmann, J.T.; Lipp, H.P. Toxicity of platinum compounds. Expert Opin. Pharmacother. 2003, 4, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Winston, J.A.; Safirstein, R. Reduced renal blood flow in early cisplatin-induced acute renal failure in the rat. Am. J. Physiol. 1985, 249, F490–F496. [Google Scholar] [PubMed]
- Ramesh, G.; Reeves, W.B. TNF-alpha mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J. Clin. Investig. 2002, 110, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Baliga, R.; Ueda, N.; Walker, P.D.; Shah, S.V. Oxidant mechanisms in toxic acute renal failure. Drug Metab. Rev. 1999, 31, 971–997. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Peng, H.; Dong, L.; Chen, L.; Ma, X.; Peng, Y.; Dai, S.; Liu, Q. Activation of the Nrf2-are signalling pathway by the Lentinula edodes polysaccharose INT alleviates ROS-mediated cisplatin nephrotoxicity. Int. Immunopharmacol. 2016, 36, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, G.; Reeves, W.B. P38 map kinase inhibition ameliorates cisplatin nephrotoxicity in mice. Am. J. Physiol. Ren. Physiol. 2005, 289, F166–F174. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Dong, Z. Regulation and pathological role of p53 in cisplatin nephrotoxicity. J. Pharmacol. Exp. Ther. 2008, 327, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, N.A.; Carvalho Rodrigues, M.A.; Martins, N.M.; dos Santos, A.C. Cisplatin-induced nephrotoxicity and targets of nephroprotection: An update. Arch. Toxicol. 2012, 86, 1233–1250. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yan, M.H.; Liu, Y.; Liu, Z.; Wang, Z.; Chen, C.; Zhang, J.; Sun, Y.S. Ginsenoside Rg5 ameliorates cisplatin-induced nephrotoxicity in mice through inhibition of inflammation, oxidative stress, and apoptosis. Nutrients 2016, 8, E566. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.M.; Ban, J.O.; Park, K.R.; Lee, C.K.; Jeong, H.S.; Han, S.B.; Hong, J.T. Potential therapeutic effects of functionally active compounds isolated from garlic. Pharmacol. Ther. 2014, 142, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Imai, J.; Ide, N.; Nagae, S.; Moriguchi, T.; Matsuura, H.; Itakura, Y. Antioxidant and radical scavenging effects of aged garlic extract and its constituents. Planta Med. 1994, 60, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, Y.; Qi, Q.; Zhang, F.; Zhang, Y.; Zhu, X.; Liu, G.; Luan, Y.; Zhao, Z.; Cai, J.; et al. Inhibitory effects of S-allylmercaptocysteine against benzo(a)pyrene-induced precancerous carcinogenesis in human lung cells. Int. Immunopharmacol. 2016, 34, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Pedraza-Chaverri, J.; Barrera, D.; Maldonado, P.D.; Chirino, Y.I.; Macias-Ruvalcaba, N.A.; Medina-Campos, O.N.; Castro, L.; Salcedo, M.I.; Hernandez-Pando, R. S-allylmercaptocysteine scavenges hydroxyl radical and singlet oxygen in vitro and attenuates gentamicin-induced oxidative and nitrosative stress and renal damage in vivo. BMC Clin. Pharmacol. 2004, 4, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maldonado, P.D.; Barrera, D.; Medina-Campos, O.N.; Hernandez-Pando, R.; Ibarra-Rubio, M.E.; Pedraza-Chaverri, J. Aged garlic extract attenuates gentamicin induced renal damage and oxidative stress in rats. Life Sci. 2003, 73, 2543–2556. [Google Scholar] [CrossRef]
- Starkenmann, C.; Niclass, Y.; Troccaz, M. Nonvolatile S-alk(en)ylthio-l-cysteine derivatives in fresh onion (allium cepa L. Cultivar). J. Agric. Food Chem. 2011, 59, 9457–9465. [Google Scholar] [CrossRef] [PubMed]
- Houghton, P.; Fang, R.; Techatanawat, I.; Steventon, G.; Hylands, P.J.; Lee, C.C. The sulphorhodamine (SRB) assay and other approaches to testing plant extracts and derived compounds for activities related to reputed anticancer activity. Methods 2007, 42, 377–387. [Google Scholar] [CrossRef] [PubMed]
- An, Y.T.; Zhao, Z.; Sheng, Y.C.; Min, Y.; Xia, Y.Y. Therapeutic time window of YGY-E neuroprotection of cerebral ischemic injury in rats. Drug Discov. Ther. 2011, 5, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Pedrycz, A.; Czerny, K. Immunohistochemical study of proteins linked to apoptosis in rat fetal kidney cells following prepregnancy adriamycin administration in the mother. Acta Histochem. 2008, 110, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.Y.; Cho, C.H.; Park, K.G.; Lee, H.J.; Lee, S.; Park, S.K.; Lee, I.K.; Koh, G.Y. Tumor necrosis factor-alpha induces fractalkine expression preferentially in arterial endothelial cells and mithramycin a suppresses TNF-α-induced fractalkine expression. Am. J. Pathol. 2004, 164, 1663–1672. [Google Scholar] [CrossRef]
- Pabla, N.; Dong, Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 2008, 73, 994–1007. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Pi, J.; Woods, C.G.; Andersen, M.E. A systems biology perspective on Nrf2-mediated antioxidant response. Toxicol. Appl. Pharmacol. 2010, 244, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, G.; Reeves, W.B. Inflammatory cytokines in acute renal failure. Kidney Int. Suppl. 2004, 91, S56–S61. [Google Scholar] [CrossRef] [PubMed]
- Nasr, A.Y.; Saleh, H.A. Aged garlic extract protects against oxidative stress and renal changes in cisplatin-treated adult male rats. Cancer Cell Int. 2014, 14, 92. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Ching, Y.P.; Liong, E.C.; Nanji, A.A.; Fung, M.L.; Tipoe, G.L. Garlic-derived S-allylmercaptocysteine is a hepato-protective agent in non-alcoholic fatty liver disease in vivo animal model. Eur. J. Nutr. 2013, 52, 179–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pecere, T.; Sarinella, F.; Salata, C.; Gatto, B.; Bet, A.; Dalla Vecchia, F.; Diaspro, A.; Carli, M.; Palumbo, M.; Palu, G. Involvement of p53 in specific anti-neuroectodermal tumor activity of aloe-emodin. Int. J. Cancer 2003, 106, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Park, H.M.; Cho, J.M.; Lee, H.R.; Shim, G.S.; Kwak, M.K. Renal protection by 3H-1,2-dithiole-3-thione against cisplatin through the Nrf2-antioxidant pathway. Biochem. Pharmacol. 2008, 76, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Sahu, B.D.; Rentam, K.K.; Putcha, U.K.; Kuncha, M.; Vegi, G.M.; Sistla, R. Carnosic acid attenuates renal injury in an experimental model of rat cisplatin-induced nephrotoxicity. Food Chem. Toxicol. 2011, 49, 3090–3097. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y. Induction of apoptosis by S-allylmercapto-l-cysteine, a biotransformed garlic derivative, on a human gastric cancer cell line. Int. J. Mol. Med. 2008, 21, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, P.; Pan, H.; Rajesh, M.; Batkai, S.; Patel, V.; Harvey-White, J.; Mukhopadhyay, B.; Hasko, G.; Gao, B.; Mackie, K.; et al. Cb1 cannabinoid receptors promote oxidative/nitrosative stress, inflammation and cell death in a murine nephropathy model. Br. J. Pharmacol. 2010, 160, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Peres, L.A.; da Cunha, A.D., Jr. Acute nephrotoxicity of cisplatin: Molecular mechanisms. J. Bras. Nefrol. 2013, 35, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. Signaling to NF-κB. Genes Dev. 2004, 18, 2195–2224. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Martinez, A.B.; Benito Martinez, S.; Lucio Cazana, F.J. Intracellular prostaglandin E2 mediates cisplatin-induced proximal tubular cell death. Biochim. Biophys. Acta 2016, 1863, 293–302. [Google Scholar] [CrossRef] [PubMed]



© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Jiang, X.; Li, A.; Zhao, Z.; Li, S. S?Allylmercaptocysteine Attenuates Cisplatin?Induced Nephrotoxicity through Suppression of Apoptosis, Oxidative Stress, and Inflammation. Nutrients 2017, 9, 166. https://doi.org/10.3390/nu9020166
Zhu X, Jiang X, Li A, Zhao Z, Li S. S?Allylmercaptocysteine Attenuates Cisplatin?Induced Nephrotoxicity through Suppression of Apoptosis, Oxidative Stress, and Inflammation. Nutrients. 2017; 9(2):166. https://doi.org/10.3390/nu9020166
Chicago/Turabian StyleZhu, Xiaosong, Xiaoyan Jiang, Ang Li, Zhongxi Zhao, and Siying Li. 2017. "S?Allylmercaptocysteine Attenuates Cisplatin?Induced Nephrotoxicity through Suppression of Apoptosis, Oxidative Stress, and Inflammation" Nutrients 9, no. 2: 166. https://doi.org/10.3390/nu9020166
APA StyleZhu, X., Jiang, X., Li, A., Zhao, Z., & Li, S. (2017). S?Allylmercaptocysteine Attenuates Cisplatin?Induced Nephrotoxicity through Suppression of Apoptosis, Oxidative Stress, and Inflammation. Nutrients, 9(2), 166. https://doi.org/10.3390/nu9020166




